Abstract
Application of chondroitinase ABC (ChABC) to injured peripheral nerves improves axon regeneration, but it is not known whether functional recovery is also improved. Recordings of EMG activity [soleus (Sol) M response and H reflexes] evoked by nerve stimulation and of Sol and tibialis anterior (TA) EMG activity and hindlimb and foot kinematics during slope walking were made to determine whether ChABC treatment of the sciatic nerve at the time of transection improves functional recovery. Recovery of evoked EMG responses began as multiple small responses with a wide range of latencies that eventually coalesced into one or two more distinctive and consistent responses (the putative M response and the putative H reflex) in both groups. Both the initial evoked responses and the time course of their maturation returned sooner in the ChABC group than in the untreated (UT) group. The reinnervated Sol and TA were coactivated during treadmill locomotion during downslope, level, and upslope walking throughout the study period in both UT and ChABC-treated rats. By 10 wk after nerve transection and repair, locomotor activity in Sol, but not TA, had returned to its pretransection pattern. There was an increased reliance on central control of Sol activation across slopes for both groups as interpreted from elevated prestance Sol EMG activity that was no longer modulated with slope. Limb length and orientation during locomotion were similar to those observed prior to nerve injury during upslope walking only in the ChABC-treated rats. Thus treatment of cut nerves with ChABC leads to improvements in functional recovery.
Keywords: compound muscle action potential, electromyographic activity, H reflex, kinematics, locomotion
the reason most often cited for poor functional outcomes following peripheral nerve injuries, both in laboratory animals and human patients, is that axons fail to regenerate completely enough to restore muscle and sensory function (Fawcett and Keynes 1990). After a peripheral nerve is cut and repaired, the environment of the distal stump is changing as axons from the proximal stump begin to form regenerative sprouts that extend toward the endoneurial tubes of the distal stump. These sprouts must enter the endoneurial tubes and extend in the distal stump of the nerve to reach their former targets (Stoll and Muller 1999; Tona et al. 1993). In the Schwann cell basal lamina in this regeneration pathway, they encounter growth-promoting molecules, such as laminin and fibronectin (Ferguson and Muir 2000; Gorio et al. 1998; Tabb et al. 1994; Trigg et al. 1998), as well as molecules that inhibit growth. The best-known of these inhibitory molecules are the chondroitin sulfate proteoglycans (CSPGs) (Dou and Levine 1994; McKeon et al. 1991, 1995; Zuo et al. 1998a).
Core glycoproteins of four CSPGs are present in peripheral nerves: versican and decorin (Braunewell et al. 1995), NG2 (Diers-Fenger et al. 2001; Martin et al. 2001), and neurite inhibitory factor (NIF) (Muir et al. 1989; Zuo et al. 1998a). On these core proteins are glycosaminoglycan (GAG) side chains composed of chondroitin sulfate. Because of their large size and negative charge, the GAGs of CSPGs are thought to hinder neurite access to growth-promoting matrix molecules, thereby inhibiting their growth (reviewed in Bovolenta et al. 1993, but cf., e.g., Jones et al. 2003). Recent evidence shows that chondroitin sulfate also inhibits growth of regenerating axons by binding to a transmembrane protein tyrosine phosphatase receptor (Shen et al. 2009).
Muir and co-workers, in a series of studies, showed that CSPGs present in the distal stump of cut peripheral nerves inhibited neurite growth from cultured sensory neurons (Krekoski et al. 2001), that this inhibition increased during the first week after nerve injury (Zuo et al. 1998a), and, most importantly, that the inhibition could be attributed to their chondroitin sulfate-containing GAG side chains (Zuo et al. 1998a, 1998b). Treatment of cultures with the bacterial enzyme chondroitinase ABC (ChABC), which degrades only the GAG side chains of CSPGs, removed the inhibition. A single injection of injured rat peripheral nerves with this enzyme at the time of transection and repair resulted in enhanced axon regeneration during the first week after transection of the sciatic nerve in vivo (Krekoski et al. 2001; Zuo et al. 2002).
In our laboratory we found that soaking either nerve grafts used to repair cut peripheral nerves or the distal stumps of cut nerves in ChABC for 1 h at the time of surgical repair of the nerve resulted in a significant enhancement of the growth of regenerating axons (Groves et al. 2005). A much greater proportion of regenerating axons entered the distal stump of treated cut nerves than in untreated controls. However, it is not completely clear whether axon regeneration enhanced with ChABC treatment also results in improved function. A recent study reported encouraging results of ChABC treatment on functional recovery after peripheral nerve injury. The direct electrical and contractile response of the triceps surae to electrical stimulation of the sciatic nerve was improved, and ankle kinematics were better during walking on a neutral incline (Beaumont et al. 2009). The goal of the present study was to expand on those findings by examining the time course of recovery of the muscle response to nerve stimulation (i.e., the M response) and the H reflex after sciatic nerve transection in rats. This study also investigated muscle activity and hindlimb kinematics during locomotion at negative, neutral, and positive slopes at 4 and 10 wk postoperatively. Treatment with ChABC shortened the time course of muscle reinnervation. Although locomotor EMG activity of reinnervated muscles does not entirely return to the pattern and intensity of activity noted prior to nerve transection, locomotor function is significantly improved compared with untreated controls.
METHODS
Surgical procedures.
Female Sprague-Dawley rats (Rattus norvegicus) weighing 270–315 g were tested in this study. Rats were housed one per cage in a temperature- and humidity-controlled room with 12:12-h light-dark cycles. They were allowed normal cage activities under standard laboratory conditions, fed with standard chow and water ad libitum, and evaluated daily by clinical veterinarians for signs of discomfort and pain. All procedures were approved by the Institutional Animal Care and Use Committee at Emory University.
The protocol for implanting stimulating and recording electrodes has been described in detail in our previous papers (Sabatier et al. 2011a, 2011b). Each rat was implanted with EMG wires under general anesthesia using pentobarbital sodium (60 mg/kg) or ketamine (75 mg/kg) and xylazine (10 mg/kg) administered intraperitoneally and supplemented as needed. All surgical procedures were performed under aseptic conditions. Pairs of multistranded (10 × 50 gauge, Cooner, AS631) stainless steel fine-wire electrodes stripped of their final 1 mm of Teflon insulation were implanted in the right soleus (Sol) and tibialis anterior (TA) muscles. A small bipolar cuff electrode was placed on the right posterior tibial nerve and was closed by a suture that encircled the cuff. This cuff electrode was constructed from a short length of Silastic tubing and the same type of wire used for EMG electrodes. The tubing was slit longitudinally, and the wires were sewn into it at a spacing of 2–3 mm. These cuff electrodes have been described in detail by others (Sweeney et al. 1990). Wires from EMG electrodes and the nerve cuff were led subcutaneously to a six-conductor plug mounted onto the skull of the rat with stainless steel bone screws and dental acrylic. All materials needed to formulate this percutaneous interface were obtained from Plastics One (Roanoke, VA).
Prior to implantation each rat was trained to walk on a single-lane Plexiglas-enclosed treadmill (53 cm × 10 cm × 14 cm; Columbus Instruments, Columbus, OH) intermittently for 3–4 days, 5–10 min/day, at 11 m/min, at each of three slopes (downslope, level, and upslope), and given a Froot Loop reward. Three to five training sessions of 5–10 min were given during a 1-wk period prior to implantation. Stress was minimized with low noise levels and gentle handling of the rats in order to help ensure acclimation and to obtain locomotion under normal conditions. Mild intensities of foot shock were used as negative reinforcement to improve performance. Rats that refused to walk on the treadmill during acclimation were excluded from the study.
The right sciatic nerve was transected just proximal to the branch point of the sural nerve with sharp scissors in an Untreated group of rats (n = 5) and a ChABC-treated group of rats (n = 5). For some analyses the sample size was <5 because of subject or equipment attrition. Therefore, sample size is identified for all figures. For all nerve repairs, the proximal and distal segments of the cut nerves were aligned as carefully as possible on a small piece of Gore-Tex and secured in place under very little tension with fibrin glue (English 2005; MacGillivray 2003; Menovsky and Beek 2001). This completed the surgical manipulation of the sciatic nerve for Untreated rats. The distal segment of the severed sciatic nerve in ChABC-treated rats was soaked with protease-free ChABC (from Proteus vulgaris, E.C. 4.2.2.4) obtained from Seikagaku (Tokyo, Japan). We showed in a previous paper that using the enzyme ChABC in this manner in our lab effectively removed the GAG side chains from the CSPGs (Groves et al. 2005).
Evoked potential recordings.
EMG activity evoked by stimulation of the cuff on the tibial nerve was recorded in intact and injured rats. Electrical activity in the Sol muscle was amplified and recorded with a laboratory computer system and custom software. Animals were allowed to move about freely in a small enclosure while tethered to the recording equipment. When spontaneous EMG activity was maintained at a low level for at least 1 s, single stimulus pulses were delivered to the tibial nerve via the cuff electrode under computer control and recordings of evoked activity were saved to hard disk. Stimulus intensity was increased gradually from a predetermined level to record a large range of muscle responses. Stimuli were delivered no less than 3 s apart.
Beginning 7 days after sciatic nerve transection, EMG activity evoked by stimulation of the cuff on the tibial nerve below the lesion was monitored on a daily basis to determine whether muscle reinnervation had begun. Once a direct muscle or M response (compound muscle action potential) could be recorded from rats with a sciatic nerve lesion, it was assumed that muscle reinnervation had begun. For each rat studied, we determined the earliest time at which a restored M response was noted. Significance of differences in this timing between Untreated and ChABC-treated rats was evaluated with an unpaired t-test (P < 0.05 considered significant).
In Intact rats, the response to electrical stimulation of the tibial nerve is two discrete periods of EMG activity in Sol. A first response typically occurs between 1 and 5 ms after simulation and is known as the direct muscle or M response. The second typically begins ∼5 ms after stimulation and lasts for 5–10 ms. It represents the synaptic activation of motoneurons in response to afferent axon stimulation—the H reflex. A typical M response and H reflex recorded in one Intact animal are shown in Fig. 1.
Fig. 1.
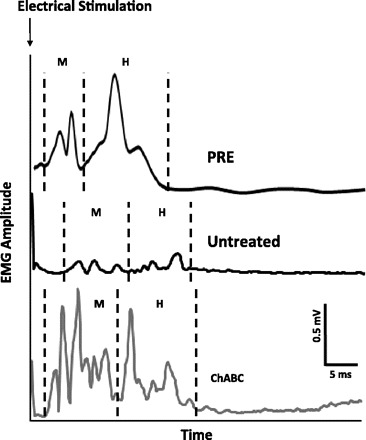
Typical soleus (Sol) EMG activity responses to tibial nerve stimulation in a representative Intact rat (PRE) and 2 rats 4 wk after sciatic nerve transection, 1 Untreated and 1 chondroitinase ABC (ChABC) treated. Traces are averaged from ≥25 stimulus presentations. Poststimulation time periods when EMG activity associated with the M response and H reflex occurs are noted at bottom (M and H, respectively).
Analysis of functional recovery.
Hair was closely clipped from around the right hindlimb while the rat was anesthetized with isoflurane. Marks for digitizing were applied with a permanent marker over the right greater trochanter, lateral malleolus, and fifth metatarsophalangeal (MTP) joints to approximate the end points of these hindlimb segments. Each rat walked on the treadmill while it was level (0°) and at two different slope conditions, i.e., ±20° (±36.4%), and slope order was randomized. Multiple 15-s locomotion trials were collected for each slope to ensure that at least five step cycles were recorded where the rat was walking at a constant speed. Step cycles were selected for analysis from video records obtained during locomotor trials. A cycle was selected if the rat was walking in the same place on the belt and did not ride the belt backward or accelerate forward in the preceding step cycles. The beginning of a step cycle was defined as the first video frame in which the foot was touching the belt and ended at the last video frame of the following swing phase. The Plexiglas treadmill enclosure helped control parallax error by minimizing lateral position variation on the treadmill belt.
Kinematics.
Sagittal plane gait kinematics was obtained from two-dimensional video records of treadmill locomotion. A single Dragonfly Express high-speed digital camera (Point-Grey Research, Vancouver, BC, Canada) was used to record motion pictures (120 fps) of the right side of the rat while walking on the treadmill. The camera was placed orthogonal to the rat, and magnification was calibrated to cover the entire length of the treadmill belt. Video was streamed through an IEEE-1394 port and recorded to the computer's hard drive as AVI files at 620 × 480 pixels and codified in 256 gray levels. MaxTRAQ (Innovision Systems, Columbiaville, MI) and ImageJ (Rasband 1997–2006) software was used to digitize the markers over the hip, ankle, and MTP joints for each frame of a step cycle, and custom-written LabView software (National Instruments, Austin, TX) was used to calculate joint angles.
To study hindlimb movements during walking, a single global kinematic variable, the vector between the MTP and hip marker positions, was computed from the digitized points with custom LabView software (Chang et al. 2009; Hamilton et al. 2011; Sabatier et al. 2011a, 2011b). The magnitude of this vector is referred to as limb length, and the direction of this vector, measured as the rostral orientation of the extensible hindlimb to the treadmill belt, is referred to as limb angle. The digitized points were also used to determine the included angle of the foot to the treadmill belt (measured caudally; shown in Sabatier et al. 2011a, 2011b).
Electromyography.
Locomotor EMG activity was recorded from the Sol and TA muscles and synchronized to concurrently collected digitized video with a common pulse. Potentials were amplified (×1,000) with a band pass of 30–1,000 Hz and then digitized for each 15-s locomotion trial with custom-written LabView software. Frame numbers corresponding with the onset (paw-on) and termination (next paw-on) of selected step cycles were recorded from videos and then used to extract the corresponding locomotor EMG activity. The extracted EMG activity from individual step cycles was rectified and low-pass filtered with a time constant of 20 ms and then time-normalized to 100 time bins. Therefore, all figures containing locomotor EMG activity have a time scale of 1–100. In figures in this article locomotor EMG intensity has been normalized to the maximum locomotor EMG intensity recorded from each rat (usually found during upslope locomotion). Average EMG activity during muscle activation was calculated as the average of such normalized EMG activity during the step cycle that was ≥5% of the maximum EMG activity recorded from each rat (Gregor et al. 2006).
Principal components analysis.
Principal components analysis (PCA) was used to evaluate differences in EMG profiles between muscles and time points. PCA is a data reduction method that uses structure detection to express two or more correlated variables with one or more uncorrelated variables that are termed principal components (Bishop 1995). The proportion of overall variance explained by each principal component is termed its eigenvalue. The first principal component necessarily counts for the largest proportion of the variance in the data (i.e., it has the largest eigenvalue). Each succeeding principal component explains the maximum amount of the remaining variance and is uncorrelated to the previous principal component.
Each average EMG profile (i.e., 1 per slope, per muscle, for each rat) was incorporated in the data set as previously described (Sabatier et al. 2011b). Varimax normalization was used to obtain the maximum variance explained by each principal component (Widmer et al. 2003). Principal component loadings were determined for each EMG profile for each principal component. Each principal component loading represents the correlation coefficient between the EMG activity profile and the regression line representing each principal component. The principal component loadings for each principal component were averaged for each muscle at each time point for each of the three groups of rats studied.
Statistical analysis.
Descriptive statistics were calculated for variables of interest, including those representing aspects of the step cycle, hindlimb kinematics, and EMG amplitudes and timing. Data are reported as means ± SE unless otherwise noted. Student's t-tests and analysis of variance with post hoc tests [Fisher's least significant difference (LSD)] were used to determine whether differences between groups were statistically significant. The significance level was set at 0.05 for all statistical tests.
RESULTS
Timing of step cycle.
The step cycle in this study was measured as the period between successive paw contacts with the treadmill belt. We studied the timing of the step cycle during locomotion in Intact rats and in rats at 4 and 10 wk after sciatic nerve transection (Table 1). Changes in both stance and step cycle duration with slope in Intact rats were reported in a previous study (Sabatier et al. 2011b). Step cycle duration and stance duration were virtually unaffected by injury. The only exception was an increase in step cycle duration with upslope walking in Untreated rats at 10 wk. Sciatic nerve transection resulted in significant increases in swing phase duration at all three slopes. In contrast, swing phase duration was increased only during downslope walking in ChABC-treated rats. Therefore, ChABC treatment at the time of injury and repair reduced the impact of sciatic nerve transection on step cycle timing.
Table 1.
Average timing of step cycle during slope walking
| Downslope | Level | Upslope | ||
|---|---|---|---|---|
| Step cycle duration, ms | 620 ± 32 | 650 ± 31 | 710 ± 25 | |
| UT | 4 wk | 636 ± 23 | 655 ± 17 | 741 ± 9 |
| 10 wk | 654 ± 16 | 667 ± 18 | 801 ± 17* | |
| ChABC | 4 wk | 649 ± 55 | 695 ± 61 | 669 ± 53 |
| 10 wk | 626 ± 18 | 630 ± 43 | 686 ± 51 | |
| Stance, ms | 474 ± 30 | 504 ± 24 | 564 ± 23 | |
| UT | 4 wk | 441 ± 35 | 457 ± 33 | 547 ± 23 |
| 10 wk | 480 ± 13 | 482 ± 24 | 624 ± 14 | |
| ChABC | 4 wk | 448 ± 45 | 517 ± 46 | 493 ± 39 |
| 10 wk | 453 ± 17 | 471 ± 37 | 529 ± 53 | |
| Swing, ms | 140 ± 6 | 141 ± 11 | 141 ± 6 | |
| UT | 4 wk | 195 ± 24* | 198 ± 26* | 193 ± 14* |
| 10 wk | 174 ± 6* | 185 ± 10* | 176 ± 8* | |
| ChABC | 4 wk | 201 ± 25* | 178 ± 18 | 176 ± 24 |
| 10 wk | 173 ± 6* | 159 ± 11 | 157 ± 3 |
Values are means ± SE. UT, Untreated; ChABC, chondroitinase ABC treated.
Significantly different (P ≤ 0.05) from value calculated for Intact rats at same slope.
Response to tibial nerve stimulation.
After sciatic nerve transection and repair, the normal responses to electrical stimulation of the tibial nerve disappear. As noted previously (English et al. 2007), by 4 wk after nerve transection and repair of the sciatic nerve in Untreated rats, evoked EMG potentials are found as a series of small potentials at longer latency (Fig. 1). At this posttransection time it is difficult to determine unequivocally which of these potentials corresponds to a direct muscle response and which represents the H reflex, so we have termed these putative M responses and putative H reflexes. In similar recordings from the same electrodes in the same rats at later survival times, the latencies are restored to nearly those of Intact rats and the temporal distinction of the two responses is clear (English et al. 2007). In animals treated with ChABC at the time of the sciatic nerve lesion, the same multiple peaks in the EMG responses were noted (Fig. 1), but because these peaks were considerably larger at the 4 wk time shown, and because discrete separation of short- and longer-latency responses is found in the same latency windows as noted in Intact rats, we felt more secure in our distinction between early M responses and restored H reflexes.
We used these evoked potentials to evaluate the timing of reinnervation of the Sol muscle. Average EMG activity in the period immediately following electrical stimulation that was at least double the prestimulus background EMG activity was considered evidence that electrical stimulation had evoked an EMG response from the Sol. The time windows used to measure the amplitudes of the different responses were adjusted based on judgments as to where the first response (putative M response) started and ended and where the second response (putative H reflex) started and ended. We used later observations from the same rat to confirm our decisions on the putative M and H responses. Results for time to detection of these earliest evoked responses are shown in Fig. 2, A and C. Treatment with ChABC hastened the return of the putative M response (16.4 ± 0.6 vs. 27.2 ± 1.6 days, ChABC vs. Untreated; P = 0.0002) and the putative H reflex by nearly 2 wk (17.8 ± 0.6 vs. 29.3 ± 1.6 days, ChABC vs. Untreated; P = 0.0001).
Fig. 2.
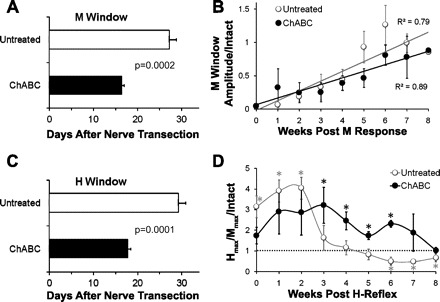
Stimulus-evoked Sol EMG activity responses in Untreated (UT) and ChABC-treated rats. A: average (+SE) time after sciatic nerve transection and repair that an evoked EMG response to tibial nerve stimulation judged to be an M wave could be discerned is shown for Untreated rats and rats in which the repaired nerve was treated with ChABC. B: time course of recovery of the M response is noted for Untreated rats and rats with ChABC-treated nerves. Mean (±SE) amplitude of the maximal evoked M response is graphed as a function of the time after the first observation of an M wave. Lines fitted to the data points used a least-squares linear regression model. C: average (+SE) time after sciatic nerve transection and repair that an evoked EMG response to tibial nerve stimulation resulted in a putative H reflex is shown for Untreated rats and rats in which the repaired nerve was treated with ChABC. D: time course of recovery of the H reflex is shown for Untreated rats and rats with ChABC-treated nerves. For each observation, the amplitude of the largest putative H reflex (Hmax) is expressed as a function of the amplitude of the largest M response (Mmax) evoked from tibial nerve stimulation. This ratio was then scaled to the ratio observed in each rat prior to sciatic nerve transection and repair. Each point represents the mean (±SE) of this scaled ratio at different times after the earliest observation of a restored H reflex. Dashed horizontal line at unity indicates the Hmax-to-Mmax ratio in Intact rats. *Mean values significantly different from 1.0.
A wide range of stimulation intensities (ranging from subthreshold to supermax) was used to determine the maximal evocable EMG activity in the M and H windows (Mmax and Hmax, respectively). The time course of the maturation of the average maximal M response (Mmax) as a function of the Mmax prior to sciatic transection is shown for both groups in Fig. 2B. The amplitude of the M response increased linearly over the 8 wk following its initial reappearance in both groups. Data from Untreated and ChABC-treated groups were fit with a linear function. No significant differences were found between groups for either the slopes or the intercepts. Correlation coefficients were high and were significantly greater than zero in both groups. We interpret this finding to mean that although recovery of muscle reinnervation started earlier in ChABC-treated rats, this recovery proceeded at the same rate after it began in both groups.
Hmax is assumed to result from activation of afferent axons in the nerves and their terminals, with subsequent monosynaptic activation of associated α-motoneurons. The amplitude of Hmax was normalized to that of Mmax, which is assumed to result from direct electrical stimulation of all of the α-motoneurons innervating Sol. This method of normalization was used to minimize interanimal variance associated with differences in 1) effectiveness of transmittance of electrical current to the tibial nerve, 2) the overall number of motoneurons with axons projecting through the tibial nerve, and 3) the sampling volume of the EMG electrodes embedded in the muscle. Values for Untreated rats and rats treated with ChABC are expressed as a percentage of the Hmax/Mmax values from the same rats, before injury (Intact), in Fig. 2D. In Untreated rats the putative H response was transiently elevated for the first 3 wk that it was detected after nerve transection. It then returned to the relative amplitude found prior to nerve transection for several weeks but then declined below intact levels by week 6. In ChABC-treated rats the H response was significantly elevated from weeks 3 to 6 after first postinjury detection and returned to Intact levels by week 7. At the longest time after reinnervation studied (11 wk after nerve transection), the amplitude of the restored H reflex remained at or slightly above the amplitude of the reflex found in Intact rats. Thus during the postinjury period evaluated in this study, ChABC treatment improves recovery of the H reflex.
Slope-related modulation of locomotor muscle activity patterns.
The Sol and TA are recruited reciprocally during treadmill walking in intact rats (Roy et al. 1991; Sabatier et al. 2011b; Thota et al. 2005). In both Untreated and ChABC-treated rats, the reinnervated Sol and TA are coactivated during treadmill locomotion at 4 wk and 10 wk after sciatic nerve transection and repair (Fig. 3). However, differences in amplitude modulation of activity were also noted between Untreated and ChABC-treated rats.
Fig. 3.
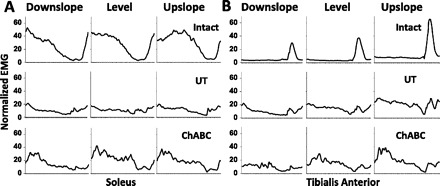
Muscle activity during walking at different slopes before and after injury. Normalized Sol (A) and tibialis anterior (TA; B) EMG activity is shown from Intact rats (n = 8), and postinjury Untreated (UT) rats (n = 3) and ChABC-treated rats (n = 4) 10 wk after sciatic lesion. Tracings start and end with paw contact and have been time-normalized to 100 x-axis units.
The significance of differences in activation patterns of the reinnervated muscles was studied with PCA. Muscle activity from multiple selected step cycles during downslope, level, and upslope conditions was averaged in each rat, and the resulting data for all rats were subjected to PCA, as described previously (Sabatier et al. 2011a). The data set for PCA included average locomotor EMG activity in TA and Sol from Intact, Untreated, and ChABC-treated rats walking on level and up and down slopes. The proportion of the variance in this data set that is accounted for by the eigenvalues for the first five principal components is shown in a scree plot in Fig. 4A. The first two principal components accounted for the majority of the variance (i.e., 67%) in the data set. The first principal component alone explained 46% of the variance. However, the amount of added variance explained for the third, fourth, and fifth principal components fell precipitously. Therefore, analysis was limited to the first two principal components.
Fig. 4.
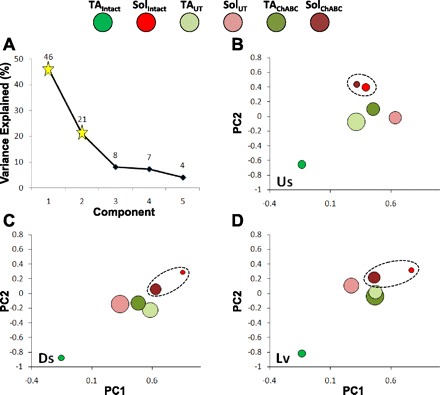
Analysis of locomotor EMG activity in Sol and TA with principal components analysis (PCA). The variance in average rectified and integrated locomotor activity of each muscle was reassigned with PCA. A: scree plot. The variance explained by each of the first 6 principal components (PCs) is shown (y-axis value is displayed above corresponding symbol). Values for the first 2 principal components (PCs) are represented by stars. Loadings on the first 2 principal components for the TA and Sol from Intact animals and Untreated (UT) and ChABC-treated animals 10 wk after transection of the sciatic nerve are illustrated as bubble plots for upslope (Us), downslope (Ds), and level (Lv) walking in B–D, respectively. Symbol size is proportional to the average SE for the 2 factors (i.e., larger bubbles indicate larger variance about the mean). Color coding of data from muscles and groups is illustrated at top. There is only 1 data point associated with intact TA (red) and 1 data point associated with intact Sol (green) in each of B–D. The circle surrounding the Intact Sol and ChABC Sol bubbles in the plot for each slope denotes an absence of statistical significance for those data on both PC1 and PC2.
Average principal component loadings for both muscles and time points (i.e., from Intact rats and those that had sciatic nerve lesions 10 wk earlier) are illustrated in Fig. 4, B–D. Values for Sol and TA are found on opposite corners of this portion of principal component loading space. Loading values on both principal components were significantly different for Sol versus TA (P ≤ 0.05) at each slope for Intact rats. Values for both ChABC-treated and Untreated rats are found in a different region of this space from either TA or Sol in Intact rats, albeit closer to the Intact Sol. After sciatic nerve transection, whether or not the distal stump was treated with ChABC, principal component loadings for Sol and TA were not significantly different from one another on either principal component (Table 2). This finding is consistent with a change in the activation patterns of Sol and TA from reciprocal in Intact rats to coactivation after sciatic nerve transection and muscle reinnervation, irrespective of whether ChABC treatment is used.
Table 2.
Comparisons of PC loading values associated with EMG activity from TA and Sol in Intact rats and both injury groups
| Group | Ds | Lv | Us |
|---|---|---|---|
| Intact | 1, 2 | 1, 2 | 1, 2 |
| UT 10 wk | NS | NS | NS |
| ChABC 10 wk | NS | NS | NS |
Comparisons of principal component (PC) loading values associated with EMG activity from tibialis anterior (TA) and soleus (Sol) in Intact rats and both injury groups at 10 wk postoperatively and at each slope. Ds, downslope; Lv, level; Us, upslope. Factor loadings for EMG profiles from TA vs. Sol were compared statistically [least significant difference (LSD) post hoc tests]. The presence of a “1” and/or a “2” means that the difference in the PC1 and/or PC2 factor loadings, respectively, was statistically significant (P ≤ 0.05). If they were not statistically significant on either factor, then “NS” (i.e., not significant) occupies the cell.
Loading values associated with Intact TA EMG activity were always significantly different (P ≤ 0.05) from those of all Intact Sol and all postoperative Sol and TA (from both groups). The same uniqueness was not true for Sol, as loading values associated with Sol EMG activity from ChABC-treated rats alone were not significantly different from loading values for either PC associated with Sol locomotor EMG activity from Intact animals (Fig. 4). The most likely explanation of this finding is that some restoration of the Intact Sol muscle activation pattern occurs when ChABC is used at the time of sciatic nerve transection and repair but not if the nerve repair is untreated.
Slope-related modulation of locomotor EMG intensity.
Average EMG activity from the Sol in Intact, Untreated, and ChABC-treated rats is shown in Fig. 5. The intensity of Sol EMG activity at the midpoint in the step cycle is significantly increased during level and upslope walking compared with downslope in Intact rats (Fig. 5A). Slope-related modulation of Sol EMG intensity at the midstep is lost in both postinjury groups at 10 wk after nerve transection and repair. Identical effects are found for TA EMG intensity during the swing-phase TA activation period across slopes in Intact rats and after injury in both postinjury groups (data not shown). Modulation of TA and Sol locomotor EMG intensity with slope is lost after reinnervation whether or not the lesioned nerve is treated with ChABC. Data for the Untreated group were presented in a previous report (Sabatier et al. 2011b).
Fig. 5.
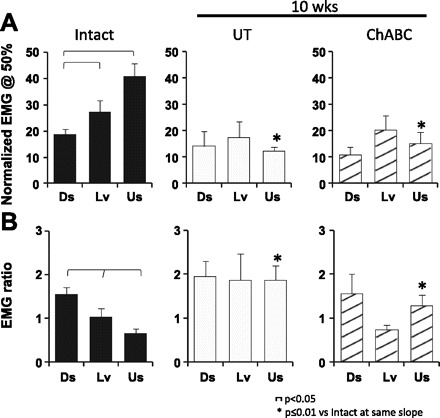
Sol EMG activity during walking at different slopes before and after injury. A: average Sol EMG activity (+SE) at the midpoint in the step cycle (Sabatier et al. 2011b). B: average EMG ratio (+SE). The precontact EMG ratio was calculated as the EMG activity before paw-on ÷ EMG activity at the midstep. Data are shown for Intact and both groups of lesioned rats 10 wk after injury.
The intensity of EMG activity in the Sol that occurs just before paw contact, during the later swing phase while the limb is still suspended and its path is unobstructed, has been used as an index of central control of Sol muscle activity (Gorassini et al. 1994; Maas et al. 2010; Sabatier et al. 2011b) since autogenic afferent modulation is not active while the paw is not in contact with the walking surface (Pearson and Collins 1993; Prochazka et al. 1989). We expressed the average precontact Sol EMG intensity as a function of the mean Sol EMG intensity at the midstep. These EMG ratios are shown for locomotion at different slopes in Intact rats and at 10 wk after transection and surgical repair of the sciatic nerve for Untreated and ChABC-treated rats in Fig. 5B. All slope comparisons were significantly different for Intact rats (P < 0.05). There was a progressive decrease in the precontact EMG ratios in Sol with increasing slope. After injury, these Sol EMG ratios did not change with slope. However, for upslope walking the EMG ratios for both postoperative groups were significantly larger than for Intact rats (P ≤ 0.01). Therefore, after sciatic nerve transection precontact Sol EMG activity remains elevated during upslope walking, which is when precontact muscle activation is minimal in Intact rats.
Kinematic analysis.
We studied the two components of a global kinematic vector as measures of overall limb movements during slope walking. The magnitude of this vector, the distance between the hip and MTP joints, was expressed as a function of femur length and termed adjusted limb length in each rat to accommodate changes in size of the rats during the course of the study and to reduce the effects of variability in body size between rats. The direction of this global vector is termed limb angle. Adjusted limb length is plotted as a function of limb angle for locomotion at three different slopes at 4 and 10 wk after sciatic nerve transection for Untreated rats (Fig. 6A) and rats treated with ChABC (Fig. 6B). The times of paw contact (PC) and paw-off (PO) in all panels are similar to those marked by open down and up arrows, respectively in Fig. 6A, left. The three traces in each panel include data from Intact rats and data from rats at 4 wk and 10 wk after transection and repair of the sciatic nerve. The sizes of the symbols in the graphs are proportional to the average SE for two components (adjusted length and angle) of each data point. The step cycle in each tracing progresses in a counterclockwise direction. These graphs have been described in detail previously (Sabatier et al. 2011a, 2011b).
Fig. 6.
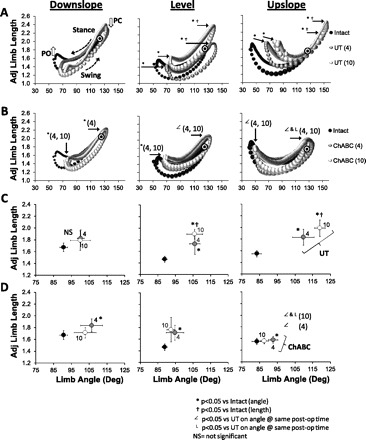
Hindlimb kinematics during treadmill locomotion. A and B: plots of hindlimb angle (x-axis) vs. adjusted hindlimb length (y-axis) are shown for downslope, level, and upslope walking. Data from Intact rats and from rats 4 and 10 wk after nerve transection are shown for Untreated rats (UT; A, n = 3 for 4 and 10 wk) and ChABC-treated rats (B, n = 5 for 4 wk and n = 4 for 10 wk). Data from injured rats are always in the foreground. The size of the symbols is proportional to the average SE from the y- and x-axes (i.e., larger bubbles indicate larger variance about the mean). In the downslope panel of A open arrows are next to inflection points in the polygon corresponding to the time at which the paw leaves the treadmill belt (i.e., paw-off, PO) and paw contact with the treadmill belt (PC). Stance and swing and the direction of the advancement of the step cycle (counterclockwise) are depicted in this panel as well. In A and B solid arrows point to angles and/or femur lengths of postoperative rats at PO and PC that were significantly different from Intact rats, and in some cases for ChABC data that were different from UT data. Black-and-white targets identify maximal femur lengths from Intact rats when postoperative data obscured Intact data. C and D: average (±SE) positions of these measures of hindlimb position from the same data shown in A (Intact and postinjury, UT) and B (Intact and postinjury, ChABC).
Average limb angles and adjusted limb lengths (±SE) are plotted against each other for Untreated rats (Fig. 6C) and ChABC-treated rats (Fig. 6D). In Untreated rats, these measures of central tendency of overall hindlimb movements are not significantly different from those measured in Intact rats during downslope walking, but during level and upslope walking they are markedly different. A similar finding was obtained from analysis of limb angle and adjusted limb length at the turning points of the step cycle (PC and PO) (Fig. 6A). In contrast, for ChABC-treated rats significant differences in these parameters from those measured in Intact rats were found only at the 4 wk sampling times. By 10 wk after sciatic nerve transection and repair, the components of the global limb vector in ChABC-treated rats were not significantly different from those in Intact rats.
A distinguishing feature of the angle-length polygons found in Intact rats is marked rotation associated with slope (Sabatier et al. 2011b). There is a clockwise rotation during upslope walking, resulting from smaller limb lengths at PC and larger limb lengths at PO, and a counterclockwise rotation for downslope walking, resulting from an opposite relationship of the limb lengths at PC and PO (compare different slopes in Fig. 6, A and B). To assess the effects of sciatic nerve transection on the ability of rats to employ these adaptations, we calculated the ratio of limb length at PC to limb length at PO for each animal at each slope (Fig. 7). A ratio of 1.0 means that limb length was identical at PC and PO. In Intact rats, this ratio declined with increasing slope, as anticipated. In Untreated rats at both 4 and 10 wk after transection and repair of the sciatic nerve, the ratios were significantly elevated compared with those for Intact rats at all slopes and no significant slope-related differences were noted. In these rats the ability to modify hindlimb length with slope is lost. For ChABC-treated rats the ratios were not significantly different from those for Intact animals at all slope conditions. Also, as in Intact animals, there was a significant slope effect for the PC-to-PO ratio in ChABC-treated rats. Thus, to the extent that these measures reflect adaptive changes of hindlimb movements made in response to the very different biomechanical demands of slope walking, slope-related adaptive changes were achieved only in ChABC-treated rats.
Fig. 7.
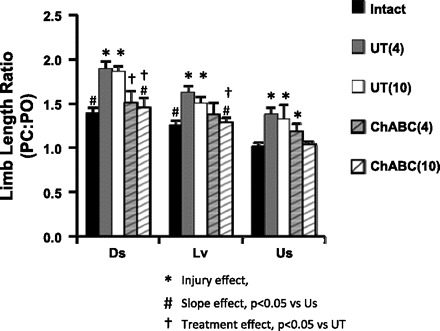
Limb length ratio at paw contact (PC) and paw-off (PO). Average (+SE) values of the ratio of paw contact to paw-off for Intact, injured and untreated (UT), and injured and ChABC-treated (ChABC) at each slope are displayed as bar graphs. In the key, numbers in parentheses to right of group notations correspond with length of time after sciatic nerve transection.
Changes in position of the foot relative to the treadmill belt throughout a step cycle are illustrated in Fig. 8 for Intact rats and at 4 and 10 wk after transection and repair of the sciatic nerve for Untreated rats (Fig. 8A) and ChABC-treated rats (Fig. 8B). The effects of slope and sciatic nerve transection on this measure were described previously (Sabatier et al. 2011b). After injury to the sciatic nerve with or without ChABC treatment and subsequent reinnervation of the muscles crossing the ankle joint, the movements of the foot remain quite different from those observed in Intact rats. The foot is placed flat on the treadmill belt at paw contact and held in that position until late in the stance phase when it is lifted. Midstep foot angle (Fig. 8) was significantly smaller for both groups of injured rats at both postinjury times and for each slope. In ChABC-treated rats, some adjustment of the midstep foot angle was found during upslope walking (vs. downslope, P = 0.03).
Fig. 8.
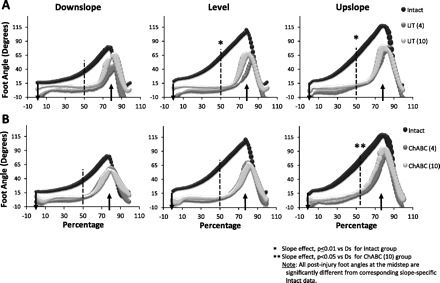
Kinematics of the foot. Values of foot angle throughout the step cycle for downslope, level, and upslope walking starting with paw contact are displayed for Intact rats (n = 8) in A and B as black bubbles in the background. A also shows injured rats at 4 and 10 wk (n = 3 for both injured groups) after sciatic nerve transection and repair without additional treatment. B also shows data for injured rats treated with ChABC at the time of injury at 4 (n = 4) and 10 (n = 3) wk after sciatic nerve transection and repair. Paw contact is identified with a vertical arrow pointing down that crosses the x-axis. An arrow pointing up crosses the x-axis where the swing phase generally begins. A vertical line is located at 50% through the step cycle. The sizes of the symbols are proportional to the SE (i.e., larger bubbles indicate larger variance about the mean).
DISCUSSION
Despite regeneration of injured axons and reinnervation of muscle targets, the lack of functional recovery after peripheral nerve injuries in humans remains an important clinical problem. Maximally hastening the return of axons to their distal end-organs should help maximize the potential for full recovery of function (Sunderland 1978). Removal of GAG side chains of CSPGs with the enzyme ChABC has been found to hasten the elongation of regenerating axons across the injury site and through the distal stump (Groves et al. 2005; Zuo et al. 2002). Therefore, it was hypothesized that functional recovery might be improved as well. To address the question of functional recovery this study used three measures: evoked EMG activity, locomotor activity of reinnervated muscles, and limb movements during slope walking. ChABC resulted in improvement in all three measures.
Reflex activity.
One of the primary findings from the present investigation is a 40% reduction in the time to detection of an EMG response elicited by tibial nerve stimulation when ChABC was used at the time of sciatic nerve transection and repair. This supports the hypothesis that ChABC-induced hastening of axon elongation results in quicker restoration of functional connectivity of regenerating axons and their muscle targets. It is also consistent with previous findings from our lab that electrical stimulation, which also hastens axon elongation after peripheral nerve injury, decreases the time to detection of an M response after sciatic nerve injury (Hamilton et al. 2011).
Quicker reinnervation and connectivity alone may not result in full restoration of function. Accurate proprioceptive feedback from skeletal muscles and integration of this information by spinal circuitry is required for normal movement. Therefore, we included the H reflex in our measurements. The H reflex is the muscle EMG response to activation of afferent neurons at the anatomical site of stimulation (Schieppati 1987). The Ia-mediated spinal reflex arc is an integral component of the sensory motor strategy for locomotion (Mazzaro et al. 2005). The time for restoration of the H reflex after sciatic nerve transection found in this study, i.e., 31 days, is consistent with what we found previously (English et al. 2007). The putative H reflex was first detected in ChABC-treated rats just slightly more than half the time compared with Untreated rats. This reduction in time to recovery is similar to that found for the M response. The magnitude of the H reflex when it returned was also similar between the groups. Therefore, quicker recovery of the H reflex is probably primarily a function of quicker reestablishment of neuromuscular connections.
The H reflex was elevated when it returned in both groups. This hyperreflexia found early in the process of muscle reinnervation has been reported by others (Udina et al. 2011). Its origin is not entirely clear, but its presence is consistent with a loss of GABA-mediated spinal inhibition after peripheral nerve injury (Castro-Lopes et al. 1993; Wall and Devor 1981), as well as lower depolarization thresholds (Eccles et al. 1958), larger monosynaptic excitatory postsynaptic potentials (EPSPs) (Hellgren and Kellerth 1989), and increased motoneuron excitability (Foehring et al. 1986) found during the first few weeks of muscle reinnervation. The more rapid reinnervation of muscle observed in ChABC-treated rats had little effect on this hyperreflexia.
The decline in the H reflex following a period of hyperreflexia in both groups is similar to what we (English et al. 2007) and others (Valero-Cabre and Navarro 2002) have reported. For Untreated rats this decline was larger than for ChABC-treated rats, and even fell below normal (i.e., values recorded before injury). This may be explained by a heterogeneous (Alvarez et al. 2010) and temporally staggered (Brannstrom and Kellerth 1998) loss of central synapses after motoneurons lose connections with peripheral targets. It is noteworthy that when recovery from peripheral nerve injury advances unaided, altered central synaptic connections between sensory afferents and spinal motoneurons seem to be permanent (Alvarez et al. 2010). Thus quicker restoration of muscle connections may avoid a more permanent loss of balance of excitation and inhibition in the new central synaptic organization. Preservation of the size of the H reflex is encouraging because it may represent a breach in the spinal circuits that can be exploited with complementary experimental treatments to enhance functional ability.
Hindlimb kinematics.
In addition to improvements in the H reflex, hindlimb kinematics during upslope walking for ChABC-treated rats were statistically indistinguishable from those for Intact rats. This represents a significant improvement compared with Untreated rats. Such an outcome was not unexpected for downslope walking. As we have shown previously (Sabatier et al. 2011b), hindlimb movements during downslope walking, but not level or upslope walking, were unchanged in Untreated rats compared with Intact rats. The most significant finding of the present study was the better outcomes in ChABC-treated rats than Untreated rats for level and upslope walking.
Upslope walking has the greatest demands for force production in extensor muscles of the limbs (Carlson-Kuhta et al. 1998; Donelan et al. 2009; Gregor et al. 2006). Therefore, it could be predicted that better ankle extensor strength would improve upslope walking. In support of this idea, a recent study reported a ChABC-induced improvement in kinematics of the hip-ankle-toe complex that was coincident with a fourfold increase in triceps surae muscle force after sciatic nerve lesion and repair (Beaumont et al. 2009). Therefore, one way that ChABC may have improved upslope walking was by improving the ability of the triceps surae to produce stronger ankle extensor moments.
Electromyographic activity.
After transection and repair of the sciatic nerve and subsequent muscle reinnervation, Sol activation during treadmill locomotion is dominated by elevated EMG activity just prior to paw contact with the treadmill belt. We propose that this new pattern of Sol EMG activity across slopes is consistent with increased reliance on central control of muscle activation. Since afferents contributing to autogenic modulation of Sol EMG activity are not activated while the paw is not touching the treadmill belt (Pearson and Collins 1993; Prochazka et al. 1989), any elevation in precontact Sol EMG activity is likely to result from central control (Maas et al. 2010; Sabatier et al. 2011b). This new centrally dominated pattern is sufficient to result in improved limb movements during upslope walking, but only if ChABC has been used to hasten muscle reinnervation.
A major factor thought to be responsible for poor functional outcomes following peripheral nerve injuries is the loss of myospecificity resulting from misdirection of regenerating axons (Fawcett and Keynes 1990). Producing muscle innervation without significant axon misdirection results in some improvement of functional recovery, but function is not returned to normal (Sabatier et al. 2011a). Treating transected nerves in mice with ChABC results in speedier axon regeneration but also results in misdirection of regenerating axons that is worse than normal (English 2005). The present study did not find a deterioration of functional recovery with ChABC treatment that might correspond with worsened axon misdirection. In fact, ChABC treatment resulted in improvements in function that are comparable to those found when axon misdirection is reduced (Sabatier et al. 2011a) and superior improvements in limb movements during upslope walking. Therefore, misdirection of regenerating axons does not necessarily correlate with worse functional recovery as might be predicted from very early reports (Sperry 1941). These results support the idea that application of chondroitinase has the potential to affect real clinical improvements in recovery from peripheral nerve injuries.
GRANTS
A.W.E., B.N.T., J.N., S.R., and M.J.S. were funded by HD32571 and HD053669. M.J.S. was also funded by a USPHS NIH Institutional Research and Academic Career Development grant (K12 GM00680-05).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.S. and A.W.E. conception and design of research; M.J.S., B.N.T., S.R., J.N., and A.W.E. performed experiments; M.J.S., B.N.T., S.R., J.N., and A.W.E. analyzed data; M.J.S., B.N.T., S.R., J.N., and A.W.E. interpreted results of experiments; M.J.S., B.N.T., S.R., J.N., and A.W.E. prepared figures; M.J.S. and A.W.E. drafted manuscript; M.J.S., B.N.T., S.R., J.N., and A.W.E. edited and revised manuscript; M.J.S., B.N.T., S.R., J.N., and A.W.E. approved final version of manuscript.
REFERENCES
- Alvarez FJ, Bullinger KL, Titus HE, Nardelli P, Cope TC. Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injuries. Ann NY Acad Sci 1198: 231–241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont E, Cloutier FC, Atlan M, Rouleau DM, Beaumont PH. Chondroitinase ABC and acute electrical stimulation are beneficial for muscle reinnervation after sciatic nerve transection in rats. Restor Neurol Neurosci 27: 297–305, 2009 [DOI] [PubMed] [Google Scholar]
- Bishop C. Neural Networks for Pattern Recognition. Oxford, UK: Oxford Univ. Press, 1995 [Google Scholar]
- Bovolenta P, Wandosell F, Nieto-Sampedro M. Characterization of a neurite outgrowth inhibitor expressed after CNS injury. Eur J Neurosci 5: 454–465, 1993 [DOI] [PubMed] [Google Scholar]
- Brannstrom T, Kellerth JO. Changes in synaptology of adult cat spinal alpha-motoneurons after axotomy. Exp Brain Res 118: 1–13, 1998 [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Pesheva P, McCarthy JB, Furcht LT, Schmitz B, Schachner M. Functional involvement of sciatic nerve-derived versican- and decorin-like molecules and other chondroitin sulphate proteoglycans in ECM-mediated cell adhesion and neurite outgrowth. Eur J Neurosci 7: 805–814, 1995 [DOI] [PubMed] [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79: 1687–1701, 1998 [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res 620: 287–291, 1993 [DOI] [PubMed] [Google Scholar]
- Chang YH, Auyang AG, Scholz JP, Nichols TR. Whole limb kinematics are preferentially conserved over individual joint kinematics after peripheral nerve injury. J Exp Biol 212: 3511–3521, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J. AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia 34: 213–228, 2001 [DOI] [PubMed] [Google Scholar]
- Donelan JM, McVea DA, Pearson KG. Force regulation of ankle extensor muscle activity in freely walking cats. J Neurophysiol 101: 360–371, 2009 [DOI] [PubMed] [Google Scholar]
- Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci 14: 7616–7628, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Libet B, Young RR. The behaviour of chromatolysed motoneurones studied by intracellular recording. J Physiol 143: 11–40, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol 490: 427–441, 2005 [DOI] [PubMed] [Google Scholar]
- English AW, Chen Y, Carp JS, Wolpaw JR, Chen XY. Recovery of electromyographic activity after transection and surgical repair of the rat sciatic nerve. J Neurophysiol 97: 1127–1134, 2007 [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci 13: 43–60, 1990 [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Muir D. MMP-2 and MMP-9 increase the neurite-promoting potential of schwann cell basal laminae and are upregulated in degenerated nerve. Mol Cell Neurosci 16: 157–167, 2000 [DOI] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. I. Long-term reinnervation. J Neurophysiol 55: 931–946, 1986 [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Prochazka A, Hiebert GW, Gauthier MJ. Corrective responses to loss of ground support during walking. I. Intact cats. J Neurophysiol 71: 603–610, 1994 [DOI] [PubMed] [Google Scholar]
- Gorio A, Vergani L, De Tollis A, Di Giulio AM, Torsello A, Cattaneo L, Muller EE. Muscle reinnervation following neonatal nerve crush. Interactive effects of glycosaminoglycans and insulin-like growth factor-I. Neuroscience 82: 1029–1037, 1998 [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. J Neurophysiol 95: 1397–1409, 2006 [DOI] [PubMed] [Google Scholar]
- Groves ML, McKeon R, Werner E, Nagarsheth M, Meador W, English AW. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp Neurol 195: 278–292, 2005 [DOI] [PubMed] [Google Scholar]
- Hamilton S, Hinkle M, Nicolini J, Rambo L, Rexwinkle A, Rose S, Sabatier M, Backus D, English A. Misdirection of regenerating axons and functional recovery following sciatic nerve injury in rats. J Comp Neurol 519: 21–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren J, Kellerth JO. A physiological study of the monosynaptic reflex responses of cat spinal alpha-motoneurons after partial lumbosacral deafferentation. Brain Res 488: 149–162, 1989 [DOI] [PubMed] [Google Scholar]
- Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci 23: 9276–9288, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekoski CA, Neubauer D, Zuo J, Muir D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci 21: 6206–6213, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ, English AW, Prilutsky BI. Locomotor changes in length and EMG activity of feline medial gastrocnemius muscle following paralysis of two synergists. Exp Brain Res 203: 681–692, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray TE. Fibrin sealants and glues. J Card Surg 18: 480–485, 2003 [DOI] [PubMed] [Google Scholar]
- Martin S, Levine AK, Chen ZJ, Ughrin Y, Levine JM. Deposition of the NG2 proteoglycan at nodes of Ranvier in the peripheral nervous system. J Neurosci 21: 8119–8128, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaro N, Grey MJ, Sinkjaer T. Contribution of afferent feedback to the soleus muscle activity during human locomotion. J Neurophysiol 93: 167–177, 2005 [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol 136: 32–43, 1995 [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci 11: 3398–3411, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menovsky T, Beek JF. Laser, fibrin glue, or suture repair of peripheral nerves: a comparative functional, histological, and morphometric study in the rat sciatic nerve. J Neurosurg 95: 694–699, 2001 [DOI] [PubMed] [Google Scholar]
- Muir D, Engvall E, Varon S, Manthorpe M. Schwannoma cell-derived inhibitor of the neurite-promoting activity of laminin. J Cell Biol 109: 2353–2362, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol 70: 1009–1017, 1993 [DOI] [PubMed] [Google Scholar]
- Prochazka A, Trend P, Hulliger M, Vincent S. Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res 80: 61–74, 1989 [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. Bethesda, MD: National Institutes of Health, 1997–2006 [Google Scholar]
- Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol 70: 2522–2529, 1991 [DOI] [PubMed] [Google Scholar]
- Sabatier MJ, To BN, Nicolini J, English AW. Effect of axon misdirection on recovery of EMG activity and kinematics after peripheral nerve injury. Cells Tissues Organs 193: 298–309, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier MJ, To BN, Nicolini J, English AW. Effect of slope and sciatic nerve injury on ankle muscle recruitment and hindlimb kinematics during walking in the rat. J Exp Biol 214: 1007–1016, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol 28: 345–376, 1987 [DOI] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326: 592–596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry R. The effect of crossing nerves to antagonistic muscles in the hind limb of the rat. J Comp Neurol 75: 1–19, 1941 [Google Scholar]
- Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol 9: 313–325, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland S. Nerve and Nerve Injuries. London: Churchill Livingstone, 1978 [Google Scholar]
- Sweeney JD, Ksienski DA, Mortimer JT. A nerve cuff technique for selective excitation of peripheral nerve trunk regions. IEEE Trans Biomed Eng 37: 706–715, 1990 [DOI] [PubMed] [Google Scholar]
- Tabb JS, Fanger GR, Wilson EM, Maue RA, Henderson LP. Suppression of sodium channel function in differentiating C2 muscle cells stably overexpressing rat androgen receptors. J Neurosci 14: 763–773, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thota AK, Watson SC, Knapp E, Thompson B, Jung R. Neuromechanical control of locomotion in the rat. J Neurotrauma 22: 442–465, 2005 [DOI] [PubMed] [Google Scholar]
- Tona A, Perides G, Rahemtulla F, Dahl D. Extracellular matrix in regenerating rat sciatic nerve: a comparative study on the localization of laminin, hyaluronic acid, and chondroitin sulfate proteoglycans, including versican. J Histochem Cytochem 41: 593–599, 1993 [DOI] [PubMed] [Google Scholar]
- Trigg DJ, O'Grady KM, Bhattacharyya T, Reinke M, Toriumi DM. Peripheral nerve regeneration: comparison of laminin and acidic fibroblast growth factor. Am J Otolaryngol 19: 29–32, 1998 [DOI] [PubMed] [Google Scholar]
- Udina E, Cobianchi S, Allodi I, Navarro X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Ann Anat 193: 347–353, 2011 [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Navarro X. Changes in crossed spinal reflexes after peripheral nerve injury and repair. J Neurophysiol 87: 1763–1771, 2002 [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M. The effect of peripheral nerve injury on dorsal root potentials and on transmission of afferent signals into the spinal cord. Brain Res 209: 95–111, 1981 [DOI] [PubMed] [Google Scholar]
- Widmer CG, Carrasco DI, English AW. Differential activation of neuromuscular compartments in the rabbit masseter muscle during different oral behaviors. Exp Brain Res 150: 297–307, 2003 [DOI] [PubMed] [Google Scholar]
- Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol 34: 41–54, 1998a [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Dyess K, Ferguson TA, Muir D. Degradation of chondroitin sulfate proteoglycan enhances the neurite-promoting potential of spinal cord tissue. Exp Neurol 154: 654–662, 1998b [DOI] [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Graham J, Krekoski CA, Ferguson TA, Muir D. Regeneration of axons after nerve transection repair is enhanced by degradation of chondroitin sulfate proteoglycan. Exp Neurol 176: 221–228, 2002 [DOI] [PubMed] [Google Scholar]


