Abstract
Objective
High-frequency (HF) changes were analysed in relation to anatomical origin of spikes, shape and occurrence within the seizure onset zone (SOZ). We evaluated whether HF changes are linked to the SOZ, as established for distinct high-frequency oscillations.
Methods
SEEG was filtered at 500 Hz and sampled at 2000 Hz. Spikes were selected by shape (spike/spike-slow wave) and location (SOZ/non-SOZ and neocortex/amygdala/hippocampus) in 15 patients. About 50 spikes were averaged for each set. Changes compared to baseline were quantified with false discovery rate controlled t-statistics using time-frequency spectra. Counts of increased or decreased time-frequency values were compared across spike categories in the 80–250 and 250–500 Hz bands.
Results
Seventy-seven spike types were analysed. Differences between spike categories were most prominent between 250 and 500 Hz. HF changes were more frequent and larger in mesial temporal than in neocortical spikes and for spikes with slow waves than spikes alone. HF changes above 250 Hz were more frequent in spikes within than outside the SOZ.
Conclusions
HF increases above 250 Hz show regional differences and are very prominent in the SOZ. Hippocampal spikes have the strongest HF components.
Significance
Analysis of HF changes during spikes may provide information on differing pathophysiological mechanisms of spikes and on epileptogenicity of the tissue.
Keywords: Spikes-slow wave, Ripples, Fast ripples, Epilepsy, Seizure onset zone
1. Introduction
Interictal epileptic spikes have always been an important diagnostic feature in patients with epilepsy (Bautista et al., 1999; Hufnagel et al., 2000). In presurgical diagnostics for patients with epilepsy, the brain areas which generate epileptic spikes are called the irritative zone (Rosenow and Luders, 2001). It is however unclear to what extent the spikes are indicators of the epileptic potential of the underlying cortex. Many patients are free of seizures after surgical removal of the seizure onset zone (SOZ), even if a large part of the irritative zone remains untouched (Hufnagel et al., 2000). This has often been described in patients with bitemporal independent interictal spikes, in whom the removal of one mesial-temporal lobe resulted in seizure freedom (Schwartz et al., 1997). Some authors even go as far as stating that spikes may represent the inhibitory potential of the epileptic cortex and thus represent a protective mechanism against epileptic seizures (Barbarosie and Avoli, 1997; Curtis et al., 2005). This hypothesis is strengthened by the observation that spikes, contrary to intuitive expectations, decrease after withdrawal of antiepileptic medication and prior to the increase of spontaneous seizures (Spencer et al., 2008). Thus the reduction of spikes seems to be correlated with a higher epileptogenic potential of the underlying tissue.
High-frequency oscillations are another marker of epileptogenicity. They can be recorded with intracranial electrodes and gained increasing interest in recent publications (Engel et al., 2009; Otsubo et al., 2008; Urrestarazu et al., 2007). Ripples (80– 250 Hz) and fast ripples (250–500 Hz) occur with the highest rates in the SOZ and they seem to be closely related to epileptogenic tissue (Bragin et al., 2002a; Jacobs et al., 2008, 2009a; Staba et al., 2007). The surgical removal of HFO generating tissue correlates with the postsurgical outcome of patients with refractory epilepsy (Jacobs et al., 2009a).
Interictal HFOs and epileptic spikes seem to be closely related to each other (Urrestarazu et al., 2007). They often co-occur at the same time in the EEG and HFOs may be riding on epileptic spikes and then can even be seen in the unfiltered intracranial EEG (Crepon et al., 2009; Jacobs et al., 2008). Nevertheless, both events seem to be independent to some extent as well. On the one hand, HFOs can be found on channels that do not show any interictal spikes and around half of HFOs that occur on the same channels as spikes occur at different times. On the other hand, only 44% of spikes co-occur with ripples and 27% with fast ripples (Jacobs et al., 2008). Additionally, HFOs show different reactions to medication withdrawal. Like seizures, they increase in rate when the medication is lowered (Zijlmans et al., 2009). Thus the pathophysiologic mechanisms of HFOs and spikes may be fundamentally different.
Not only distinct HFOs but also HF power changes after spikes vary with anatomical region and may represent excitability or inhibition of the underlying tissue. Postspike HF decreases were observed in mesial temporal spikes and most prominent in the hippocampus independent of a visible postspike slow wave (Urrestarazu et al., 2006). This was interpreted as a reflection of the hippocampal ability to inhibit the excitability during spikes. In contrast, spikes in the amygdala were followed by postspike increase of HF power, maybe reflecting a lack of postspike inhibition. Thus postspike HF power changes were found useful to analyse pathophysiological mechanisms after spikes and compare different brain regions independent of an occurring postspike slow wave. The study however did not analyse HF changes during spikes and their relationship towards the SOZ.
Spikes with co-occurring HFOs are more specific to the SOZ than spikes without HFOs. They therefore may represent a specific subpopulation of spikes, which has to be interpreted differently in regard to their diagnostic value than spikes in general. At this time however the clinical value of differentiation between spikes with and without HFOs in presugical planning still has to be evaluated in more detail.
For HF power changes during spike the link to epileptogenic areas is not evaluated yet. The present study focuses on HF power changes during and after spikes. We previously developed a statistical time-frequency analysis method for HF during spikes, which is based on the Gabor transform (Kobayashi et al., 2009). This method easily visualizes HF changes during spikes and therefore allows a rapid analysis of HF content of a group of spikes. A first analysis in three patients suggested that HF augmentation during spikes and HF decrease immediately after the spike are clearly visible with the method. They are also most prominent in spikes in the SOZ and in hippocampal structures.
Here we present the analysis of a large number of different spike types selected from 15 consecutive patients who were implanted with depth electrodes. The HF augmentation during spikes is analysed and compared for spikes within and outside the SOZ, for spikes with and without slow waves and for spikes in the hippocampus, neocortex and amygdala. We hypothesize that HF content within a spike may be representative of the epileptogenic potential of the underlying tissue, and thus that HF increase is larger in the seizure onset zone and in susceptible anatomical areas such as the hippocampus.
2. Methods
2.1. Patient and spike selection
Between 2004 and 2009, 47 patients were implanted with intracranial depth electrodes at the Montreal Neurological Institute. It was the aim of this study to select spike sets from several different spike categories distinguishing between the following parameters: anatomical regions (amygdala vs. hippocampus vs. neocortex), SOZ (SOZ vs. non-SOZ), and occurrence of postspike slow waves (with SW vs. without SW). All possible combinations of these parameters resulted in 12 different spike categories. The EEGs of consecutive patients were then marked and the spikes were classified in the different categories. Spikes sets corresponding to each category were only included in the analysis if at least 50 single spikes with similar shapes could be marked from the same patient. It was not possible to find the 12 categories in a single patient as some patients did not have sufficient spikes in some of the categories. We continued marking consecutive patients until we had at least 5 spikes sets (of 50 spikes each) from different patients for each spike category. No patient was excluded a priori. Spikes were selected from consecutive patients. At the end of the study two consecutive patients were skipped, as already enough spike types in the mesial temporal structures had been obtained and these two patients were solely implanted in the mesial temporal structures. After this the following patients were implanted in neocortical areas again and therefore spike types from neocortical areas could be included. In total, data from 15 patients were included; the patient clinical data and implantation sides are summarized in Table 1.
Table 1.
Detailed clinical information on the included patients and their implantation sites.
| Number | Gender | MRI | Implantation | SOZ |
|---|---|---|---|---|
| 1 | M | L MT atrophy | LA, HC, PHC; RA, HC, PHC | LA, HC, PHC; |
| 2 | M | R hippocampal malrotation | L-A, L-HC, R-A, R-HC | R-A, R-HC |
| 3 | M | Normal | L-A, L-HC, L-PHC, R-A, R-HC, R-PHC | L-A, L-HC, L-PHC, R-A, R-HC, R-PHC |
| 4 | M | Bilateral MT atrophy | L-A, L-HC, L-PHC; R-A, R-HC, R-PHC | L-A, L-HC, L-PHC; R-A, R-HC, R-PHC |
| 5 | M | Tuberous sclerosis R-superior Fr gyrus, L-OF, L-TP | L-A, L-HC, R-A, R-HC, L-OF (Le), L-C, R-OF, R-C | Inner cont of L-OF |
| 6 | F | R MT sclerosis | L-A, L-HC, L-PHC; R-A, L-HC, L-PHC | L-A, L-HC, L-PHC; R-A, R-HC, R-PHC |
| 7 | F | Neuro-cutaneous syndrome | L-A, L-HC, L-PHC, R-A, R-HC, R-PHC | L-A, L-HC, L-PHC, R-A, R-HC, R-PHC |
| 8 | F | Normal | L-A, L-HC R-A, R-HC | L-A, L-HC |
| 9 | F | FCD R Fr basal region and anterior insular cortex | L-AC, L-PC, L-OF, R-AC, R-PC, R-OF, R-Le (lesional: superior Fr gyrus aiming at the anterior insular) | External contacts of R-OF |
| 10 | F | Nodular heterotopia + overlying cortical malformation | R-HC, R-PHC, R-A, R-P (posterior to R-PHC towards the heterotopia) R-S and R-O inserted from occipital aiming obliquely at the heterotopia | Superficial contacts of RS and RO |
| 11 | M | Nodular heterotopia | L-A, L-HC, L-O, L-OT, R-A, R-HC, R-PHC, R-OT | L-A, L-HC R-A, R-HC, R-PHC |
| 12 | M | L central FCD | L-SM, L-IM, L-SPC, L-IPC | Inner cont. of L-IM and L-SM |
| 13 | M | L hemispheric atrophy | L-HC, L-PHC, L-OT, L-O | Inner cont L-HC and L-OT |
| 14 | M | Normal | R-A, R-HC, R-AC, R-PC, R-IC, R-SC, R-OF | Inner cont. R-SC external cont. R-HC |
| 15 | M | L Ischemic lesion CP | L-A, L-HC, L-PHC, L-OF, L-AC, L-PC | Inner cont L-OF and L-AC |
Abbreviations: Bold print: more common SOZ, A = amygdala, AC = anterior cingulate, cont. = contacts, C = cingulate, CP = centro-parietal, FCD = focal cortical dysplasia, F = female, Fr = frontal, HC = hippocampus, IM = inferior motor, IPC = inferior postcentral, M = Male, MT = mesial temporal, L = left, Le = lesion, O = occipital, OF = orbito-frontal, OT = occipito-temporal junction, PHC = parahippocampus, PC = posterior cingulate, R = right, SC = supra-calcarine, SM = superior motor, SPC = superior postcentral, T = temporal, TP = temporal pole.
In the bitemporal mesial implantations SOZ was always in the inner contacts located in amygdala and hippocampus, if not indicated otherwise.
This study was approved by the Montreal Neurological Institute and Hospital Research Ethics Committee and all patients signed an informed consent.
2.2. EEG recordings and segment selection
Depth electrodes were implanted stereotactically using an image-guidance system (SNN Neuronavigation System, Mississauga, Ontario, Canada) (Olivier et al., 1994). All electrodes were manufactured onsite (9 contacts per electrode with a contact surface of 0.8 mm2), as described earlier (Urrestarazu et al., 2006; Jacobs et al., 2008). SEEGs were low-pass filtered at 500 Hz, sampled at 2000 Hz and recorded using Harmonie software (Stellate, Montreal, Canada). The recording was performed referentially with an epidural reference electrode placed in the parietal lobe of the hemisphere least likely to include the main epileptic focus. Analyses were performed on bipolar montages.
For this analysis, EEG segments were chosen during wakefulness to avoid high spiking rates, where spikes would follow each other too closely. This was important as only one spike could be analysed for each spike segment. The segments were defined to last 2.5 s before and after the spike, thus the minimum inter-spike distance was 2.5 s. EEG segments were taken from 1 of the first 3 days of the intracranial investigation to reduce influences of antiepileptic medication reduction and all EEG segments were selected to have a distance to the closest seizure of at least 2 h.
2.3. Visual marking of spikes
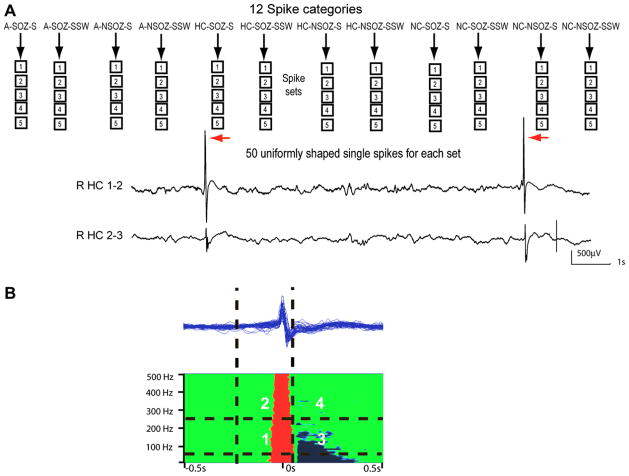
SEEG recordings were visually reviewed to identify the interictal epileptic spikes. As described above, for each patient, spike sets containing 50 consecutive spikes with similar spatial distribution, shape, and morphology were marked for each spike category present in that patient. The polarity of the spike peak was negative in some discharges and positive in others depending on the relation between the generator and the electrode locations, but the identified spike types had a consistent polarity within each spike set. We selected spikes and not sharp waves thus events with an appearance of less than 70 ms duration, but we did not measure the length of each event. Single spikes had to have a minimum interspike distance of 2.5 s to allow enough surrounding background activity for the analysis. Spikes following each other more closely were excluded. At the end of the marking process, the selected spike segments were displayed in one diagram to confirm that they had similar shapes (Fig. 1). If necessary, spikes with a morphology standing out from the rest were removed and replaced by new spikes with matching morphology. An automated correction for the peak time (time of largest negativity or positivity) was performed using Harmonie Systems (Stellate, Montreal, Canada) to ensure that the marker was placed exactly on the peak, which is difficult in manual markings.
Fig. 1.
Part A demonstrates the method of selecting and grouping spikes in spike categories and sets. In total 12 different spike categories were defined. For each category 5 spikes sets from different patients were selected. Each spike set consisted of 50 uniformly shaped single spikes. Part B demonstrates a typical time-frequency spectra. In Sections 1 and 2 of the spectra HF increase during the spike coloured in red were analysed for frequencies below 250 Hz (Section 1) and above 250 Hz (Section 2). The time window analysed for HF increases ranged from 0.3 s prior to the spike to 0.2 after the spike peak. In Sections 3 and 4 postspike decreases in HFs coloured in blue were analysed below 250 Hz (Section 3) or above 250 Hz (Section 4). Postspike decreases were analysed in the time window between the time of the spike peak and 0.5 s following it.
With respect to each spike set, non-overlapping EEG segments lasting 2 s (comprising 4000 data points) were sampled for analysis, each segment including a marked spike and surrounding background activity.
For each channel on which a spike was marked, eight baseline segments lasting at least 10 s were selected. These segments did not include any spikes, and were necessary for the subsequent statistical comparison.
2.4. Analysis of statistical time-frequency spectra
A time-frequency power spectrum was built for each spike segment of EEG data by using the Gabor (Windowed Fourier) Transform with a sliding Gaussian window of 50 ms FWHM (full width half maximum). The frequency range was 50–500 Hz. The Fourier Transform was performed on 512 data-points (256 ms; frequency resolution 3.9 Hz) of the window at each time-step, and the step of the sliding window was 2 ms. The signal power was converted to logarithmic scale to obtain a more Gaussian distribution. The average spike-related spectra were obtained by averaging the 50 data segments regarding each spike type.
Statistical comparison of the power of fast activity was performed between the spikes and the baseline control data (Fourier Transform of a total of 300 non-overlapping segments lasting 256 ms in the above mentioned baseline periods). The unpaired t-test was performed between the spike-related and control spectral data to obtain the t value and the corresponding p value at each pixel of the time-frequency spectrum.
To solve the problem of declaration of too many pixels as active (type I errors), we used a statistical procedure for controlling the false discovery rate (FDR) (Genovese et al., 2002), as in our previous study (Kobayashi et al., 2009). The FDR is defined as the ratio of the number of false positive pixels to the number of pixels declared active, and this procedure keeps the FDR below the specified bound q on average. In the present study, the two-tailed test was used with q = 0.025 to detect the pixels of significant power increase or decrease in the spike segments.
The computation was done with an in-house program written in MATLAB (version 6.5.1; MathWorks, USA).
2.5. Analysis and spike category comparisons
For all comparisons, we analysed separately high-frequency changes above and below 250 Hz. This division was defined according to the separation between ripples (80 to 250 Hz) and fast ripples (>250 Hz), with the idea that frequencies above 250 Hz may be better able to distinguish between epileptic and non-epileptic sites than lower frequencies (Bragin et al., 2002b; Staba et al., 2007). We then calculated spectral increases as well as post-spike decreases.
While comparing the categories, we performed three analyses. First, we visually analysed the time course of increase and decrease in the high-frequency domain using the plot of the Gabor transform. Second, we analysed the number of pixels that showed a significant increase or decrease compared to baseline. These numbers of pixels were then compared across each spike category. As this calculation only took into account the number of significant pixels, but not the strength of differences, we also analysed in a third step the sum of significant t-values across all pixels for each spike category. For the calculation of the number of significant pixels and the sum of their t-values, spike-related increases (visualized as red dots in the spectra) were analysed between −0.3 and +0.2 s around the spike peak (time zero of the automatically corrected spike peak). Postspike decreases (visualized as blue dots) were calculated from 0 to +0.5 s after the spike peak. The increases and decreases were analysed separately, so the overlap between 0 and 0.2 s did not result in problems to differentiate between increases and decreases of HF power.
3. Results
About 15 consecutive patients were included, providing a minimum of five different spike sets for each spike category (Table 1). In some categories, more than five spike sets could be included. This resulted in a total of 77 spike sets, each consisting of 50 single spikes. The number of spike sets per category can be found in Table 2.
Table 2.
Number of spikes sets for each category.
| Spikes
|
Spike-slow wave
|
Total | |||
|---|---|---|---|---|---|
| SOZ | Non-SOZ | SOZ | Non-SOZ | ||
| Neocortex | 7 | 8 | 5 | 8 | 28 |
| Hippocampus | 6 | 5 | 8 | 6 | 25 |
| Amygdala | 7 | 5 | 7 | 5 | 24 |
| Total | 20 | 18 | 20 | 19 | 77 |
3.1. Anatomical regions
Twenty-four spike sets were marked in amygdala, 25 in hippocampal and 28 in neocortical contacts. In general, no striking differences were seen between the results in pixel numbers and in sums of t-value scores; thus there were no real differences between the time-frequency extent of the changes and their strength. Detailed results below are therefore given for only one measure, the pixel numbers.
3.1.1. Spike-related increase in high frequencies
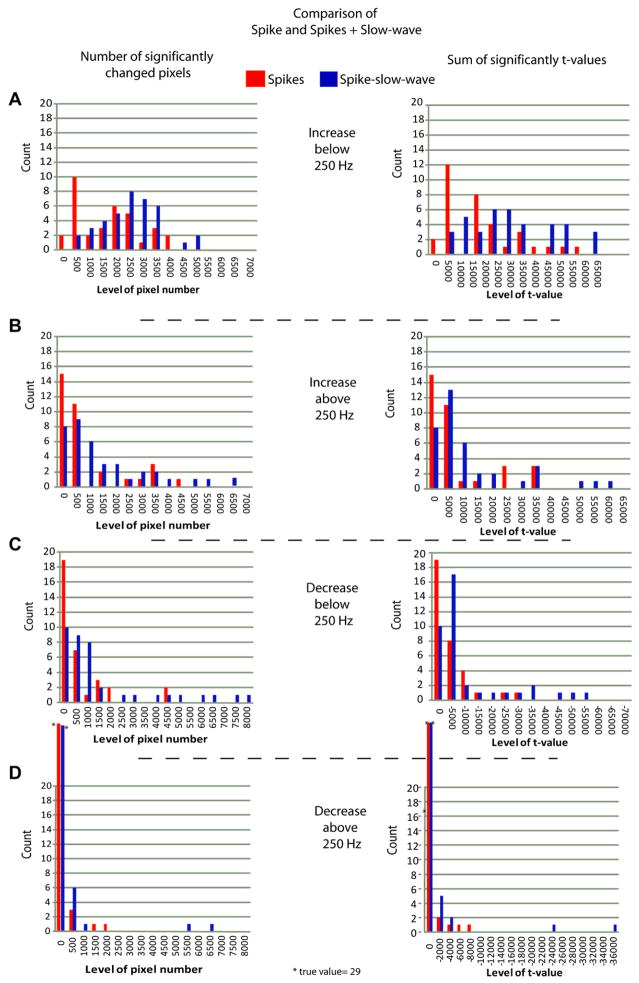
Differences in high-frequency increases below 250 Hz were seen in all spike sets in the amygdala and hippocampus and in 92.5% of spike sets in the neocortex. In the neocortex 71.4% of spike sets involved more than 500 and 57.1% more than 1000 pixels. In the amygdale 91.7% of spike sets showed increases of high frequencies below 250 Hz in more than 500 and 87.5% in more than 1000 pixels. Hippocampal spike sets showed increases below 250 Hz in more than 500 pixels in 84% and above 1000 pixels in 80% of spikes sets (see Fig. 2 a).
Fig. 2.
Histograms showing results from the comparison of spikes generated in the amygdala, hippocampus and neocortex. In the column on the left, the number of significant pixels in the time frequency spectra is given on the y-axes and the number of spikes sets showing these changes on the x-axes. The right column gives sums of significant t-values. The first column of each graph represents the number of spike set which did not show any HF changes. Panel A shows the increase of HF power below 250 Hz. Differences between anatomical regions are visible but smaller than in Panel B with fewer HF changes in the neocortex than in mesial temporal areas. Panel B: HF increases above 250 Hz are more frequent and larger in the hippocampus and amygdala than in neocortex. Panels C and D: HF power decreases below and above 250 Hz are more frequent and larger in hippocampus and amygdala than in the neocortex. Decreases above 250 Hz are rare, especially in the neocortex.
Above 250 Hz, increases in the neocortex occurred in 46.4% of all spike sets and only 3 spikes sets (10.7%) showed a significant increase in more than 500 pixels and 2 (7.1%) in more than 1000 pixels (see Fig. 2b). In the amygdala, in contrast, 87% of spike sets showed increase in frequencies above 250 Hz, 37.5% of spike sets showed an increase in more than 500 pixels and 33.3% above 1000 pixels. Increases above 250 Hz were most extensive in the hippocampus; 80% of spikes showed differences and 64% of increases involved more than 500 and 56% more than 1000 pixels. Striking changes with large number of pixels and high values of t-changes only occurred in the hippocampus.
3.1.2. Postspike decrease in high frequencies
Postspike decreases below 250 Hz occurred in 60.7% of neocortical spike sets, with 28.6% involving more than 500 pixels and 7.1% more than 1000 pixels. In the amygdala, 50% of spike sets show decreases below 250 Hz, with 16.6% involving more than 500 and the same number more than 1000 pixels. In the hippocampus, 76% of spike sets showed postspike decreases below 250 Hz, with 68% having an extent over 500 pixels and 56% over 1000 pixels (Fig. 2c).
Decreases above 250 Hz were less common in all anatomical areas. In the neocortex, 3.6% of spikes sets showed decreases above 250 Hz, none of them with an extent of more than 500 pixels. In the amygdala, 16.6% of spike sets showed this type of decrease, with 4.2% involving more than 500 and 1000 pixels. 40% of spike set in the hippocampus showed decreases above 250 Hz with 16% involving more than 500 and 1000 pixels (figure 2d).
3.2. Spikes with and without slow waves
Thirty-nine spikes sets occurred with slow wave and 38 without. Again, no obvious differences could be seen between the number of significant pixels and the sum of t-values.
3.2.1. Increase in high frequencies
Most spike sets without slow waves (95%) showed some increase in frequencies below 250 Hz. About 68.4% of spike sets had an increase of more than 500 pixels and 63.2% of more than 1000 pixels (Fig. 3a). In spike-slow wave sets, an increase below 250 Hz occurred in all spike sets, involving more than 500 pixels in 97.4% and more than 1000 pixels in 87.1%.
Fig. 3.
Structure of the figure is the same as Fig. 1. Comparison of HF power changes in spike sets with and without slow waves. All HF power changes are more frequent and of larger extent in the spikes with slow waves than those without. The occurrence of postspike HF decreases however is not dependent on the occurrence of a slow wave.
Increases in frequency above 250 Hz were seen in 60.5 of spike sets, with 21.1% of spike sets having more than 500 and more than 1000 pixels. Spike-slow wave sets showed increases above 250 Hz in 79.5%, involving more than 500 pixels in 56.4% and more 1000 in 41% (Fig. 3b). Thus HF changes during spikes were more common in spikes with slow waves.
3.2.2. Postspike decreases
Postspike decreases below 250 Hz were seen in 50% of spike sets and 74.4% of spike-slow waves sets. In the spike sets, the decrease involved more than 500 pixels in 31.6% and more than 1000 pixels in 28.9%. In spike-slow wave sets the corresponding numbers were 51.3% and 30.8% (Fig. 3c).
Postspike decreases above 250 Hz were rarer, they were seen in 13.2% of spike sets and 23.1% of spike-slow wave sets. More than 500 and 1000 pixels showed significant decrease in 5.3% of spike sets. Spike-slow wave sets showed these decreases in 7.7% involving more than 500 pixels and in 5.1% involving more than 1000 pixels (Fig. 3d).
3.3. SOZ vs. non-SOZ
Forty spike sets were located within and 37 spike sets outside the SOZ. Again no striking differences were found between the two analysis methods.
3.3.1. Increases in high frequencies
Increases below 250 Hz during spikes were always seen in the SOZ and in 97.3% of spike sets outside the SOZ. Spike sets inside the SOZ showed increases below 250 Hz with an extend of more than 500 pixels in 82.5% and of more than 1000 pixels in 80% of spike sets. Outside the SOZ, spike sets showed increases involving more than 500 pixels in 81,1% and more than 1000 pixels in 70.3% of spike sets (Fig. 4a).
Fig. 4.
Structure of the figure is the same as Fig. 1. Comparison of HF power changes in spike sets inside and outside the SOZ. HF power changes are larger and more frequent in spikes generated in the SOZ than outside. Differences between both groups are more prominent in the frequency band above than below 250 Hz.
Increases above 250 Hz were seen in 72.5% of spike sets inside the SOZ with 50% involving more than 500 pixels and 47.5% involving more than 1000 pixels. Spike sets outside the SOZ showed significant increases in 67.6%, with 37.8% of spikes sets involving more than 500 pixels and 24.6% more than 1000 (Fig. 4b).
3.3.2. Postspike decreases
Postspike decreases below 250 Hz were seen in 70% of spikes in the SOZ and in 54.1% outside the SOZ. Inside the SOZ, 47.5% of spike sets showed decreases involving more than 500 pixels and 35% more than 1000 pixels. Outside the SOZ the respective numbers were 35.1% and 24.3% (figure 4c).
Postspike decreases above 250 Hz were seen in 27.5% of the spike sets in the SOZ, involving more than 500 pixels in 12.5% and more than 1000 pixels in 10%. Outside the SOZ postspike decreases were only seen in 8.2% of spike sets, none involving more than 500 pixels (Fig. 4d).
3.4. HF decrease following HF increase
Twenty-seven spike sets showed an increase limited to frequencies below 250 Hz during the spike. This was followed by a postspike decrease in the frequency band below 250 Hz in five cases (18%). We never observed a decrease in the band above 250 Hz after a spike that did not have these frequencies during the spike.
Forty-four spike sets showed an increase of frequencies above 250 Hz during the spike. All increases above 250 Hz were occurring together with increases below 250 Hz. This combined increase above and below 250 Hz was followed by a decrease in 27 spike sets (61%); the decrease was in the band above 250 Hz in seven sets and in the band below 250 Hz in 20 sets.
In regard to the location of a spike set within the SOZ or outside and in regard to the occurrence with slow waves no specific dynamics could be observed. Higher frequency increases were followed by higher decreases as described above. As higher increases were more frequent within the SOZ and within spike sets with slow waves, these were also more often followed by decreases.
Postspike decreases especially in the high-frequency range were rare in the amygdala and neocortex. Decreases in the frequency above 250 Hz did not occur at all in the neocortex. On the contrary in the hippocampus all but two spikes that showed an increase above 250 Hz during the spike also showed a postspike decrease above 250 Hz.
3.5. Individual patients
Spike sets were not selected to allow within patient comparisons in this study. Nevertheless, we could compare features of SOZ and non-SOZ spike sets in nine patients who had spikes of both categories (Fig. 5). In all but one patient, the increase in high frequencies during the spike was larger in the spike set in the SOZ than outside the SOZ. In the ninth patient (patient 5), the spike set outside the SOZ in the hippocampus showed a larger increase of high frequency than the spike set inside the SOZ in the neocortex.
Fig. 5.
Example of different HF-power changes in two patients comparing a spike set generated in the SOZ with one generated outside. It is clearly visible that the changes in the time frequency spectra are more extended in the hippocampal spike (top, patient 6) than in neocortex (bottom, patient 12). For each patient however, HF power changes are relatively larger in the spike set from the SOZ compared to the one from outside for both patients. Thus HF power changes during spikes can help to identify the SOZ. The comparison of patients with a mesial temporal SOZ with one with a neocortical SOZ is difficult and if one patient is implanted in both structures results are hard to interpret.
4. Discussion
This study demonstrates a method that uses time-frequency spectra to visualize statistically significant changes in HF during inter-ictal epileptic spikes. High-frequency power is usually much smaller than low frequency power; therefore raw power spectra are not suitable to illustrate small HF power changes. This problem can be overcome by using FDR-controlled time-frequency t-spectra, as demonstrated in this study and before (Kobayashi et al., 2009). Different HF patterns were observed for varying spike characteristics. Spikes within the mesial temporal structures showed more frequent and higher changes in the HF band than neocortical spikes. Spikes with slow waves tended to have larger HF increases and postspike HF decreases than spikes without slow waves, but postspike decreases were not limited to those spikes showing slow waves. Moreover, spikes within the SOZ were more likely to show HF increases and postspike decreases than those outside the SOZ.
The above described differences were always more prominent for HFs above 250 Hz than for those between 80 and 250 Hz. This is in agreement with the observation that distinct HFOs above 250 Hz have more potential to indicate epileptogenic areas than those below (Bragin et al., 2002b, 2007). Additionally, HF power increases above 250 Hz were always present with postspike HF decreases above 250 Hz. Therefore the decrease in high frequency seemed to be dependent on the prior increase.
The idea of differentiating the high-frequency content of spikes with different characteristics derives from the observation that spikes with a larger amount of HF power may have specific patho-physiological meanings. This was first described when analysing the relationship between spikes and distinct HFOs. Rates of spikes with HFOs were better in predicting SOZ areas than rates of spikes in general (Jacobs et al., 2008) and seem to be more closely linked to epileptogenic areas. Therefore, spikes with and without HFOs, which could not be distinguished by looking at the raw EEG, might have a different relevance when defining epileptogenic areas in patients with refractory epilepsy. It is important to note that the above studies worked with distinct HFOs and usually analysed single events and rates of events (Jacobs et al., 2008, 2009a; Urrestarazu et al., 2007). The present study however analyses HF power changes. This allows averaging groups of spikes from the same contact and has two main advantages. First, it is a faster and less reviewer-dependent approach to analyse spikes from the same region and second it gives the possibility to apply statistics on HF changes. In contrast to the actual identification of distinct HFOs, measuring HF power changes does not allow recognizing the actual distinct HFOs and may not be as specific to the epileptic tissue.
Before being able to analyse the connection between spike-related HF changes and epileptic tissue, it is important to evaluate the influence of different anatomical regions as well as spike shapes on the occurrence of HF power changes. Urrestarazu and co-workers analysed HF changes following spikes. The study mainly found HF power decreases, which were interpreted as prolonged depression of excitability after the spike (Neckelmann et al., 2000, neuroscience). Large postspike decreases were coupled with large increases in HF power during the spike, as observed in the present study. HF power decreases were more prominent in the hippocampus than in the amygdala or neocortex (Urrestarazu et al., 2006). Especially in the amygdala, postspike decreases were less common and this was interpreted as an inability to inhibit excitability after a spike. The present study confirmed the large extent of HF power changes within the hippocampus; it however also found larger HF changes in the amygdala than in the neocortex. Generally, HF power increases and decreases were more frequent and of larger extent within the mesial temporal structures. This observation correlates with the one made in studies marking distinct HFOs, where HFO rates, length and amplitude in the mesial temporal structures exceed the ones in neocortex (Crepon et al., 2009; Jacobs et al., 2008). It is believed that the large HF amount and changes in hippocampus and amygdala may result from the specific cortical layering of these structures and reflect their epileptogenic potential. Several studies established a direct link between pathological changes in the mesial temporal structures and the amount of HFOs generated in these structures (Ogren et al., 2009; Staba et al., 2007).
A second goal of this study was to analyse whether the spike shape or more precisely the occurrence of a postspike slow wave reflects HF power changes, especially postspike decreases. It is believed that the postspike slow waves reflect postspike inhibition (Ayala et al., 1973; Neckelmann et al., 2000). Thus, spike-slow waves should show a larger post-spike decrease in HF power than spike alone. In the present study, clear differences in HF power changes between spikes and spike-slow waves were observed. These were however not limited to the postspike HF decrease, as HF increase during the spikes as well as postspike HF decrease were more common and of larger extent in spikes with than without slow waves. Moreover, large postspike decreases seemed to be coupled with preceding large HF increases during the spike. Thus the degree of postspike depression of HF power and degree of postspike inhibition seemed largely dependent of the extent of HF power increase and excitability during the spike. Large changes were less common but still observed in spikes without slow waves and the occurrence of a slow wave therefore does not necessarily reflect the degree of HF postspike decrease.
The core question of this study was whether HF power changes were different within and outside the SOZ. Distinct HFOs have been closely linked to areas of seizure onset and epileptogenic areas (Bragin et al., 2002b, 2004; Jacobs et al., 2008, 2009a). Additionally, spikes co-occurring with HFOs were more exact in predicting the SOZ than spikes in general. Therefore measuring the HF content during spike may be a tool to improve the delineation of the SOZ. A first study with three patients using the FDR-controlled time-frequency t-spectra, gave some indication that the HF changes in spikes within the SOZ are larger than those outside the SOZ (Kobayashi et al., 2009). These differences were confirmed in the present study with HF power increases as well as postspike power decreases being more prominent and frequent in the SOZ than outside. Nevertheless, differences were not as striking as with rates of distinct HFOs and there was a considerable overlap in the amount of HF power changes inside and outside the SOZ. When analysing those patients who had spikes in the SOZ as well as outside, all but one patient had larger HF power changes in the SOZ than outside. The one patient in whom this was not the case (patient 5) was implanted in the mesial temporal structures and the frontal lobe. His SOZ was located within a lesion in the frontal lobe, but spikes in the mesial temporal structures had larger HF power changes than those generated in the frontal lesion. This electrode constellation may provide the explanation for the HF power change pattern in this patient. As spikes in the mesial structures are compared with neocortical spikes the comparison is complicated by the fact that mesial temporal spikes, as described above, are accompanied by larger HF power changes around the spike than neocortical ones in general. Thus is might not be possible to compare mesial temporal with neocortical spikes, when trying to locate the SOZ. Smaller HF power changes around the neocortical spike may actually reflect more pathological changes than higher ones in the mesial temporal structures. This may also explain why patterns of HF power changes between spikes in and outside the SOZ are not completely different in this study, as spikes inside and outside the mesial temporal structures were grouped for the comparison inside vs. outside the SOZ.
Besides this, there is the possibility that averaged measurement of HF power changes of spikes actually do not allow to delineate the SOZ as well as it is possible with measuring rates of distinct events. This may be especially true, as it is known that a distinction of spikes just by the visual appearance and shape in the unfiltered EEG may not reflect their high-frequency content (Jacobs et al., 2008). Thus the averaged spikes, which appeared to have the same shape, may actually have had very different extents of HF power changes and be badly represented by an average.
The technique used here does not allow differentiating between HF components that result from the fast slope of the spikes and those which are distinct HF oscillations co-occurring with spikes. In non-statistical frequency spectra, spike-related components would be associated with a broadband increase of power in all frequencies while distinct HFOs would be seen as small band limited increase in a few frequencies. As this study analysed statistical changes from baseline only in the HF domain, it is not possible to differentiate between the two types of HF activity.
Previous analysis of distinct HFOs distinguished between HFOs visible and invisible during the spike on the unfiltered EEG {Urrestarazu, Chander, et al. 2007 43/id}. HFOs which are invisible on the spike can always result from either an actual distinct event or as a result of the fast slope of the spike. Nevertheless, regardless of the distinction between these 2 possibilities, spikes with HF component were more specific to the SOZ {Jacobs, LeVan, et al. 2008 2/id}. Thus the present study was based on the assumption that even if HF components within a spike are just resulting from the spike’s peaky appearance, this still may be relevant to a better identification of the SOZ. The fact that the present study showed less prominent differences between averaged spike inside and outside the SOZ may however suggest that a differentiation and single spike analysis may be relevant after all.
Moreover our analysis of HF power changes may be influenced by HFOs occurring outside the spike as well as physiological HF power changes. Spontaneous HFOs, which occur independently of spikes, could have interfered with the baseline segments, as we did not select the latter as a function of HF activity. These events however are distinct and very short in regard to the whole baseline segment. Therefore their influence on the statistics should be minimal. Another problem when analysing epileptic HF changes is to differentiate them from physiological HF changes. Physiological HF changes have been seen during wakefulness in sensory- and motor areas and during sleep in the mesial temporal structures. The likelihood that these physiological HFOs largely influenced our results however is small for two reasons. First physiological events are not described to co-occur with epileptic spikes. Second in the mesial temporal structures they are mainly seen during memory consolidation in sleep (Axmacher et al., 2008) and not during wakefulness; in the neocortex they have been mainly described in primary motor and sensory regions (Ball et al., 2008; Curio, 1999), which were not targeted in this study.
In conclusion, the use of time-frequency t-spectra allowed analysing HF power changes around spikes in a large number of spike types. The extent of the HF power increase during a spike and the following postspike decrease were closely correlated. HF power changes were strongest and most frequent in the mesial temporal structures, in spikes with associated postspike slow wave and in spikes generated in the SOZ. HF power changes seem to reflect the amount of excitability during a spike as well as the degree of postspike depression.
References
- Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–17. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- Ayala GF, Dichter M, Gumnit RJ, Matsumoto H, Spencer WA. Genesis of epileptic interictal spikes new knowledge of cortical feedback systems suggests a neurophysiological explanation of brief paroxysms. Brain Res. 1973;52:1–17. doi: 10.1016/0006-8993(73)90647-1. [DOI] [PubMed] [Google Scholar]
- Ball T, Demandt E, Mutschler I, Neitzel E, Mehring C, Vogt K, et al. Movement related activity in the high gamma range of the human EEG. Neuroimage. 2008;41:302–10. doi: 10.1016/j.neuroimage.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–14. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista RE, Cobbs MA, Spencer DD, Spencer SS. Prediction of surgical outcome by interictal epileptiform abnormalities during intracranial EEG monitoring in patients with extrahippocampal seizures. Epilepsia. 1999;40:880–90. doi: 10.1111/j.1528-1157.1999.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–23. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002a;52:407–15. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002b;22:2012–21. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, et al. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2009 doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- Curio G. High frequency (600 Hz) bursts of spike-like activities generated in the human cerebral somatosensory system. Electroencephalogr Clin Neurophysiol. 1999;49(Suppl 1):56–61. [PubMed] [Google Scholar]
- Curtis M, Tassi L, Lo RG, Mai R, Cossu M, Francione S. Increased discharge threshold after an interictal spike in human focal epilepsy. Eur J Neurosci. 2005;22:2971–6. doi: 10.1111/j.1460-9568.2005.04458.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hufnagel A, Dumpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia. 2000;41:467–78. doi: 10.1111/j.1528-1157.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chatillon CE, Olivier A, Dubeau F, Gotman J. High-frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009a;132:1022–37. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy. Surgery. 2009b:120. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Jacobs J, Gotman J. Detection of changes of high-frequency activity by statistical time-frequency analysis in epileptic spikes. Clin Neurophysiol. 2009;120:1070–7. doi: 10.1016/j.clinph.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckelmann D, Amzica F, Steriade M. Changes in neuronal conductance during different components of cortically generated spike-wave seizures. Neuroscience. 2000;96:475–85. doi: 10.1016/s0306-4522(99)00571-0. [DOI] [PubMed] [Google Scholar]
- Ogren JA, Wilson CL, Bragin A, Lin JJ, Salamon N, Dutton RA, et al. Three-dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol. 2009;66:783–91. doi: 10.1002/ana.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier A, Germano IM, Cukiert A, Peters T. Frameless stereotaxy for surgery of the epilepsies: preliminary experience. Technical note. J Neurosurg. 1994;81:629–33. doi: 10.3171/jns.1994.81.4.0629. [DOI] [PubMed] [Google Scholar]
- Otsubo H, Ochi A, Imai K, Akiyama T, Fujimoto A, Go C, et al. High-frequency oscillations of ictal muscle activity and epileptogenic discharges on intracranial EEG in a temporal lobe epilepsy patient. Clin Neurophysiol. 2008;119:862–8. doi: 10.1016/j.clinph.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Bazil CW, Walczak TS, Chan S, Pedley TA, Goodman RR. The predictive value of intraoperative electrocorticography in resections for limbic epilepsy associated with mesial temporal sclerosis. Neurosurgery. 1997;40:302–9. doi: 10.1097/00006123-199702000-00014. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Goncharova II, Duckrow RB, Novotny EJ, Zaveri HP. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia. 2008;49:1881–92. doi: 10.1111/j.1528-1167.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields T, Bragin A, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–8. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–66. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Jirsch JD, LeVan P, Hall J, Avoli M, Dubeau F, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–76. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–86. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]