Abstract
The visually driven responses of macaque area V4 neurons are modulated during the preparation of saccadic eye movements, but the relationship between presaccadic modulation in area V4 and saccade preparation is poorly understood. Recent neurophysiological studies suggest that the variability across trials of spiking responses provides a more reliable signature of motor preparation than mean firing rate across trials. We compared the dynamics of the response rate and the variability in the rate across trials for area V4 neurons during the preparation of visually guided saccades. As in previous reports, we found that the mean firing rate of V4 neurons was enhanced when saccades were prepared to stimuli within a neuron's receptive field (RF) in comparison with saccades to a non-RF location. Further, we found robust decreases in response variability prior to saccades and found that these decreases predicted saccadic reaction times for saccades both to RF and non-RF stimuli. Importantly, response variability predicted reaction time whether or not there were any accompanying changes in mean firing rate. In addition to predicting saccade direction, the mean firing rate could also predict reaction time, but only for saccades directed to the RF stimuli. These results demonstrate that response variability of area V4 neurons, like mean response rate, provides a signature of saccade preparation. However, the two signatures reflect complementary aspects of that preparation.
INTRODUCTION
Visual perception and oculomotor control are known to interact. In one direction, the features of a visual scene influence the patterns of saccadic eye movements (Vishwanath and Kowler 2003; Yarbus 1967). Underlying this influence is presumably the projection of visual cortical representations onto oculomotor structures (Edelman and Keller 1996; Keller and Edelman 1994; Moore 1999). Conversely, psychophysical evidence demonstrates that the preparation of saccadic eye movements informs perception of visual targets, enhancing visual sensitivity at the intended saccade location (Deubel and Schneider 1996; Hoffman and Subramaniam 1995). Correspondingly, the mean firing rates of single neurons in some areas of visual cortex have been shown to be modulated during the preparation of saccades to receptive field stimuli, suggesting a direct influence of saccade preparation on these neurons (Chelazzi et al. 1993; Fischer and Boch 1981; Mazer and Gallant 2003; Moore et al. 1998; Nakamura and Colby 2002; Sheinberg and Logothetis 2001; Tolias et al. 2001). Mimicking endogenous saccade signals by electrically stimulating sites within the frontal eye field (FEF) yields similar modulation of visually driven responses in visual area V4 (Armstrong and Moore 2007; Moore and Armstrong 2003), suggesting that the perisaccadic modulation observed during voluntary saccades originates from oculomotor structures (Moore et al. 2003). In spite of the preceding evidence, our understanding of the nature of the oculomotor influence on visual cortex and the contribution of extrastriate areas to saccade preparation remains incomplete.
Thus far, evidence of an influence of saccade preparation on extrastriate neurons has been exclusively examined in terms of perisaccadic modulations in mean firing rate (Fischer and Boch 1981; Moore and Chang 2009; Tolias et al. 2001). However, a recent study suggests that the across-trial variability of neuronal firing rate provides a more robust signature of motor preparation (Churchland et al. 2006). This study examined the relationship between the activity of neurons in dorsal premotor cortex and the reaction time of monkeys performing a delayed reach task. Although the mean firing rate of premotor neurons did not predict reaction time, changes in the across-trial variability of firing rate did. This observation suggests that firing rate variability may be a more sensitive measure of behavioral state than mean firing rate and thus may be a more robust signature of motor preparation. A recent study of extrastriate area V4 observed attention-dependent changes in across-trial variability of neuronal response rates (Mitchell et al. 2007). Given the well-established relationship between attention and saccade preparation (Moore 2006; Schafer and Moore 2007), across-trial variability of response rates of V4 neurons may also provide an index of motor preparation.
To assess the interaction between saccade preparation and visual cortical representations, we measured the mean firing rate and variability across trials of spike trains recorded from area V4 neurons in monkeys trained to make saccades to visual targets. Response variability was measured by the Fano factor (FF), which was computed by dividing the across-trial variance in spike counts within a small window by the mean count. As expected, the mean firing rate of V4 neurons was enhanced when saccades were prepared to stimuli within a neuron's receptive field (RF) in comparison with saccades to a non-RF location. In contrast, we found robust decreases in FF prior to saccades both to RF and non-RF stimuli, and these decreases predicted saccadic reaction times for saccades to all stimuli. Mean firing rate also predicted reaction time, but only for saccades directed to the RF stimuli. For saccades directed away from the RF, no mean firing rate change was observed yet FF still predicted saccadic reaction time. These results demonstrate that response variability of area V4 neurons, like mean response rate, provides a signature of saccade preparation. However, the two signatures reflect complementary aspects of that preparation.
METHODS
Subjects
Two male monkeys (Macaca mulatta, 8–12 kg) were used in these experiments. All experimental procedures were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Society for Neuroscience Guidelines and Policies. General surgical procedures have been described previously (Graziano et al. 1997).
Behavioral task
Monkeys performed a visually guided, delayed saccade task which was initiated by fixation to within 1.0° of the central fixation spot (Fig. 1). Immediately following fixation, an oriented bar stimulus appeared in the RF of the neuron under study and remained there until the end of the trial. Following the onset of the RF stimulus, the monkey was required to maintain fixation for a fixed delay (0.5–1 s, for a given experiment) while it waited for the appearance of a saccade target (0.25° diam) at one of two locations distant from the RF. In 2/3 of the trials, the target appeared, the fixation spot was extinguished, and the monkey was rewarded for making a saccade to the target. In these conditions, the saccade target could appear either directly upward from the fixation spot (“up” condition) or in the opposite visual hemifield to the RF stimulus (“opposite” condition). In the remaining one-third of trials (“toward” condition), the saccade target did not appear. Instead, when the fixation spot was extinguished, the monkey was rewarded for saccades to the RF stimulus. All conditions were identical until the cue to saccade (disappearance of the fixation spot) and were randomly interleaved. During all behavioral trials, eye position was measured via the scleral search coil method, and digitized at 200 Hz for off-line analysis. Trials in which the monkey broke fixation prematurely or made a saccade to an incorrect target were discarded.
Fig. 1.
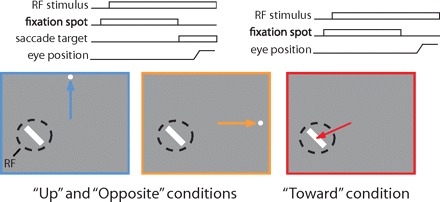
The visually guided delayed saccade task. In the task, the monkey fixates a central dot while an oriented bar is displayed in the receptive field (RF; dashed circle) of a single V4 neuron. After a delay, the monkey is cued (by fixation spot offset) to make a saccade in 1 of 3 directions. On 2/3 of the trials, a target dot appears in 1 of 2 locations, conditions up (left) and opposite (middle), and the monkey is rewarded for making a saccade to that dot. If no target appears, the monkey is rewarded for executing a saccade to the RF stimulus (right).
Recording
The activity of single V4 neurons was recorded via glass-coated platinum-iridium electrodes lowered into the dorsal surface of the prelunate gyrus. Neural activity was sampled at 32 kHz, digitized and stored. The waveforms of single neurons were isolated by off-line clustering (DataWave Technologies).
RF stimuli
RF stimuli were displayed on a 34 × 27-cm Sony video monitor that was driven by a Number Nine graphics board (640 × 480) at a 60 Hz, noninterlaced, refresh rate. The video display was positioned 57 cm in front of the monkey. Visual stimuli consisted of gray-, red-, green-, or blue-colored bars appearing at one of four orientations (0, 45, 90, or 135° θ), presented at the center of a V4 neuron's RF. The contrast of the oriented bars varied between 5 and 80%, and the sizes varied between 1.0 × 0.1 and 8.0 × 0.8°. In a single block of trials, the RF stimulus varied along only one of the four stimulus dimensions (color, orientation, contrast, or size). The fixation spot was a small (0.25° diam) circle displayed at the center of the video display. The non-RF saccade target stimulus used in some behavioral conditions was identical to the fixation spot but located peripherally (>5.0°).
Data analyses
We distinguished between tuned and untuned neurons by performing an unpaired t-test between firing rates for trials of each stimulus identity (i.e., “red” or “green”) and trials of each other stimulus along the same dimension (size, contrast, color, or orientation). If any comparison was significant (P < 0.05), then the neuron was defined as tuned and the maximal stimulus was taken as “preferred,” whereas the minimal was “nonpreferred.” For trials corresponding to each neuron, each stimulus identity, and each saccade direction [10–20 trials (mean 15.6), hereafter defined as a “neuron-condition”], we used the median reaction time (RT) for that neuron-condition to determine the faster (“short RT”) and slower (“long RT”) trials. Thus the long RT and short RT trials are exactly controlled for the effects of stimulus identity, neuron identity, stimulus preference, and saccade direction. Trials with RTs equal to the median of the neuron-condition were randomly assigned to the short or long RT groups.
Fano factor (FF) was computed by calculating the variance divided by mean of the spike counts across trials for an 80-ms window centered on successive 1-ms time bins for each neuron-condition, i.e., those trials with the same recording site, visual stimulus, and saccade direction. For example, for a time bin centered at −45 ms relative to saccade onset, counts were made within the 80-ms window around that time point (−85 to −5 ms) on each of the 10–20 trials of the neuron-condition, and both the mean and the variance were computed on the resulting set of 10–20 numbers. Finally the variance was divided by the mean to yield the FF, and the population estimate was simply the average of the FF values from all neuron-conditions. Note that the FF measures across-trial variability (Churchland et al. 2006) as opposed to within-trial variability of spike times or interspike intervals (de Ruyter van Steveninck et al. 1997) or variability across neurons (Cohen et al. 2007). Windows with no spikes on any of the trials were excluded from FF calculations. Eighty milliseconds was chosen as a window size, prior to computing any statistics, after trying values between 5 and 150 ms and selecting visually for a window that yielded traces retaining salient features of those generated with shorter windows while smoothing the noise effectively. Mean firing rates were likewise computed with the same 80-ms window.
To determine whether mean firing rate or FF traces at a particular point deviated significantly from a baseline period, we performed Wilcoxon ranked sum tests on the difference between data at the point of interest and data from a set of baseline period time points chosen to include the entire delay period without overlap because each point contains data from an 80-ms window. For saccade aligned data, the delay period was −640 to −320 ms relative to saccade onset, so the selected data points composing the delay period were −600, −520, −440, and −360 ms.
To control for a possible effect of variable firing rates on FF, we employed a “mean-matching” procedure in which the population distribution of mean spike counts was equalized across time (see Fig. 4 of Churchland et al. 2007). The algorithm computed the mean spike counts for all neuron-conditions, where each neuron-condition consists of a complete set of trials, 10–20 total, from a particular neuron, visual stimulus, and saccade direction. Each plotted dot in Fig. 2G represents the mean and variance across the trials of one neuron-condition. The algorithm determined a common distribution of these mean spike counts that can be found at all time points. It then randomly eliminated neuron-conditions until this common distribution was achieved at each time point. Because individual trials were never deleted from within neuron-conditions, the relationship between the mean and variance of spike counts for any neuron-condition was never altered by this procedure; rather, a different selection of the neuron-conditions (i.e., variance/mean pairs) is taken at each time point to meet the common distribution. The elimination was independent at each time point. The algorithm discarded a minority of the data in each case, keeping 69% for the upward saccade condition, 63% for opposite saccades, and 53% for saccades toward the RF. The FF was then computed only on these remaining data. The process was repeated 10 times, and the results averaged to control for variation due to the randomness of the procedure. We performed this analysis using the “Variance Toolbox” for MATLAB provided by M. M. Churchland.
Fig. 4.
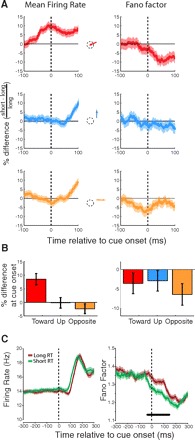
Relationship of presaccadic mean firing rate and FF to saccadic RT for the population of V4 neurons. A, left: traces show percent difference in mean firing rate between short and long reaction time (RT) trials for each saccade condition. Right: percent differences in FF. B: differences in mean firing rate and FF for short and long RT trials in each saccade condition at the time of the movement cue (t = 0). C: same data as in A but collapsed across the 3 saccade conditions. Horizontal bar indicates a significant difference between long and short RT traces.
Fig. 2.
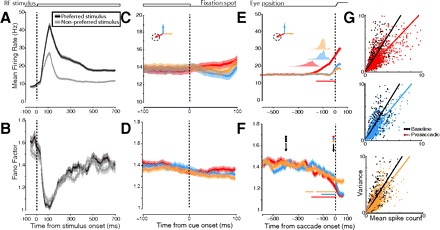
Effects of RF stimulation and saccade preparation on the mean firing rate and response variability for the population of area V4 neurons. Left: mean firing rate (A) and Fano factor (FF; B) aligned to the time of RF stimulus onset and divided into responses to preferred vs. nonpreferred visual stimuli. These traces, as well as those in C–F, are all smoothed with an 80-ms box filter (see methods). C and D: mean firing rate and FF aligned to movement cue onset (i.e., fixation offset) and split by direction of saccade. E and F: the same but aligned to saccade onset. In all traces, means (dark lines) and SE (shading) are shown. In E and F, horizontal bars indicate significant difference from delay period. In E, translucent plots above traces show distributions of cue onset times relative to saccade onset. G: data from individual neuron-conditions for 2 time points: baseline and immediately prior to saccade onset. Each dot represents the mean and variance of spike counts within an 80-ms window for just 1 neuron-condition (those trials corresponding to a particular neuron, stimulus, and saccade direction). Black dots represent variance/mean pairs taken from windows during the baseline period of each saccade condition (1st arrow in F). Colored dots represent variance/mean pairs taken from windows just prior to saccade onset (2nd arrow in F). Thick lines are linear regressions on the data.
To assess the possible influence of microsaccades on the mean rate and variability of V4 responses, we performed control analyses in which trials containing microsaccades within relevant time windows were eliminated. Thus for analyses of presaccadic firing rates and FFs, we excluded trials with microsaccades occurring within 200 ms of saccade onset (0.6% of trials). Likewise, for analysis of RT effects around the time of cue onset, we excluded trials with microsaccades occurring within 200 ms of cue onset (2.4% of trials). Microsaccade detection was performed as in Armstrong et al. (2006). Microsaccades were defined as eye movements that exceeded 0.1° amplitude and had maximum velocity >10°/s for ≥10 ms.
For comparison of two conditions (for example, long RT trials vs. short RT trials), we computed a Wilcoxon signed-rank test on the mean firing rate or FF values for all neurons under the first condition versus those for the second condition at a certain time point. For stimulus aligned responses, we used t = 100 ms poststimulus onset, approximately at the peak of responsiveness. For cue aligned, we used t = 0 ms (exactly at cue onset) and for saccade aligned, we used t = −45 ms (just prior to saccade onset without including any postsaccadic visual responses). Because a window of 80 ms was used for the computation of both mean firing rate and FF, the values at these points include spikes from 40 ms on either side of the point. For comparisons in which many time points were examined to determine the time course of an event, the Simes procedure was used to control the false discovery rate (Benjamini and Hochberg 1995).
To analyze differences in the magnitude of the presaccadic decline in FF between saccade directions, we performed an analysis of covariance (ANCOVA) on the change in FF over the final 80 ms of saccade preparation with the change in mean firing rate over the same time period as a covariate and the saccade direction as a factor. Thus for each neuron-condition, without mean-matching, we subtracted the FF and mean rate values at −80 ms relative to saccade from the values at the time of saccade onset. These two sets of numbers, ΔFF (dependant variable) and Δ mean firing rate (independent variable), were grouped according to saccade direction and analyzed with the ANCOVA. The y intercept of the ΔFF versus Δ mean firing rate measures the component of the change in FF that is independent of change in mean firing rate.
RESULTS
We computed the FF and the mean firing rate for 102 single neurons recorded in area V4 of two macaque monkeys (n = 28 neurons from one and n = 74 from the other) during the visually guided saccade task. Neurons were visually stimulated with single oriented bars that varied in orientation, color, size, or contrast. Figure 2 shows both the mean firing rate and mean FF changes in the population following onset of the RF stimulus, around the time of cue onset, and at the time of saccades to the RF stimulus or to non-RF targets. Stimulus-onset-aligned data from the most effective (preferred) and least effective (nonpreferred) RF stimuli are plotted separately; cue- and saccade-aligned data are divided according to the direction of the saccade. Overall, the sample of V4 neurons was highly selective for the RF stimuli employed, shown by the roughly twofold difference in mean firing rate following stimulus onset between the preferred and nonpreferred stimuli (Fig. 2A). In contrast, the FF exhibited a marked decrement following stimulus onset (Fig. 2B), and there was no significant difference in that decrement between the preferred and nonpreferred responses (Wilcoxon signed-rank test, P = 0.234; see methods for further details on statistical procedures). Instead the dynamics of the stimulus-driven FF changes were similar for the two stimulus divisions during both the initial onset transient and the sustained response in the delay period. The overall decrement in the FF following stimulus presentation is consistent with the stimulus-driven changes in variability reported across many other cortical areas (Churchland et al. 2010). Cue-aligned firing rate and FF are shown only to emphasize that at the time of cue onset the rewarded direction of saccade was unknown to the monkey, and therefore the overall mean firing rate and FF did not differ between the three saccade direction conditions (Fig. 2, C and D).
The mean firing rate changes we observed prior to saccade onset (Fig. 2E) confirmed previous findings. Specifically, there was a significant increase in mean firing rate for saccades to the RF stimulus (toward condition; Wilcoxon rank sum test, P < 0.001). However, there was no change in mean firing rate for saccades to the opposite hemifield or upward saccade target locations (opposite and up conditions; Wilcoxon rank sum test, P = 0.155 and P = 0.069, respectively) (Fischer and Boch 1981; Moore and Chang 2009; Moore et al. 1998). In contrast to the mean firing rate effects, the FF decreased significantly for all saccade directions when compared with its value during the delay period (Wilcoxon rank sum test, P < 0.001 for each direction; Fig. 2F). The decrement in the FF was present within the final 100 ms of saccade preparation for each of the three saccade directions, shown by the decreased slope in the presaccadic variance/mean relationship relative to baseline (Fig. 2G). Saccades to the RF stimulus generally had much longer RTs than saccades to non-RF targets (RTs, mean ± SD: toward = 224 ± 50 ms; up = 115 ± 28 ms; opposite = 115 ± 24 ms; toward vs. up, P < 0.001; toward vs. opposite, P < 0.001). The larger RTs of the saccades to the RF stimuli is presumably due to the lack of an abrupt onset of the target (i.e., the RF stimulus) in this condition in contrast to the other two conditions (Yantis and Jonides 1984). Nonetheless the pattern of presaccadic FF decline was largely similar to the other saccade conditions.
Mean-matched control for presaccadic firing rate changes
Neural firing patterns are commonly approximated as Poisson processes for which the variance of spike counts across trials is equal to the mean and thus FF is unity. However, this assumption may be violated and FF may decrease for extraneous reasons, for example due to an increasing influence of the refractory period at high firing rates. Although average firing rates were low (less than ∼40 Hz), and thus the refractory period is unlikely to have a large impact on spike train variability (Mitchell et al. 2007), we nonetheless performed an analysis to control for the influence of firing rate dynamics on the FF (Fig. 3,A and B). In this analysis, neuron-conditions (sets of trials corresponding to each neuron and stimulus condition; see methods) were discarded randomly at each time point to equalize the distribution of mean firing rates across the entire presaccadic period. This was done separately using data for each saccade condition (toward, up, and opposite), and the FF was computed on the remaining data (see methods). Thus this procedure eliminated changes in mean firing rate preceding saccades. Nevertheless, the significant decline in FF prior to saccade onset persisted for all three saccade conditions (Wilcoxon signed rank, P < 0.001 for toward condition; P < 0.05 for up and opposite) even in this mean-matched data set. Thus the observed presaccadic decreases in FF were not due to the changes in mean firing rate.
Fig. 3.
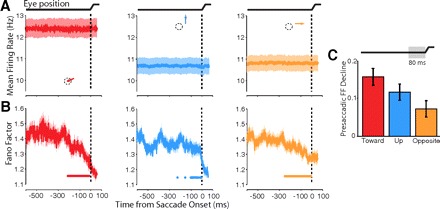
Presaccadic changes in FF for “mean-matched” conditions. The mean-matching algorithm was applied to presaccadic spike trains from the population of recorded V4 neurons to equalize firing rate distributions across time for each of the saccade directions. A: mean-matched firing rates for each of the saccade directions (toward, left; up, middle; opposite, right), which no longer vary over time. B: FF of the mean-matched data, which still declines presaccadically despite removing variation in firing rate. C: the magnitude of FF decline in the final 80-ms period before the saccade for each of the 3 saccade directions. The FF decline plotted corresponds to the component of the FF decline independent of the presaccadic change in mean firing rate, computed in an ANCOVA. Error bars represent 95% confidence intervals from ANCOVA.
Dependence of FF changes on saccade direction
We compared the magnitude of the presaccadic decline in FF between the three saccade directions during the final 80-ms period prior to the saccade onset. We used an ANCOVA to factor out the effect of presaccadic changes in mean firing rate. We found main effects of saccade direction (P < 0.016) and mean firing rate (P < 10-4) on the magnitude of FF decline (Fig. 3C). The latter effect demonstrates that firing rate indeed influences the presaccadic change in FF. The main effect of saccade direction, however, demonstrates that the FF declines with different magnitude for the different saccade directions and that this difference is independent of changes in mean firing rate. The overall decline for all saccade directions (P < 0.01 for all directions) corroborates the results of the mean-matching analysis in that it confirms a presaccadic decline in FF that is independent of changing firing rates. However, the FF decline was greatest for saccades directed toward the RF compared with the up and opposite conditions. Thus in addition to a robust overall decline in FF for all saccade directions, we observed a component of that decline that depended on saccade direction.
Predicting saccadic RT
A recent study found that across trial firing rate variability provides a better predictor of motor preparation than does the mean firing rate (Churchland et al. 2006). We sought to determine whether the variability of V4 responses, measured by FF, might reflect the state of saccade preparation. To do this, we examined the extent to which the FF was predictive of saccadic RT. We divided the trials obtained from all saccade directions and all RF stimuli into two subsets, long and short RT trials, with equal numbers of all conditions in each subset. We then recomputed mean firing rate and FF on these new trial divisions. We reasoned that if either FF or mean firing rate reflects the state of saccade preparation, then we should observe differences in these measures between short and long RT saccades at the time of the movement cue. Because the analysis window was 80 ms in duration, it included spikes occurring from 40 ms prior to the movement cue onset ≤40 ms after. V4 neurons have visual onset latencies of ∼50 ms (Maunsell 1987), and in our data closer to 70 ms (Fig. 2A). Thus the analysis window includes only the activity of neurons prior to any measurable responses to the movement cue (fixation offset) or target onset.
Despite the lack of differential visual stimulation at the time of cue onset, the FF of V4 neurons was significantly different between long and short RT trials, although mean firing rate was not (Fig. 4). We computed the mean firing rate and FF around the time of movement cue onset separately for trials corresponding to each RT group and saccade direction and depict these data as percent changes from short to long RT trials, plotted for each saccade direction separately (Fig. 4A). A two-way repeated-measures ANOVA revealed no main effect of either RT or direction on mean firing rate at exactly the time of cue onset, although there was an interaction between the two (Fig. 4B, P < 0.001). Considering only those saccades directed toward the RF, there was a difference in mean firing rate between short and long RT trials at the time of cue onset with 8.6% larger mean firing rate for short RT trials (P < 0.001). Mean firing rate did not differ significantly between RT groups for saccades to other locations, although the trend was toward a suppression of mean firing rate for saccades to the opposite hemifield on short RT trials relative to long (2.4% lower mean firing rate for short RT trials; P = 0.16).
We observed a main effect of RT on FF, with short RT saccades having lower FF across directions (Fig. 4A, right; P = 0.003). However, there was no main effect of saccade direction (P = 0.66) and no interaction between RT and saccade direction (P = 0.59). Thus for saccades toward the RF, both mean firing rate and FF predicted RT. However, for saccades directed to the upward and opposite saccade targets, the FF predicted saccadic RT even though there was no change in mean firing rate. Due to the interaction between saccade direction and RT for mean firing rate, collapsing the data across saccade direction largely eliminated the difference between short and long RT trials (P = 0.15). In contrast, collapsing the FF data across saccade directions yielded a robust difference between short and long RT trials (P = 0.006). FF was significantly lower for short RT trials than long from −35 to 148 ms relative to cue onset (P < 0.014; Fig. 4C). The difference between FF of short and long RT trials (4.5%) is similar in magnitude to the effects reported in the study of premotor cortical neurons in a reaching task (5%) (Churchland et al. 2006). Our results demonstrate that in contrast to the presaccadic decline in FF, a component of which depended on saccade direction, the relationship between FF and RT at the time of cue onset was independent of saccade direction.
Possible influence of microsaccades
Because it is known that fixational saccades (i.e., microsaccades) can affect the firing rates of V4 neurons (Leopold and Logothetis 1998), we considered their possible influence on the rate and variability of V4 activity in this study. For example, because the rate of microsaccades necessarily (and empirically) decreases in the time leading up to a saccade, this decline in the incidences of microsaccades might have contributed to the decline in FF (Fig. 2D). To control for any influence of microsaccades, we discarded all of the trials in which a microsaccade occurred within the time window of interest and re-performed the analyses described in the preceding text. There were no differences in the primary effects in this reduced data set compared with the data set in which all trials with microsaccades were included. In particular, the presaccadic decline in FF remained significant for all saccade directions (Wilcoxon rank sum test, P < 0.001). The magnitude of the presaccadic decline still depended on saccade direction (P < 0.018). The mean firing rate still predicted saccadic RTs for saccades toward the RF but not for other directions (P < 0.001), and the FF predicted saccadic RTs for all directions (P = 0.05).
DISCUSSION
We measured the mean and variability of firing rates across trials of spike trains recorded from area V4 neurons during visually guided saccades. As expected, the mean firing rate of V4 neurons was enhanced when saccades were prepared to stimuli within a neuron's RF in comparison with saccades to a non-RF location. In contrast, we found robust decreases in FF prior to saccades both to RF and non-RF stimuli with only a small influence of saccade direction on the magnitude of the FF decrease. These FF decreases predicted saccadic RTs for all saccade directions. Although mean firing rate also predicted RT, this effect depended on saccades being directed to the RF stimuli. These results demonstrate that mean firing rate and FF exhibit different and complementary signatures of saccade preparation in area V4: while mean firing rate conveys more information about the direction of an imminent saccade, FF primarily reflects the progress of saccade preparation.
The way in which saccades toward the RF were cued differed from that of saccades directed to up or opposite targets. Specifically, in the latter case, the appearance of a saccade target indicated the location of the rewarded saccade, whereas in the former case, the absence of such a target indicated that the rewarded saccade was to the RF stimulus. It could be argued that this unbalanced task design could confound the interpretation. For example, the presaccadic enhancement of mean firing rate for toward saccades might be explained by a difference in the cueing method or by increased RTs in that condition. A previous report has shown that the presaccadic enhancement of mean firing rate is independent of the cue (Moore and Chang 2009), and thus these mean firing rate changes are not due to the differences in cueing method. The novel changes in FF that we report were also independent of cueing method. Specifically, the ability of FF to predict RT and the presaccadic decline in FF were largely independent of saccade direction and thus cannot be explained by the task design.
FF is a measure of variability normalized to the mean, so it reflects changes in variance relative to concurrent changes in mean firing rate. Nevertheless the FF may vary due to indirect effects caused by changes in mean rate that do not reflect true changes in variability. For example, spike trains may be regularized by an increasing influence of the refractory period at high mean firing rates, such as those observed in our task prior to saccades toward RF stimuli. In some conditions, such indirect effects could not possibly account for the dynamics in the FF. For example, although saccades in different directions were preceded by either enhanced or unchanged mean firing rates, the FF decreased for all directions uniformly (Fig. 2, C and D). Thus differences in the dynamics of mean firing rate across conditions do not necessarily result in the same direction of differences in the dynamics of FF. In addition, we controlled for the effect of changes in mean rate on FF by matching the mean across-trial firing rate distributions across time. The effect of this manipulation is to produce a subset of the data that has stable mean firing rate across time. We found that the FF decrease during saccade preparation was still present in the mean-matched data, indicating that the dynamics of the FF response were independent of changes in mean firing rate. The dissociation of responses of these two measures of neural activity demonstrates that they represent different information about the state of the visuosaccadic network.
Firing rates of single neurons predict behavioral RTs in many frontal and parietal cortical regions, such as motor and premotor cortex (Riehle and Requin 1993), the parietal reach region (Snyder et al. 2006), the frontal eye fields (Hanes and Schall 1996), and the lateral intraparietal area (Ipata et al. 2006). These correlations may reflect the role of these individual neurons in generating motor behaviors such as arm and eye movements. Recent studies have also shown that several measures of visual cortical activity predict RT, such as LFP in striate and extrastriate cortex (Zhang et al. 2008), spike-field coherence in area V4 (Womelsdorf et al. 2006), and multiunit activity in area V1 (Super and Lamme 2007) as well as single-unit activity in areas MT and VIP (Cook and Maunsell 2002). Our results are thus consistent with a growing body of evidence that neural activity in visual cortex can predict RT. Our results also demonstrate that the FF of V4 responses provides a reliable prediction of RT in that it predicted RTs of saccades in all tested directions rather than simply those that target the neuron's RF. Taken together, these findings argue for a more integrated view of the role of visual cortical areas in visually guided behavior, a view that could take advantage of the myriad signatures that predict that behavior.
FF has been interpreted as reflecting the true underlying variability of neuronal firing rate across trials (Churchland et al. 2006). In this view, every spike train recorded from a neuron is a noisy instantiation of some “true” firing rate for that trial. This true firing rate may itself be variable across trials so that the recorded spike trains are in fact noisy realizations of a different true firing rate on each trial. While averaging the firing rate eliminates both sources of variability, FF instead estimates the extent of the underlying true variability with the assumption that spiking noise is invariant. With this context, we can interpret our data in much the same way as did Churchland et al. (2006). The decreased FF for short RT trials relative to long at the time of the cue to move reflects less variability in underlying firing rate, i.e., that more of the trials had the same true firing rate at cue onset for the short RT condition than the long. The precise value of this true firing rate may depend on the particular task, such as in our results in which neurons exhibited higher firing rates for saccades toward the RF and were on average unresponsive to saccades away from the RF. Nevertheless variability decreased in all three conditions, so the FF provides an index of the state of saccade preparation.
Importantly, FF revealed a signature of saccade preparation in the responses of area V4 neurons even when there was no change in mean firing rate. Traditionally, a neuron without changes in mean firing rate would be viewed as nonmodulated and its activity as uninformative during these conditions. Our results indicate that such a view is inaccurate. Even though a neuron may not be modulated in terms of its mean firing rate, a measure of the firing rate distribution may reveal that the activity of such a neuron is indeed modulated. Our results show that such modulation present during saccade preparation occurs to such a degree that the activity predicts saccadic RTs. FF therefore provides a sensitive measure of the influence of saccade preparation on V4 activity that is complimentary with mean firing rate, revealing that neuronal responses are influenced by saccade preparation even when mean firing rate is neither enhanced nor suppressed.
Nonetheless our results do not in any way undermine the important role that firing rate likely plays in determining how neurons drive behavior. On the contrary, likely it is not the variability per se but rather the particular firing rates on individual trials, as indexed by the FF, which relates to the state of saccade preparation. We assume that the proximity of the firing rate on individual trials to some optimal mean firing rate relates directly to motor preparation (Churchland et al. 2006), an assumption consistent with our result that groups of trials with shorter RTs tend to have firing rates closer to the mean (i.e., lower FF). This view is depicted schematically in Supplemental Fig. S1,1 where the difference between baseline and presaccadic periods, as well as the difference between short and long RT trials, can be understood as a narrowing of the width of the firing rate distribution across trials. Due to a relatively small number of trials for each neuron-condition, and the noisiness of the firing rate measure, these firing rate distributions cannot be visualized directly, but are instead estimated from both the mean and variance of the measured single trial spike counts.
There may be some relationship between the presaccadic modulation of variability reported here and modulation of the gamma frequency (30–70 Hz) spectral power of visual cortical responses. Gamma modulation has been demonstrated to occur presaccadically at least for microsaccades (Bosman et al. 2009), and coherence effects in the gamma range are predictive of behavioral RTs (Womelsdorf et al. 2006) on some tasks, as is the FF reported here. Despite these similarities, it should be re-emphasized that we have measured variability across trials rather than variability in the spike times within trials, which would be most directly related to oscillatory processes. Future studies might address the potential relationship between observed decline in across-trial variability and frequency domain properties of neural activity.
The predictive activity of the mean firing rate for some, and FF for all, saccade directions can also be interpreted in the context of the influence of attention on saccadic RT (Kustov and Robinson 1996). Because the interval between fixation onset and cue onset during a particular experiment was fixed, monkeys might have anticipated the impending saccade and directed spatial attention accordingly prior to the cue to move. In fact, increased anticipation of a behaviorally relevant stimulus does increase the magnitude of attentional modulation of the firing of area V4 neurons (Ghose and Maunsell 2002). Thus for example, on some trials the monkey may have anticipated the cue and attended to the RF stimulus, which could have resulted both in reduced FF (Mitchell et al. 2007) and “short” RTs on those trials for which that stimulus became the saccade target (Posner et al. 1980). Likewise, higher FFs and “long” RTs may have resulted from allocation of attention to incorrect target locations or lack of attentional allocation altogether.
Our results do not allow us to determine whether the two measures (mean firing rate and FF) are signatures solely of attentional deployment or saccade preparation. However, given the preponderance of evidence that the effects of attention and saccade preparation on V4 neurons are very similar, if not identical (Moore et al. 2003), it is unclear to what extent such a distinction is possible in this area. However, our results cannot be explained solely by the known influences of covert spatial attention on variability (Mitchell et al. 2007). Because we observed a robust decline in FF even when the monkey directed saccades, and thus spatial attention (Deubel and Schneider 1996; Hoffman and Subramaniam 1995), away from the neuron's RF, any known influence of covert spatial attention on FF must have been combined with some other influence that is independent of the saccade direction. For example, there may be a saccade-direction-independent influence of attention, perhaps merely related to the disengagement of fixation prior to saccades of any direction. On the other hand, such a nonspatial influence need not be directly related to the preparation of the eye movement per se. A number of studies have observed neural correlates of other spatially nonselective factors such as stimulus and reward expectation as well as elapsed time (e.g., Ghose and Maunsell 2002; Janssen and Shadlen 2005). Moreover, although the magnitude of presaccadic decline in FF depended on saccade direction, there was a substantial decline for all saccade directions. Thus a more global influence, for example arousal or reward anticipation, could be considered to explain the nonspatial component of the effects. Indeed like attention, these other influences may be associated with saccade preparation but may not require an actual movement to produce the dynamics we observe. Nonetheless the FF predicts saccadic RTs for saccades in all tested directions and thus provides a reliable signature of saccade preparation.
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron 50: 791–798, 2006 [DOI] [PubMed] [Google Scholar]
- Armstrong KM, Moore T. Rapid enhancement of visual cortical response discriminability by microstimulation of the frontal eye field. Proc Natl Acad Sci USA 104: 9499–9504, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Roy Stat Soci Series [B] 57: 289–289, 1995 [Google Scholar]
- Bosman CA, Womelsdorf T, Desimone R, Fries P. A Microsaccadic rhythm modulates gamma-band synchronization and behavior. J Neurosci 29: 9471–9480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature 363: 345–347, 1993 [DOI] [PubMed] [Google Scholar]
- Churchland M, Yu B, Cunningham J, Sugrue L, Cohen M, Corrado G, Newsome W, Clark A, Hosseini P, Scott B, Bradley D, Smith M, Kohn A, Movshon A, Armstrong K, Moore T, Chang S, Snyder LH, Ryu S, Santhanam G, Sahani M, Shenoy K. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nature Neurosci In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Ryu SI, Santhanam G, Shenoy KV. Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci 26: 3697–3712, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Sahani M, Shenoy KV. Techniques for extracting single-trial activity patterns from large-scale neural recordings. Curr Opin Neurobiol 17: 609–618, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Pouget P, Woodman GF, Subraveti CR, Schall JD, Rossi AF. Difficulty of visual search modulates neuronal interactions and response variability in the frontal eye field. J Neurophysiol 98: 2580–2587, 2007 [DOI] [PubMed] [Google Scholar]
- Cook EP, Maunsell JHR. Dynamics of neuronal responses in macaque MT and VIP during motion detection. Nat Neurosci 5: 985–994, 2002 [DOI] [PubMed] [Google Scholar]
- de Ruyter van Steveninck RR, Lewen GD, Strong SP, Koberle R, Bialek W. Reproducibility and variability in neural spike trains. Science 275: 1805–1808, 1997 [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res 36: 1827–1837, 1996 [DOI] [PubMed] [Google Scholar]
- Edelman JA, Keller EL. Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol 76: 908–926, 1996 [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R. Enhanced activation of neurons in prelunate cortex before visually guided saccades of trained rhesus monkeys. Exp Brain Res 44: 129–137, 1981 [DOI] [PubMed] [Google Scholar]
- Ghose GM, Maunsell JHR. Attentional modulation in visual cortex depends on task timing. Nature 419: 616–620, 2002 [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Hu XT, Gross CG. Visuospatial properties of ventral premotor cortex. J Neurophysiol 77: 2268–2292, 1997 [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996 [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys 57: 787–787, 1995 [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J Neurosci 26: 3656–3661, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nat Neurosci 8: 234–241, 2005 [DOI] [PubMed] [Google Scholar]
- Keller EL, Edelman JA. Use of interrupted saccade paradigm to study spatial and temporal dynamics of saccadic burst cells in superior colliculus in monkey. J Neurophysiol 72: 2754–2770, 1994 [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature 384: 74–77, 1996 [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Microsaccades differentially modulate neural activity in the striate and extrastriate visual cortex. Exp Brain Res 123: 341–345, 1998 [DOI] [PubMed] [Google Scholar]
- Maunsell JHR. Physiological evidence for two visual subsystems. In: Matters of Intelligence. Boston: Reidel, 1987, p. 59–87 [Google Scholar]
- Mazer JA, Gallant JL. Goal-related activity in V4 during free viewing visual search evidence for a ventral stream visual salience map. Neuron 40: 1241–1250, 2003 [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55: 131–141, 2007 [DOI] [PubMed] [Google Scholar]
- Moore T. Shape Representations and visual guidance of saccadic eye movements. Science 285: 1914–1917, 1999 [DOI] [PubMed] [Google Scholar]
- Moore T. The neurobiology of visual attention: finding sources. Curr Opin Neurobiol 16: 159–165, 2006 [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature 421: 370–373, 2003 [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron 40: 671–683, 2003 [DOI] [PubMed] [Google Scholar]
- Moore T, Chang MH. Presaccadic discrimination of receptive field stimuli by area V4 neurons. Vision Res 49: 1227–1232, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Tolias AS, Schiller PH. Visual representations during saccadic eye movements. Proc Natl Acad Sci USA 95: 8981–8984, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Colby CL. Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proc Natl Acad Sci USA 99: 4026–4031, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and the detection of signals. J Exp Psychol Gen 109: 160–174, 1980 [PubMed] [Google Scholar]
- Riehle A, Requin J. The predictive value for performance speed of preparatory changes in neuronal activity of the monkey motor and premotor cortex. Behav Brain Res 53: 35–49, 1993 [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Moore T. Attention governs action in the primate frontal eye field. Neuron 56: 541–551, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinberg DL, Logothetis NK. Noticing familiar objects in real world scenes: the role of temporal cortical neurons in natural vision. J Neurosci 21: 1340–1350, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LH, Dickinson AR, Calton JL. Preparatory delay activity in the monkey parietal reach region predicts reach reaction times. J Neurosci 26: 10091–10099, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Super H, Lamme VAF. Strength of figure-ground activity in monkey primary visual cortex predicts saccadic reaction time in a delayed detection task. Cereb Cortex 17: 1468–1475, 2007 [DOI] [PubMed] [Google Scholar]
- Tolias AS, Moore T, Smirnakis SM, Tehovnik EJ, Siapas AG, Schiller PH. Eye movements modulate visual receptive fields of V4 neurons. Neuron 29: 757–767, 2001 [DOI] [PubMed] [Google Scholar]
- Vishwanath D, Kowler E. Localization of shapes: eye movements and perception compared. Vision Res 43: 1637–1653, 2003 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature 439: 733–736, 2006 [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: evidence from visual search. J Exp Psychol Hum Percept Perform 10: 601–621, 1984 [DOI] [PubMed] [Google Scholar]
- Yarbus AL. Eye Movements and Vision. New York: Plenum, 1967 [Google Scholar]
- Zhang Y, Wang X, Bressler SL, Chen Y, Ding M. Prestimulus cortical activity is correlated with speed of visuomotor processing. J Cognit Neurosci 20: 1915–1925, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


