Abstract
We have recently shown that a single injection of mature, antigen-pulsed, human dendritic cells (DCs) rapidly elicits CD4+ and CD8+ T-cell immunity in vivo. The DCs were pulsed with 2 foreign proteins, keyhole limpet hemocyanin (KLH) and tetanus toxoid (TT), as well as an HLA A2.1-restricted influenza matrix peptide (MP). Responses to all 3 antigens peaked at 30–90 days after immunization and declined thereafter. To determine if the foreign helper proteins (TT and KLH) were essential for CD8+ T-cell responses to the viral peptide, we reinjected 3 of the HLA-2.1 subjects with mature DCs pulsed with MP alone. All 3 volunteers showed a rapid boost in MP-specific immunity, and freshly sampled blood from 1 contained cytolytic T cells. In all 3 subjects, CD8+ T-cell responses to booster DCs were faster and of greater magnitude than the responses to the first DC injection. Importantly, the T cells that proliferated after booster DC treatment secreted interferon-γ upon challenge with much lower doses of viral peptide than those elicited after the first injection, indicating a higher functional avidity for the ligand. These data begin to outline the kinetics of T-cell immunity in response to DCs and demonstrate that booster injections of mature DCs enhance both qualitative and quantitative aspects of CD8+ T-cell function in humans.
This article may have been published online in advance of the print edition. The date of publication is available from the JCI website, http://www.jci.org. J. Clin. Invest. 105:R9–R14 (2000).
Introduction
Although several approaches (such as DNA vaccines, viral vectors) generate protective T-cell immunity in mice, it has proven difficult to generate potent T-cell immunity in humans with current vaccines (1). Dendritic cells (DCs), nature’s adjuvant, are antigen-presenting cells (APCs) specialized to initiate T-cell immunity (2). It is known that exposure to inflammatory stimuli leads to terminal differentiation or maturation of DCs, with enhanced capacity to stimulate T-cell immunity (2). We have shown recently that a single injection of antigen-bearing, monocyte-derived, mature DCs leads to rapid enhancement of T-cell responses in humans (3). These data demonstrated that mature DCs are potent immune adjuvants in humans and provided the first controlled evidence of their immunogenicity.
More quantitative T-cell assays have now made it possible to study the kinetics and longevity of the T-cell immune response in humans after DC immunization. In previous work, serial quantitative measurements of immune parameters were not performed (4, 5). Here we serially characterize the kinetics and durability of CD4+ and CD8+ T-cell responses after DC injection in humans using a panel of quantitative assays. We also show that a booster injection of mature DCs leads to greater and more rapid enhancement of the CD8+ T-cell response without the need for “foreign helper epitopes.” Importantly, we demonstrate that T cells elicited after booster DCs are functionally superior and recognize lower doses of antigen than those elicited after the first injection.
Methods
Study subjects
Seven of 9 subjects who were injected with antigen-pulsed DCs in the initial study (3) were available for long-term monitoring and are subjects of the present analysis. Two subjects (P2, P3) had left the New York area and were unavailable for follow-up.
Schema for immune monitoring and booster injections
All subjects were monitored for immune responses to study antigens every 1–4 months after the first antigen-pulsed DC injection. In 3 of 4 HLA A2.1+ subjects (P4, P5, P6), booster DC injections were performed at 7–9 months after the first antigen-pulsed injection. A booster injection was not considered in the fourth HLA A2.1+ subject (P1) because of intervening pregnancy. All subjects who received a booster injection signed an updated informed consent. Subjects were monitored 2 days, 1 week, 1 month after the booster and every 1–4 months thereafter. The study was approved by the Rockefeller University Institutional Review Board and the U.S. Food and Drug Administration.
Measurement of immune responses
Antigen-specific proliferation.
Antigen-specific proliferation to keyhole limpet hemocyanin (KLH), tetanus toxoid (TT), and staphylococcal enterotoxin A (SEA) as a control, was performed as described earlier (3). All assays were performed on fresh PBMCs. When possible, cryopreserved samples from before and after immunization were also thawed and assayed together. In some cultures, the phenotype of the responding T cells was monitored at the end of culture by staining for CD4 and quantifying the percentage of CD4+ T-cell blasts (high forward scatter) using flow cytometry.
Enzyme-linked immunospot assay for IFN-γ release from single antigen-specific T cells.
Enzyme-linked immunospot (ELISPOT) assay for detection of influenza matrix protein peptide (MP) and influenza-specific T cells was performed as described earlier on freshly isolated PBMCs (3). To assess the peptide sensitivity of antigen-specific T cells, pre- and postimmunization specimens were thawed together and cocultured overnight (16 hours) with freshly generated autologous mature DCs pulsed with graded doses (0.01–100 ng/mL) of MP (unpulsed DCs as control) at a DC/T cell ratio of 30:1. The number of antigen-specific IFN-γ–producing cells was quantified as noted above.
Bulk cytolytic effector assays.
For detection of MP-specific killers in bulk uncultured PBMCs, freshly isolated PBMCs were directly added to labeled T2 targets pulsed with 1 μM MP (unpulsed T2 as controls).
T-cell recall assays for bulk cytolytic effectors and IFN-γ–secreting cells.
Recall T-cell memory was quantified using 2 methodologies, ELISPOT and cytolytic effector (CTL) assay. Recall CTL assay was performed as described earlier, using antigen-pulsed DCs pulsed with MP (1 μM) or infected with live influenza virus (moi = 2) (3). For recall ELISPOT assay, pre- and postimmunization specimens were thawed together and cocultured with freshly generated mature DCs pulsed with MP (versus no peptide as control) as in the recall CTL assay. After 7 days, cells were transferred to an ELISPOT plate at 105 cells per well and cultured for 14–18 hours with or without restimulation with MP.
Generation of dendritic cells
DCs were generated from blood monocyte precursors and maturation was induced by culture in monocyte-conditioned medium as described (3).
Booster DC injection
DCs were pulsed overnight 1 day before injection with 1 μM HLA A*0201–restricted influenza MP (manufactured under Good Laboratory Practice [GLP] by Alan Houghton, Sloan Kettering Cancer Center, New York, New York, USA). On the day of injection, the DCs were resuspended in normal saline containing 5% autologous plasma and injected as a superficial subcutaneous. injection as described earlier (3). All injected DC preparations tested negative for bacterial and fungal contamination.
Detection of KLH-specific antibodies
KLH-specific antibodies were detected by an ELISA adapted from methods described by Holtl et al. (6). Quantitation was performed by subtracting OD (450 nm) in KLH wells from that in control wells. Pre- and postimmunization sera from each volunteer were assayed together, and all experiments included a positive control serum known to contain high-titer KLH-specific antibodies.
Statistical analysis
For comparison of the immune response between first and booster DC injections, ratios of pre- and postimmunization values were compared by paired t test after logarithmic transformation of the data. P values less than 0.05 were considered significant.
Results
Longevity of KLH and TT-specific T-cell responses.
All 9 subjects injected with KLH-pulsed DCs showed priming of KLH-specific immune response (3). T-cell proliferative responses to KLH peaked at a median of 30 days (range 30–90 days) after antigen-pulsed DC injection and declined thereafter (Figure 1a). However, at ≥ 6 months after the DC injection, proliferative responses to KLH were still detectable above baseline in 4 of 7 (P1, P5, P6, P9) subjects. Five of 6 subjects (P2, P4, P5, P7, P9) injected with TT-pulsed DCs had boosting of TT-specific immunity (3). TT-specific T-cell response also peaked at a median of 30 days after injection and then declined, but remained detectable above baseline at ≥ 6 months in 2 (P5, P7) of 4 responding (P4, P5, P7, P9) subjects available for follow-up (Figure 1b). In the 3 subjects not injected with TT-pulsed DCs, as well as those injected with antigen alone, there was no significant change in TT-specific responses during follow-up (data not shown). Injection of booster DCs (P4, P5, P6) that were pulsed with MP only also did not lead to a nonspecific increase in KLH- or TT-specific immunity.
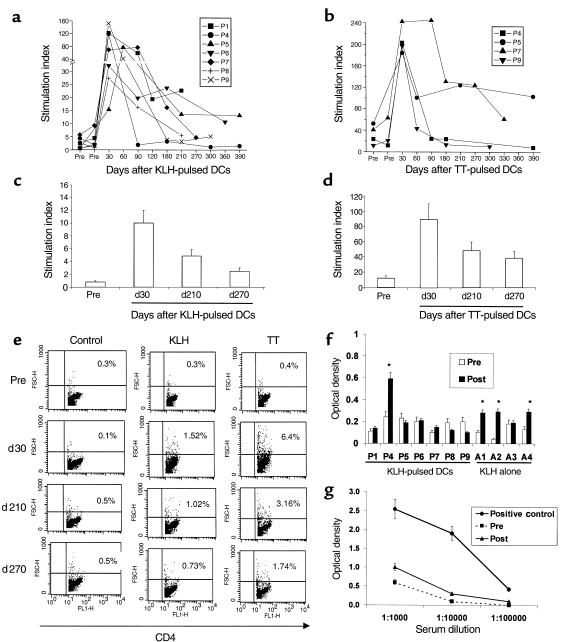
Figure 1.
TT- and KLH-specific immunity after DC injection. (a) Longevity of KLH-specific immune response after single DC injection. For each measurement shown, 105 freshly isolated PBMCs were incubated in the presence or absence of 10 μg/mL KLH for 5 days, and proliferation was measured as 3H-TdR incorporation. Results are expressed as stimulation index. SEM < 30%; cpm without antigen < 5 × 103. (b) Longevity of TT-specific immune response after single DC injection. For each measurement shown, 105 freshly isolated PBMCs were incubated in the presence or absence of 3 μg/mL TT for 5 days, and proliferation was measured as 3H-TdR incorporation. Results are expressed as stimulation index. SEM < 30%. (c) Evaluation of longevity of KLH-specific response using cryopreserved specimens. Pre- and postimmunization specimens were thawed together and cultured in the presence or absence of 10 μg/mL KLH for 5 days. Antigen-specific proliferation was measured as 3H-TdR incorporation. Results are shown as stimulation index. Data shown are for 1 subject (P5), representative of 3 subjects tested. (d) Evaluation of longevity of TT- specific response using cryopreserved specimens. Pre- and postimmunization specimens were thawed together and cultured in the presence or absence of 3 μg/mL TT for 5 days. Antigen-specific proliferation was measured as 3H-TdR incorporation. Results are shown as stimulation index. Data shown are for 1 subject (P5), representative of 2 subjects tested. (e) Quantification of CD4 proliferative response as percent of CD4 blasts. T-cell cultures from the experiment in c and d were stained for CD4 and analyzed by flow cytometry. Percent of CD4 blasts were quantified as percent of CD4+ T cells with high forward scatter, noted in the upper-right quadrant. Data shown are gated for CD4+ cells. (f and g) Detection of KLH-specific antibodies. (f) Presence of KLH-specific antibodies was determined in sera before and 3 months after immunization by ELISA as described in Methods. Data shown are at a serum dilution of 1:104. Because of variable background, reactivity was assayed by comparing pre- and postimmunization samples in the same individual. All assays were repeated to verify results. *P < 0.05. (g) Representative data from a subject (A1) injected with KLH alone. KLH-specific antibodies were detected in only 1 of 7 volunteers (P4) injected with KLH-pulsed DCs. Positive control was an individual with known high-titer anti-KLH antibodies.
To further confirm that the differences between baseline measurements and ≥ 6-month postimmunization time points were not because of interassay variability, samples from these time points, when possible, were thawed and assayed together. Data from a representative volunteer (P5) are shown (Figure 1, c and d). The CD4 nature of the proliferative response in these cultures and the frequency of responding cells was also evident when we monitored T-cell phenotype and proportion of CD4 T-cell blasts (high forward scatter) using flow cytometry (Figure 1e). The maximum number of CD4 T blasts was detected at 30 days after immunization, consistent with the data from proliferation assays on both fresh and thawed cells. We showed previously that KLH and TT responses to antigen-pulsed DCs were CD4 dependent (3).
Development of KLH-specific humoral immunity.
Sera from before immunization and 3 months after immunization were examined for the presence of KLH-specific antibodies in 7 volunteers injected with KLH-pulsed DCs (P1, P4–P9) and those injected with KLH alone (A1–A4). KLH-specific antibody responses (at much lower titer than our positive control serum), were detected in 3 of 4 volunteers injected with KLH alone (Figure 1, f and g). In contrast, KLH-specific antibodies were detected in only 1 (P4) of 7 subjects tested who received KLH-pulsed DCs, and these were at similarly low titer.
Durability of MP-specific T-cell immunity after the first antigen-pulsed DC injection.
Circulating MP-specific IFN-γ–producing CD8+ T cells peaked at a median of 60 days (range 7–90) after DC injection. In 3 of 4 HLA A 2.1+ volunteers (P4, P5, P6), the number of circulating MP-specific CD8+ T cells declined back to baseline over the next 3–6 months (Figure 2, a–c). However, in 1 volunteer (P1; Figure 2d), the number of MP-specific T cells leveled off at a higher level.
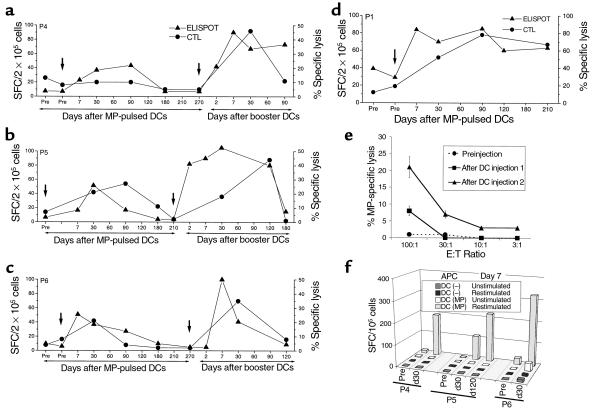
Figure 2.
Enhancement of antigen-specific CD8+ T cells in vivo. (a–d) Kinetics of antigen-specific CD8+ T cells following DC injection(s). MP-specific interferon-γ–producing T cells in freshly isolated uncultured T cells were quantified using an ELISPOT assay (triangles). Results are shown as the number of spot-forming cells (SFC)/2 × 105 PBMC. MP-specific CTLs were quantified after 7-day coculture with MP-pulsed mature DCs (DC/T cell ratio 30:1). Data shown are percent of MP-specific lysis at each time point (effector/target [E/T] ratio 20:1) after subtracting lysis with unpulsed T2 targets and that using unpulsed DCs (circles). SEM < 25%. Arrows indicate the timing of DC injections. The subjects were: a, P4; b, P5; c, P6; and d, P1. (e) Detection of circulating lytic effectors after booster DC injection. Freshly isolated bulk PBMCs of a study subject (P4) from before injection and 7 days after booster DC injections 1 and 2 were directly tested for the presence of MP-specific lytic effectors using MP-pulsed T2 cells as targets at varying E/T ratios. Data shown are percent of MP-specific lysis after subtracting lysis of unpulsed T2 cells. (f) Enhancement of MP-specific IFN-γ–producing T cells after booster DC injection, recall ELISPOT assay. Cryopreserved PBMCs from before (pre) and 30 or 120 days after booster DC injection were thawed together and cocultured for 7 days with freshly generated mature DCs (DC/PBMC ratio 1:30), either unpulsed, DC(–), or pulsed with 1 μg/mL MP, DC (MP), without exogenous cytokines. On day 7, T cells were either left unstimulated or restimulated by the addition of 10 μg/mL MP peptide and transferred to an ELISPOT plate. MP-specific IFN-γ–producing T cells were quantified using a 16-hour ELISPOT assay. SEM < 30%.
When T cells were boosted in culture with autologous DCs, there was an increase in MP-specific lytic effectors after immunization in 3 of 4 subjects (P1, P5, P6; Figure 2, a–d). In these 3 subjects, the response peaked at a mean of 70 days (range 30–90 days) after injection (Figure 2, b–d). Similar to the ELISPOT assay, MP-specific recall CTL responses had returned back to baseline by 6 months after the first injection in 3 of 4 subjects.
Booster DC injection: toxicity.
All 3 HLA A2.1+ subjects with declining circulating MP-specific T cells (P4, P5, P6) were boosted with mature DCs pulsed with MP alone. The dose of booster DCs was 4.5–5 × 106 cells, and purity ranged from 39–74%. KLH and TT were omitted from booster DCs to determine if these epitopes were required for eliciting MP-specific CD8+ T-cell responses using mature DCs. All booster injections were well tolerated with no greater than grade 1 toxicity or evidence of autoimmunity.
Local reaction to DC injection.
Two of 3 subjects developed a local reaction at the injection site. In these 2 subjects these reactions were larger and developed earlier (at 24 versus 48 hours), as compared with those after the first injection (data not shown).
T-cell response to booster DC injections: assays on uncultured PBMCs.
All 3 subjects who received a booster DC injection had an increase in the number of circulating MP-specific IFN-γ–producing T cells in freshly isolated PBMCs (Figure 2, a–c). This response was evident as early as 2 days after the injection in 2 subjects and by 7 days in all subjects. Immune response to booster injection was more rapid, with mean 18.4-fold increase in MP-specific T cells in the first week, compared with 4.8-fold increase after the first injection (P = 0.01). In addition, the peak responses following booster DCs were higher than those after the first injection (19.7-fold versus 7-fold; P = 0.008). As with the first injection, increase in MP-specific IFN-γ–producing T cells after booster DCs in 2 subjects with longer follow-up (P5, P6) has not been sustained and has declined back to preinjection baseline within 6 months.
Increased peptide sensitivity of the expanded CD8+ T-cell response.
We were unable to detect cytolytic effectors in bulk uncultured T cells in any of the subjects after the first DC injection. However, bulk lytic effectors were detected after booster DCs in 1 of 3 subjects 7 days after injection (P4; Figure 2e). The detection of bulk lytic effectors in this subject (and not in others) could not be explained simply by the frequency of MP-specific IFN-γ–producing T cells. Therefore, we next examined peptide sensitivity of elicited T-cell populations as a measure of functional maturation of the immune response. To control for variable thawing of resident APCs in these samples, freshly prepared autologous mature DCs pulsed with various doses of peptides were used as APCs in a 16-hour ELISPOT assay for IFN-γ–secreting cells with thawed uncultured PBMCs from 30 days after the first MP-pulsed DC immunization or booster DCs. T cells elicited after booster DCs demonstrated greater peptide sensitivity than those after the first injection in all 3 subjects. Remarkably, T cells in P4 (who developed circulating lytic effectors), showed the greatest peptide sensitivity, with half-maximal recognition at less than 0.01 ng/mL. (Figure 3).
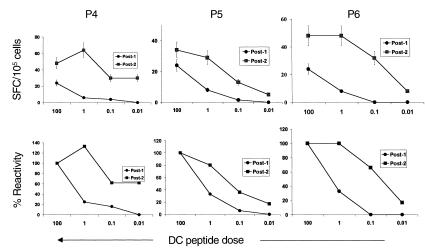
Figure 3.
MP-specific T cells after booster DCs show greater peptide sensitivity than those after the first injection. Cryopreserved PBMCs from 30 days after the first (post-1; circles) and second booster antigen-pulsed (post-2; squares) DC injection were thawed together and cocultured with freshly generated mature DCs (DC/PBMC ratio 1:30), pulsed with graded (0.01–100 ng/mL) MP. After overnight culture, the presence of MP-specific T cells (expressed as SFCs) was quantified using an ELISPOT. (Top) Effect of peptide dose pulsed on DCs on the detection of MP-specific IFN-γ–producing cells. (Bottom) Data expressed as percent of reactivity seen with DCs pulsed with the highest dose of MP (100 ng/mL).
T-cell response to booster DCs: assays in cultured T cells.
When T cells were boosted in culture with peptide-pulsed DCs, there was greater expansion of MP-specific CTLs after booster DCs, relative to the initial DC injection in all 3 volunteers (Figure 2, a–c). When T cells from before and after the second immunization were thawed together and cocultured with peptide-pulsed DCs, there was a greater expansion of MP-specific IFN-γ–producing T cells after booster DCs, which correlated with the CTL data (Figure 2f). As with the ELISPOT assays in uncultured cells, the increase in T-cell recall assays after the booster DCs was not sustained and was followed by a contraction phase similar to that after the first injection.
Discussion
In this study, we have followed several aspects of the T-cell response to 2 DC immunizations in humans. The number of circulating antigen-specific IFN-γ–secreting CD8+ T cells is rapidly enhanced following DC injection and peaks by 7–30 days after injection. The measured responses then decline over the next 3 months. This decline in T-cell reactivity may either be because of loss of measured function (e.g., the capacity to secrete cytokines or to proliferate in recall assays) or loss of T cells because of activation-induced cell death (AICD) and the rapid disappearance of antigen-bearing DC in vivo. Persistence of CD8+ T cells may also depend on the presence of influenza-specific CD4+ T cells, which are not induced by MP. These data about the kinetics of response have important implications for both the optimal schedule of immune monitoring and DC immunizations in clinical trials. Thus, peak immune responses may not be manifest until 1–3 months after the DC injection and frequent (e.g., weekly/bimonthly) injections of DCs may actually be detrimental by inducing AICD of recently activated T cells.
CD4+ T-cell responses to both a priming (KLH) and boosting (TT) antigen after a single DC injection also involves a similar rapid increase followed by a decline. However, these responses remained detectable above baseline for ≥ 6 months in some subjects. The observed lack of KLH-specific antibody response after KLH-pulsed DCs can be attributed to a lack of free/soluble antigen when delivered with DCs.
In following the kinetics of T-cell activity using quantitative assays in the blood of humans after DC immunization, we find that the responses can be detected without the need to expand the T cells in vitro before the assay. The immune responses in humans last several months, in contrast to a recent study in mice wherein response was evident only in terms of weeks (7).
Recent studies in mice have shown that CD4+ T cells help the generation of CD8+ T-cell responses through CD40 ligand-mediated activation of DCs (8). Thus, KLH and TT may have provided help for the generation of CD8+ T-cell responses after the first DC injection. Alternatively, mature DCs may already be activated and therefore may not require CD4-mediated help, as has been observed in vitro (9). All 3 subjects rapidly responded to MP-pulsed mature DCs without KLH or TT, indicating that these epitopes are not required for eliciting CD8+ T-cell responses using mature DCs. However, exclusion of these foreign helper epitozpes does not stringently exclude CD4 help generated inadvertently during DC culture (10). Ongoing studies will determine if the inclusion of these epitopes alters the generation of immune response.
Booster DCs led to significantly higher and more rapid T-cell responses, detectable as early as 2 days after injection. Thus, although all measured immune responses such as ELISPOT as well as recall assays using DCs had returned to baseline before booster DCs, these subjects had retained “memory” of the first DC injection at the level of the whole individual. Therefore, humans retain a memory for the antigen that was administered on the first dose of DCs that we are unable to measure with current functional assays.
A more effective form of protective immunity may be provided by circulating antigen-specific killers. Such responses have been detected in humans previously only following acute viral infections (e.g., acute HIV or Epstein-Barr virus infection) or certain live attenuated vaccines (e.g., measles), but not with subunit vaccines. The development of circulating lytic effectors in 1 subject after booster DCs in this study suggests that this may be feasible by using DCs in humans, particularly when one optimizes variables such as DC dose, route of administration, maturation status, helper epitopes, and DC survival in vivo.
Another important and novel aspect of immune response to booster DCs observed here is the enhanced functional avidity of CD8+ T-cell response, manifest as greater peptide sensitivity of T cells elicited after booster DCs. Similar findings were made independently in mice; i.e., T cells elicited during secondary viral infection exhibited greater peptide sensitivity (11–13). To our knowledge, this is the first evidence of qualitative enhancement of T-cell function in humans after a vaccination strategy. Higher-affinity T cells may be essential to achieve protective immunity against viruses and tumors in vivo (14, 15). Additional research will be needed to pursue and therapeutically exploit the intriguing potential of DC immunization that is revealed in the current study. A first dose of DCs expands effector and memory T cells, whereas a second dose elicits more rapid, readily measurable responses that include lytic effectors and CD8+ effector T cells with higher functional avidity.
Acknowledgments
This work was supported in part by an Investigator Award from the Cancer Research Institute and a Clinical Research Career Development Award from the American Society of Clinical Oncology (both to M.V. Dhodapkar), grants from the National Institutes of Health (AI-40874 to R.M. Steinman, AI-39516 and AI-44628 to N. Bhardwaj), American Cancer Society (ROG-98-355-01 to R.M. Steinman), the SLE foundation (N. Bhardwaj), and a General Clinical Research Center grant (M01-RR00102) from the National Center for Research Resources at the National Institutes of Health. We would like to thank all volunteers for their interest and participation in this study, Coraleen Fossella for help with clinical monitoring, Rockefeller University nursing staff for their help with patient care, and Judy Adams for help with graphics.
References
- 1.Raychaudhuri S, Rock KL. Fully mobilizing host defense: building better vaccines. Nat Biotechnol. 1998;16:1025–1031. doi: 10.1038/3469. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Dhodapkar MV, et al. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A020-specific peptides from prostate-specific peptides from prostate-specific membrane antigen. Prostate. 1996;29:371–380. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 5.Nestle FO, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 6.Holtl L, et al. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol. 1999;161:777–782. [PubMed] [Google Scholar]
- 7.Ludewig B, et al. Protective antiviral cytotoxic T cell memory is most efficiently maintained by restimulation via dendritic cells. J Immunol. 1999;163:1839–1844. [PubMed] [Google Scholar]
- 8.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj N, et al. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J Clin Invest. 1994;94:797–807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingstone AM, Kuhn M. Dendritic cells need T cell help to prime cytotoxic T cell responses to strong antigens. Eur J Immunol. 1999;29:2826–2834. doi: 10.1002/(SICI)1521-4141(199909)29:09<2826::AID-IMMU2826>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann MF, Speiser DE, Ohashi PS. Functional maturation of antiviral T cell response. J Virol. 1997;71:5764–5768. doi: 10.1128/jvi.71.8.5764-5768.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 14.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeh HJ, III, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–994. [PubMed] [Google Scholar]