Abstract
Effective therapeutic interventions and clinical care of adults infected with HIV-1 require an understanding of factors that influence time of response to antiretroviral therapy. We have studied a cohort of 118 HIV-1–infected subjects naive to antiretroviral therapy and have correlated the time of response to treatment with a series of virological and immunological measures, including levels of viral load in blood and lymph node, percent of CD4 T cells in lymph nodes, and CD4 T-cell count in blood at study entry. Suppression of viremia below the limit of detection, 50 HIV-1 RNA copies/mL of plasma, served as a benchmark for a successful virological response. We employed these correlations to predict the length of treatment required to attain a virological response in each patient. Baseline plasma viremia emerged as the factor most tightly correlated with the duration of treatment required, allowing us to estimate the required time as a function of this one measure.
Introduction
It has been clearly demonstrated that HIV-1 protease inhibitor–containing regimens (1–4) induce effective suppression of HIV replication. There is, however, large variation in the time observed for the levels of viremia to decrease below the limit of detection of the standard assays used for the determination of viremia (i.e., 50 HIV-1 RNA copies/mL). The decrease of viremia below the level of 50 HIV-1 RNA copies is widely accepted to reflect effective virological response to antiretroviral therapy (5). Previous studies have shown that the extent and the duration of viral suppression after combination antiretroviral therapy were dependent upon the levels of plasma viremia at study entry and at the nadir, respectively (6–10). However, the ability of virological and immunological factors in blood and lymphoid tissue to predict the time of the virological response to treatment has not been fully delineated, and the estimates of the average time of treatment required to decrease the levels of viremia below 50 copies have not yet been determined. To address these issues, it is crucial to study cohorts of HIV-infected subjects naive to highly active antiretroviral therapy (HAART), because in HAART-experienced patients the antiviral activity of antiretroviral drugs may be negatively influenced by preexisting protease inhibitor–resistant and nucleoside reverse transcriptase inhibitor–resistant virus mutants (11).
Methods
Study cohorts.
In this study, 118 HIV-infected adults were included. All patients were naive to any antiretroviral drug, had a baseline CD4+ T-cell count greater or equal to 250 cells/μL, and a baseline viremia greater or equal to 5,000 HIV-1 RNA copies/mL of plasma. All patients were enrolled in different clinical trials: (a) 33 HIV-1–infected patients enrolled in the CNAB2006 study (12) are being treated with abacavir (300 mg every 12 hours) and amprenavir (1,200 mg every 12 hours); (b) 22 HIV-1–infected patients enrolled in the AVIB study (13) are being treated either with abacavir (300 mg every 12 hours), nelfinavir (1,250 mg every 12 hours), and saquinavir soft gel capsules (1,200 mg every 12 hours) (n = 12) or abacavir (300 mg every 12 hours), nelfinavir (1,250 mg every 12 hours), and amprenavir (1,200 mg every 12 hours) (n = 10); (c) 19 HIV-1–infected patients, enrolled in the outpatient cohort of the Department of Infectious Diseases, San Raffaele Scientific Institute, Milan, Italy, are being treated with stavudine (40 mg every 12 hours), lamivudine (150 mg every 12 hours), and nelfinavir (750 mg every 8 hours); (d) 34 HIV-1–infected patients enrolled in the ADAM and NUCB.2019 studies (14, 15) have been treated with either stavudine (40 mg every 12 hours), lamivudine (150 mg every 12 hours), nelfinavir (750 mg every 8 hours), and saquinavir hard gel capsules (600 mg every 8 hours) or zidovudine (300 mg every 12 hours), lamivudine (150 mg every 12 hours), and ritonavir (600 mg every 12 hours); (e) 10 HIV-1–infected patients enrolled in the CNA3005 study (16) are being treated with Combivir (zidovudine 300 mg/lamivudine 150 mg) twice daily in combination with either indinavir (800 every 8 hours) or abacavir (300 mg every 12 hours). Fifty-three out of 118 subjects underwent an excisional lymph node biopsy before the initiation of HAART. In all subjects, plasma viremia was measured at study entry, at weeks 2 and 4, once every 4 weeks until week 24, and every 12 weeks thereafter. All patients gave informed consent.
Determination of plasma viremia.
Determination of plasma HIV-1 RNA concentration was routinely performed with the Amplicor HIV Monitor assay (Roche Pharma, Basel, Switzerland) with a limit of detection of either 400 or 50 RNA copies/mL.
Virological measures in lymph nodes.
Viral load in lymph nodes was distinguished between virus-expressing cells and RNA encapsidated in virions on the surfaces of follicular dendritic cells (FDC) (17–24). Quantification of these 2 forms of virus in lymph nodes was performed by in situ hybridization using HIV-specific radiolabeled active RNA probes (20, 25) and imaging analysis (26, 27).
For determinations of quantitative imaging of in situ hybridizations 2 reference standards were used. First, a viral control reference was prepared by constructing an artificial tissue (26) containing a known number of viral particles per unit of volume (27). This was done by suspending a preparation of a known number of viral particles (e.g., 6.8 × 1010 vp/mL; ABI, Columbia, Maryland, USA) in “fibrin glue” (available commercially as Tiseel VH from Baxter Healthcare Corp., Deerfield, Illinois, USA). Then, bovine thrombin was added vigorously to the fibrin-virus suspension, resulting in a very firm clot that entrapped the virus particles. The clot was formed in a plastic cylinder and could be removed easily and transferred to 20 volumes of 1.3 M aqueous formaldehyde and fixed for 24 hours at room temperature. After fixation the clot was processed and embedded in paraffin, sectioned, and mounted on microscope slides for in situ hybridization. Second, a radiation imaging control consisting of a microscope slide with known amounts of 14C plastic rectangles (ranging between 0 and 2.15 mCi/g of plastic) (ARC-146A; American Radiolabeled Chemicals Inc., St. Louis, Missouri, USA) was used to determine the relative amounts of beta radiation striking the imaging plate. Carbon was selected as a reference standard for isotopic sulfur because the decay energy of 14C (0.156 Mev) is only slightly less than that of 35S (O.167 Mev). This slide was exposed with each group of test slides. By including a control radiation standard and hybridization standard we could compensate for differences in lots of radiolabeled riboprobe. Tissues were hybridized with either 35S HIV sense or antisense probes by a well-established protocol (25). The resulting slides were exposed on BAS 5000 imaging plates (Fuji Medical Systems Inc., Stamford, Connecticut, USA) for 4 days at room temperature. The image plates were read, and the data were transferred to compact disks for storage and analysis. The slides were then made into autoradiograms by dipping into Kodak NPT 2 emulsion (Eastman Kodak Co. Scientific Imaging Systems, New Haven, Connecticut, USA) followed by 4 days exposure at 4°C. Determination of single HIV-expressing cells was done by imaging sections of lymph nodes on Fuji 5000 phosphorstorage imager (Fuji Medical Systems Inc.) at a resolution of 25 mm, and results were expressed as the number of virus-expressing cells/100 mm2. The resulting images were compared with the autoradiograms. HIV-1 RNA–expressing cells were identified and circled with MacBAS 2.5 software (Fuji Medical Systems Inc.), and the number of photo-stimulated luminescence (PSL) radiation units were measured. For calculation, the total PSL per cell and/or per lymph node tissue section were determined using MacBAS 2.5 software. To compare results obtained from different runs using varying lots of radiolabeled riboprobe, the radioactivity of the tissue sections in relation to the carbon reference standards was determined, measured in picocuries. To this extent, 16 dilutions of the 14C radiation standard were used. The number of PSL for each dilution was measured and divided by the corresponding carbon-specific activity expressed in picocuries, and the average number of PSL per picocuries of the 16 dilutions was calculated. For each sample lymph node section obtained from individual patients the number of PSL per square millimeter was divided by the average number of PSL per picocurie of the carbon standard run in the same session. This allowed expression of the number of picocuries per square millimeter for each patient’s tissue section analyzed. Then, to determine the numbers of viral particles in reference to the numbers of picocuries, the virus control reference was used. Varying known amounts of viral particles per square millimeter were divided by the corresponding value of picocuries per square millimeter obtained using the carbon standard as explained previously, and the number of viral particles per picocurie was calculated. Based on these calculations, the average number of virus particles per picocurie was 1650.9; this number was used as a constant to calculate the number of viral particle equivalents per square millimeter. Therefore, the number of picocuries per square millimeter of tissue sections from individual patients was multiplied by this constant number (1650.9) to calculate the estimates of the number of viral particles per square millimeter for each patient. The results were expressed as viral genomic equivalents (VGE) per square millimeter, where 1 VGE corresponds to 2 HIV-1 RNA copies. With this approach it is possible therefore to: (a) count the number of HIV-1–expressing cells on lymph node sections and thus assess the number of cells per 100/mm2 of tissue and (b) calculate for each sample the number of VGE per square millimeter, which corresponds to the FDC-associated HIV-1 RNA. To distinguish between cell-associated and FDC-associated HIV-1 RNA, the percentage of PSL due to virus-expressing cells were calculated for each sample and then subtracted from the total PSL; this allows a precise quantitation of the FDC-associated HIV-1 RNA. The mean percent of PSL due to virus-expressing cells in 53 lymph node samples was 3.16, ranging between 0.29 and 10.9%. Therefore, consistent with previous studies (28, 29), the FDC-associated HIV-1 RNA in HIV-1–infected persons with established chronic infection represented ≥ 90–95% of the total virus detected in the lymph nodes. Furthermore, using this technique the estimates of the number of viral particles per cell were also similar to those reported previously (28, 29). The average number of viral particles per PSL was 34. Because the number of PSL per cell was never greater than 6, the estimated number of viral particles per cell was generally below 200.
Flow cytometry.
Flow cytometry analysis was performed as described previously (21). Anti-CD3, anti-CD4, and anti-CD8 mAbs conjugated with either FITC, phycoerythrin (PE), or peridinin chlorophyll (PerCP) were used (Becton-Dickinson Immunocytometry Systems, San Jose, California, USA). Determination of percent of CD4 and CD8 was performed on freshly isolated blood and lymph node mononuclear cells.
Statistical analysis.
A series of virological and immunological measures were assessed in both blood and lymph node before the initiation of HAART and evaluated for their ability to serve as a predictor of the time of response to HAART. The virological response was defined as a plasma viremia below 50 copies/mL. To estimate the date at which the viral load dropped below 50 copies, we reviewed each patient’s time series to find the “last sample” at which load was greater than 50 and the “first sample” at which RNA load was less than 50. The second phase of viral decline was fitted by a linear regression on the log data starting after week 4 and ending with the first sample at which RNA was greater than 50. By the regression line we calculated the exact date at which the RNA load was expected to be 50 copies. If this predicted date was not between the dates of the last and first samples, we dropped the first data point of the time series and recalculated the regression line. This was repeated until the prediction fulfilled the constraint of being between the last and first samples. The worst case of this procedure is a simple interpolation, between the last and first samples. The 95% confidence intervals (see Figure 3) is twice the standard error, because the Student t value is 2 for P values less than 0.05 and df = 116.
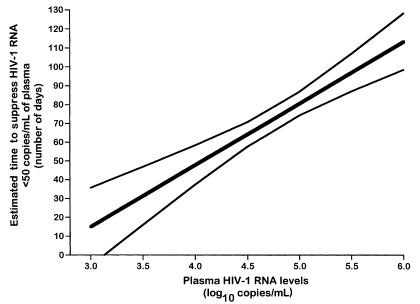
Figure 3.
Estimated time to suppress plasma HIV-1 RNA below 50 copies/mL based upon varying levels of baseline plasma HIV-1 RNA. The estimated required number of days (thick continuous line) with 95% confidence limits (thin continuous lines) are shown. To use this prediction for patients who have a certain baseline value, the population SEs for several baseline values were calculated; the 95% confidence level is t multiplied by the SE, where t = 2 is the Student’s t value for P < 0.05 and df = 116. Time is expressed in number of days, and plasma HIV-1 RNA levels are expressed in log10 copies per milliliter.
Results
In this study, 118 treatment-naive HIV-infected adults with CD4+ T-cell count of ≥ 250 cells/μL and plasma HIV-1 RNA ≥ 5,000 copies/mL, were treated with HAART (12–16). Fifty-three out of 118 subjects underwent an excisional lymph node biopsy before the initiation of therapy. All patients adhered well to HAART, and the mean follow-up after the initiation of therapy was 72 weeks.
Correlation among virological and immunological measures in blood and lymph node.
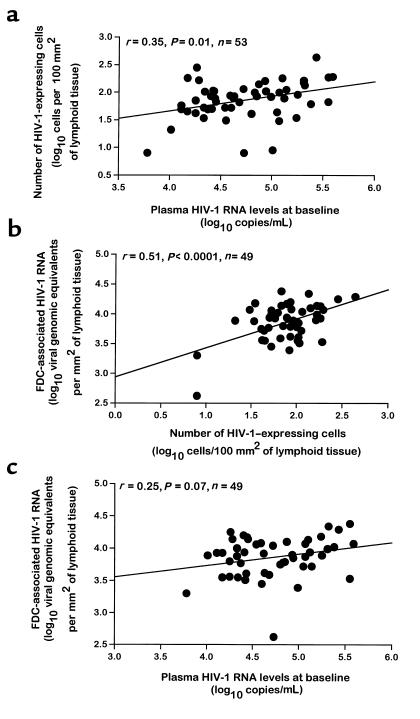
The relationships among the different virological and immunological measures in both blood and lymph node were first analyzed. In previous studies, the combination of quantitative imaging analysis of viral load in lymphoid tissue with mathematical modeling provided evidence that the viral load in lymphoid tissue was correlated with the amount of virus detected in blood (28, 29). These findings were confirmed with a large number of patients (n = 53) in the present study. The numbers of HIV-1–expressing cells measured in lymph nodes of HIV-1–infected subjects at baseline, i.e., before the initiation of HAART, were significantly correlated with both the levels of plasma viremia (r = 0.35; P = 0.01; n = 53) and with the FDC-associated HIV-1 RNA (r = 0.51; P < 0.0001; n = 49) (Figure 1). There was, however, only a trend toward correlation between FDC-associated HIV-1 RNA and plasma viremia (r = 0.25; P = 0.07; n = 49) (Figure 1). Similar to previous studies (30, 31), the CD4 T-cell count was inversely correlated with levels of plasma viremia (r = –0.34; P = 0.002; n = 83). Furthermore, the percent values of CD4 T cells in lymph node were inversely correlated with both the numbers of virus-expressing cells (r = –0.33; P = 0.014; n = 53) and FDC-associated HIV-1 RNA (r = –0.43; P = 0.002; n = 49). These analyses were necessary to validate the use of these measures for the correlation with the time of response to treatment and for the assessment of the estimates of the time required to attain the virological response.
Figure 1.
Correlation analyses among virological measures in blood and lymph nodes of 53 HIV-1–infected persons before the initiation of HAART. (a) Correlation between the number of HIV-1–expressing cells in lymph nodes and plasma HIV-1 RNA levels. (b) Correlation between FDC-associated HIV-1 RNA and the number of HIV-1–expressing cells in lymph node. (c) Correlation between FDC-associated HIV-1 RNA and plasma HIV-1 RNA levels. Plasma HIV-1 RNA levels are expressed in log10 copies per milliliter, the number of HIV-1–expressing cells is expressed in lymph node log10 cells per 100 mm2 of lymphoid tissue, and FDC-associated HIV-1 RNA levels in lymph node are expressed in log10 viral genomic equivalents (VGE) per square millimeter of lymphoid tissue; 1 VGE corresponds to 2 HIV-1 RNA copies. The regression lines are shown. Quantitation of plasma viremia and of virological measures in lymph node were performed as described in Methods. A 2-tailed P value < 0.05 was considered significant.
Relationship between virological and immunological measures and the time of virological response to HAART.
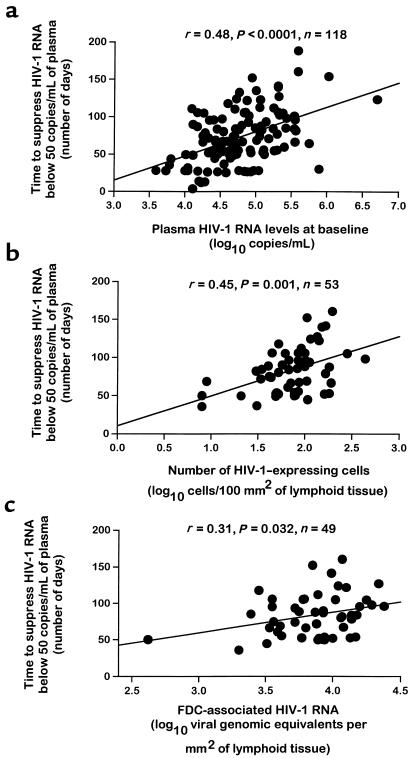
To determine the relationship between the virological and immunological measures discussed above and the time of the virological response to HAART, i.e., viremia below 50 HIV-1 RNA copies/mL, it was important to study cohorts of subjects in whom the institution of HAART was associated with a suppression of plasma viremia below 50 HIV-1 RNA copies/mL over time. Indeed, after HAART, a decrease of plasma viremia below 50 HIV-1 RNA copies/mL was observed after varying periods of time, ranging between week 2 and week 24, in all 118 subjects studied. It was, therefore, tested whether each of the virological and immunological measures in blood and lymph node were correlated with the time to decrease plasma viremia below 50 copies/mL (see Methods). Among the virological measures, baseline plasma viremia (r = 0.48; P < 0.0001; n = 118), baseline numbers of virus-expressing cells in lymph node (r = 0.45; P = 0.001; n = 53), and FDC-associated HIV-1 RNA (r = 0.31, P = 0.032; n = 49) were significantly correlated with the time to suppress viremia below 50 copies/mL (Figure 2).
Figure 2.
Correlation analyses between baseline virological measures in blood and lymph and the time required to suppress HIV-1 RNA below 50 copies/mL. (a) Correlation between baseline plasma HIV-1 RNA levels and time to suppress HIV-1 RNA below 50 copies/mL. (b) Correlation between baseline number of virus-expressing cells in lymph node and time to suppress HIV-1 RNA below 50 copies/mL. (c) Correlation between baseline FDC-associated HIV-1 RNA in lymph node and time to suppress HIV-1 RNA below 50 copies/mL. Plasma HIV-1 RNA levels are expressed in log10 copies per milliliter, the number of HIV-1–expressing cells is expressed in lymph node log10 cells/100 mm2 of lymphoid tissue, and FDC-associated HIV-1 RNA levels in lymph node are expressed in log10 VGE per square millimeter of lymphoid tissue; 1 VGE corresponds to 2 HIV-1 RNA copies. The regression lines are shown. Quantitation of plasma viremia and of the number of virus-expressing cells in lymph node were performed as described in Methods. A 2-tailed P value < 0.05 was considered significant.
For each patient we determined the exponential slope of viral decay between week 2 and the time point at which viremia dropped below 50 copies/mL. This “second phase” exponential slope of viremia of each individual patient was not correlated with baseline viremia (r = –0.055, P = 0.61). There was little variation in our estimate of the late decay rate (mean ± SD, –0.049 ± 0.04), which corresponded to a half-life of 14 days. This is in good agreement with the second-phase virus dynamics obtained by Perelson et al. in previous studies (32, 33). In our cohort there were too few data points before week 2 to estimate the first-phase decay rate. The fact that patients differ little in the second-phase slopes explains the good correlation between baseline plasma viremia and baseline virological measures in lymph node and baseline plasma viremia and the time of response to HAART.
Regarding the immunological measures, both baseline percentage of CD4 T cells in lymph node and blood CD4 T-cell count were weakly correlated with the time to suppress viremia below 50 copies/mL (r = –0.26, P = 0.06; n = 53; and r = –0.26, P = 0.02; n = 83, respectively).
Analysis of the ability of virological measures in blood and lymph node to serve as predictors of the time of response to HAART.
Based on these results, it was determined whether baseline plasma viremia and virological measures in lymph nodes may serve as a predictor for the expected time of response to HAART. This estimated time period (see Methods) was correlated with the log10 baseline viremia, with the log10 baseline number of HIV-1–expressing cells per 100 mm2 of lymphoid tissue and with the log10 baseline amount of FDC-associated HIV-1 RNA. The regression lines in Figure 2 provide a prediction for the expected time to drop levels of viremia below 50 HIV-1 RNA copies/mL, as a function of each baseline value. In the cohort of 118 subjects studied, the average baseline viremia was 4.78 log10 copies/m. The baseline numbers of virus-expressing cells (n = 53) and of FDC-associated HIV-1 RNA (n = 49) were 1.86 log10 cells per 100 mm2 of lymph node tissue and 3.86 log10 VGE/mm2 (which corresponds to the FDC-associated HIV-1 RNA), respectively. For the average baseline numbers of virus-expressing cells and VGE in the lymph node, the expected time of treatment required to attain a virological response was 83 days, whereas the time predicted by the mean baseline plasma viremia was 73 days. Therefore, these virological measures from both blood and lymph nodes had comparable ability to predict the length of treatment required to attain a virological response.
Estimates of the duration of treatment required to suppress viremia below 50 HIV-1 RNA copies/mL.
Estimates of the duration of treatment required to attain a virological response at the study population level can be calculated according to different values of baseline viremia. As mentioned previously, the correlations between baseline viremia and the time of response to therapy allowed calculation of the average time of treatment required (73 days) to attain a virological response in our population of HIV-infected adults with a mean plasma viremia of 4.78 log10 copies/mL. The regression line in Figure 2a can be used for estimating the average time of response to therapy for different baseline viremia. On average, for patients with baseline levels of viremia of 3.0 (1,000 HIV-1 RNA copies), 3.5 (3,200 HIV-1 RNA copies), 4.0 (10,000 copies), 4.5 (32,000 copies), 5.0 (100,000 copies), 5.5 (317,000 copies), and 6.0 (1,000,000 copies) log10 HIV-1 RNA copies/mL, the estimated required number of days (with 95% confidence limits of the mean) was 15 ± 20, 31 ± 15, 48 ± 10, 64 ± 7, 81 ± 6, 97 ± 10, and 113 ± 15, respectively (Figure 3).
Discussion
Overall, these results indicate that baseline plasma viremia is the best predictor of the time of the virological response to HAART. Therefore, baseline viremia is not only the best predictor of the rate of progression of HIV-1 disease (30, 31) but also of the time of response to treatment. Most importantly, evidence is provided that viral load in blood and in lymph nodes has comparable power in predicting the time of the virological response to HAART. This is particularly important from a practical standpoint, because it indicates that an accurate evaluation of the time to attain the virological response to HAART can be obtained by the rather simple determination of viral load in the plasma.
Furthermore, the results shown in this study provide the first accurate estimate of the duration, i.e., number of days, of treatment required to suppress viremia below 50 copies/mL according to varying levels of baseline plasma viremia. This analysis has been performed at the study population level. As expected, at the level of the individual patient, a larger variability in the response time to HAART has been observed (see Figure 2a). This is likely because of a series of factors, including suboptimal adherence to therapy and food- and/or drug-to-drug interactions that might partially affect the efficacy of HAART. Nonetheless, the estimates at the study population level are representative of the large majority of the 118 HIV-infected adults studied, because 73% of patients had a time of response to treatment within the upper 95% confidence interval of the mean estimate calculated according to varying baseline levels of plasma viremia. These estimates conceivably can be applied to any effective antiretroviral treatment. In fact, the antiviral potency of the HAART combinations used in this study was at least comparable to that observed in previous studies (1–3). These estimates are potentially of great practical value in evaluating the efficacy of the therapeutic regimen used and in guiding the clinical management of HIV-infected patients.
Acknowledgments
The authors thank the patients who participated in this study. This work was supported by the Swiss National Science Foundation (Grant No. 3239-047330) and by Research Grants from GlaxoWellcome, Greenford, United Kingdom and Roche Pharma, Basel, Switzerland.
References
- 1.Deeks SG, Smith M, Holodniy M, Kahn JO. HIV-1 protease inhibitors. A review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 2.Gulick RM, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 3.Hammer SM, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 4.Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 5.Hammer SM, Yeni P. Antiretroviral therapy: where are we? AIDS. 1998;12(Suppl. A):S181–S188. [PubMed] [Google Scholar]
- 6.Kempf DJ, et al. The duration of viral suppression during protease inhibitor therapy for HIV-1 infection is predicted by plasma HIV-1 RNA at the nadir. AIDS. 1998;12:F9–14. doi: 10.1097/00002030-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Raboud JM, et al. Suppression of plasma viral load below 20 copies/ml is required to achieve a long-term response to therapy. AIDS. 1998;12:1619–1624. doi: 10.1097/00002030-199813000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Katzenstein DA, et al. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. AIDS Clinical Trials Group Study 175 Virology Study Team [erratum 1997, 15:1097] N Engl J Med. 1996;335:1091–1098. doi: 10.1056/NEJM199610103351502. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien WA, et al. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. Veterans Affairs Cooperative Study Group on AIDS. N Engl J Med. 1996;334:426–431. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 10.Delta Coordinating CommitteeDelta Virology Committee. HIV-1 RNA response to antiretroviral treatment in 1280 participants in the Delta Trial: an extended virology study. AIDS. 1999;13:57–65. doi: 10.1097/00002030-199901140-00008. [DOI] [PubMed] [Google Scholar]
- 11.Montaner JS, Hogg R, Raboud J, Harrigan R, O’Shaughnessy M. Antiretroviral treatment in 1998. Lancet. 1998;352:1919–1922. doi: 10.1016/S0140-6736(98)07532-1. [DOI] [PubMed] [Google Scholar]
- 12.Rizzardi GP, et al. Quantitative normalization of CD4+ T cells in blood and lymph nodes of HIV-1-infected therapy-naive adults at early stage of chronic infection treated with abacavir plus amprenavir. Interscience Conference on Antimicrobial Agents and Chemotherapy 39th, San Francisco. 1999;508:1822. (Abstr.) [Google Scholar]
- 13.Rizzardi GP, et al. Effect of HAART and immune-based strategies in HIV-1-infected antiretroviral naive adults. Interscience Conference on Antimicrobial Agents and Chemotherapy 39th, San Francisco. 1999;488:691. (Abstr.) [Google Scholar]
- 14.Reijers MH, et al. Maintenance therapy after quadruple induction therapy in HIV-1 infected individuals: Amsterdam Duration of Antiretroviral Medication (ADAM) study. Lancet. 1998;352:185–190. doi: 10.1016/s0140-6736(98)06193-5. [DOI] [PubMed] [Google Scholar]
- 15.Notermans DW, et al. Decrease of HIV-1 RNA levels in lymphoid tissue and peripheral blood during treatment with ritonavir, lamivudine and zidovudine. Ritonavir/3TC/ZDV Study Group. AIDS. 1998;12:167–173. doi: 10.1097/00002030-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Staszewski S, et al. Comparison of antiviral response with abacavir/combivir to indinavir/combivir in therapy-naive adults at 48 weeks (CNA3005) Interscience Conference on Antimicrobial Agents and Chemotherapy 39th, San Francisco. 1999;472:505. (Abstr.) [Google Scholar]
- 17.Pantaleo G, et al. Evolutionary pattern of human immunodeficiency virus (HIV) replication and distribution in lymph nodes following primary infection: implications for antiviral therapy. Nat Med. 1998;4:341–345. doi: 10.1038/nm0398-341. [DOI] [PubMed] [Google Scholar]
- 18.Embretson J, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 19.Emilie D, et al. Production of interleukins in human immunodeficiency virus-1-replicating lymph nodes. J Clin Invest. 1990;86:148–159. doi: 10.1172/JCI114678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox CH, et al. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA [erratum 1992, 6:1161] J Infect Dis. 1991;164:1051–1057. doi: 10.1093/infdis/164.6.1051. [DOI] [PubMed] [Google Scholar]
- 21.Pantaleo G, et al. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantaleo G, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 23.Spiegel H, Herbst H, Niedobitek G, Foss HD, Stein H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am J Pathol. 1992;140:15–22. [PMC free article] [PubMed] [Google Scholar]
- 24.Tenner-Racz K, Racz P, Dietrich M, Kern P. Altered follicular dendritic cells and virus-like particles in AIDS and AIDS-related lymphadenopathy. Lancet. 1985;1:105–106. doi: 10.1016/s0140-6736(85)91994-4. [DOI] [PubMed] [Google Scholar]
- 25.Fox, C.H., and Cottler-Fox, M. 1993. In situ hybridization for the detection of HIV RNA in cells and tissues. In Current protocols in immunology. J. Coligan, A. Kruisbeek, D. Margulies, E. Shevach, and W. Strober, editors. John Wiley & Sons. New York, NY.
- 26.Cottler-Fox M, Fox CH. Examining cells for infectious agents: a novel approach. J Infect Dis. 1991;164:1239–1240. doi: 10.1093/infdis/164.6.1239-a. [DOI] [PubMed] [Google Scholar]
- 27.Fox CH, Hoover S, Currall VR, Bahre HJ, Cottler-Fox M. HIV in infected lymph nodes. Nature. 1994;370:256. doi: 10.1038/370256a0. [DOI] [PubMed] [Google Scholar]
- 28.Haase AT, et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 29.Cavert W, et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 30.Mellors JW, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma [erratum 1997, 5296:14] Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 31.Mellors JW, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 33.Perelson AS, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]