Abstract
Only some taste cells fire action potentials in response to sapid stimuli. Type II taste cells express many taste transduction molecules but lack well-elaborated synapses, bringing into question the functional significance of action potentials in these cells. We examined the dependence of adenosine triphosphate (ATP) transmitter release from taste cells on action potentials. To identify type II taste cells we used mice expressing a green fluorescence protein (GFP) transgene from the α-gustducin promoter. Action potentials were recorded by an electrode basolaterally attached to a single GFP-positive taste cell. We monitored ATP release from gustducin-expressing taste cells by collecting the electrode solution immediately after tastant-stimulated action potentials and using a luciferase assay to quantify ATP. Stimulation of gustducin-expressing taste cells with saccharin, quinine, or glutamate on the apical membrane increased ATP levels in the electrode solution; the amount of ATP depended on the firing rate. Increased spontaneous firing rates also induced ATP release from gustducin-expressing taste cells. ATP release from gustducin-expressing taste cells was depressed by tetrodotoxin and inhibited below the detection limit by carbenoxolone. Our data support the hypothesis that action potentials in taste cells responsive to sweet, bitter, or umami tastants enhance ATP release through pannexin 1, not connexin-based hemichannels.
INTRODUCTION
Taste buds detect sapid substances in the oral cavity and transmit the information to gustatory afferent nerves. Taste bud cells have been classified into four categories (type I, type II, type III, and basal cells) according to their cytological and ultrastructural features. Recognizable synapses are formed between type III cells and gustatory afferents (Murray 1986). In addition, type III cells have been found to express presynaptic molecules including synaptosomal-associated protein, voltage-gated Ca2+ channel, and neural cell adhesion molecule (e.g., DeFazio et al. 2006). Gustatory afferent fibers also come into close contact with type II cells (Clapp et al. 2004). In contrast, type II cells express proteins involved in sweet, bitter, and umami taste detection and transduction, e.g., G protein–coupled taste receptors, gustducin, and downstream effectors. Furthermore, type II cells lack synaptic vesicles and presynaptic molecules (DeFazio et al. 2006), raising questions regarding signal transmission from taste bud to afferent.
Despite their lack of conventional presynaptic machinery, type II cells may release transmitters. Previous reports have proposed several transmitter candidates: glutamate, serotonin (5-HT), acetylcholine, neuropeptide Y, and adenosine triphosphate (ATP) (Roper 2006). Of these, ATP is likely to be key. Finger et al. (2005) found that double knock-out mice lacking ionotropic purinergic receptor P2X2 and P2X3 genes, known to be expressed in nerve endings innervating taste cells (Bo et al. 1999), disrupted afferent nerve responses to taste stimuli. More recent reports using ATP biosensors showed that isolated type II cells release ATP on serial depolarization (Romanov et al. 2007) or intracellular Ca2+ increase in response to sweet/bitter mixtures (Huang et al. 2007). Romanov et al. (2007, 2008) showed that a hemichannel blocker octanol and mimetic peptide GAP26 affected voltage-gated outward currents through ATP-permeable ion channels in type II cells. Huang et al. (2007) revealed that another hemichannel blocker, carbenoxolone, abolished ATP release from type II cells. The hemichannel-dependent machinery may explain transmitter output from type II cells, although the activating mechanism remains uncertain.
A subset of taste bud cells is known to generate action potentials in response to taste stimuli (e.g., Roper 1983), which was recently supported by molecular identity of voltage-gated Na+ channels in type II and type III cells (Gao et al. 2009). Yoshida et al. (2006a,b) correlated breadth of responsiveness of action potential generating taste cells with that of the innervating fibers and concluded that taste cells with action potentials contribute significantly to taste information coding. Although it has been hypothesized that action potentials drive transmitter release in taste cells, there is little experimental evidence for the functional roles of action potentials.
To assess the potential roles of ATP in taste cell signaling we developed a method to capture ATP from type II taste cells in buds firing in response to sweet, bitter, or umami compounds. We find that ATP release from type II cells depends on action potentials. Our results indicate that action potentials enhance ATP release from type II cells through hemichannels.
METHODS
Animals
All experimental procedures were approved by the committee for Laboratory Animal Care and Use at Kyushu University, Japan. Two lines of mice were used in our experiments: 1) transgenic mice in which α-gustducin (Gust) promoter drives expression of green fluorescent protein (GFP) (Huang et al. 1999), i.e., Gust-GFP mice; and 2) knock-in mice in which the glutamate decarboxylase 67 (GAD67) promoter controls expression of GFP (Tamamaki et al. 2003), i.e., GAD67-GFP mice.
Recording of action potentials
The procedures used have been described elsewhere (Yoshida et al. 2006a, 2009b). Briefly, taste epithelia were peeled from the anterior part of tongues after treatment with 1 mg/ml elastase (Elastin Products, Owensville, MO) in Tyrode solution (in mM: NaCl 140, KCl 5, CaCl2 1, MgCl2 1, Glc 10, sodium pyruvate 10, HEPES 10; pH 7.4 adjusted with NaOH). Individual fungiform taste buds with a piece of epithelium were excised from the epithelial sheets using a glass capillary under a binocular microscope. The apical pore of a single taste bud was drawn into the orifice of the stimulating pipette. Gentle suction on the pipette was maintained by a peristaltic pump to deliver taste solutions and to hold the taste bud in place. The basolateral side of the taste bud was perfused with Tyrode solution by a peristaltic pump at about 2 ml/min. The recording electrode with 10 μl Tyrode solution (∼1.5 MΩ) was attached to the basolateral membrane of a single GFP-positive cell under an FV1000 confocal laser scanning microscope (Olympus, Tokyo). Action potentials were recorded and analyzed with an Axopatch 200B amplifier, a Digidata 1320A interface, and pCLAMP software (Axon Instruments, Foster City, CA). As standard taste stimuli, we used saccharin, quinine hydrochloric acid, cyclohexamide, monosodium glutamate (MSG), NaCl, and HCl dissolved in distilled water (D.W.).
Quantitative determination of ATP
To measure ATP release from a single taste cell the recording electrode solution was subjected to an ATP luciferase assay. Within 1 min after the end of stimulation for 30 s, the recording electrode was removed from the experimental setup and the recording electrode solution was collected into a 96-well plate on ice to avoid degradation of ATP (Supplemental Fig. S1A).1 The 96-well plate was placed into a Tropix TR717 luminometer (Applied Biosystems, Carlsbad, CA) and 25 μl of luciferin–luciferase reagent (ATP Bioluminescence Assay Kit HS II; Roche, Penzberg, Germany) was automatically injected into each well at room temperature (∼25°C) to measure the intensity of luminescence. For luminescence detection, each well was scanned at 10/s for 10 s and the intensity of luminescence was calculated in the form of mean relative light units (RLUs) with WinGlow (Applied Biosystems) and Microsoft Excel 2007. Preliminary tests with 10 μl of ATP standard solutions, the same volume as the recording electrode solutions, demonstrated that the mean RLU was proportional to ATP concentrations at ≥40 pM on double-logarithmic plots (Supplemental Fig. S1B). Standard curves for mean RLU with 10 μl of ATP standard solutions were drawn in each ATP determination. For determination of significant differences, data were analyzed by t-test or ANOVA by using Microsoft Excel 2007. After removal of the recording electrode, in some cases, the newly prepared recording electrode was subsequently attached to the same GFP-positive cell for the second measurement of the ATP release. The ATP levels detected by the second measurements did not significantly differ from those by the first measurements in gustducin-expressing cells responding to a taste compound (also see Fig. 5).
Fig. 5.

Two measurements of ATP release from a type II cell. A: representative records: the first (top) and second (bottom) measurements from the same type II cell in response to cyclohexamide (Chx 1 mM). B: no significant difference was observed in ATP levels detected between the first and the second measurements in gustducin-expressing cells responding to Chx (P = 0.39, paired t-test). Values are means ± SE (n = 4).
Data analysis
Electrophysiological records were analyzed according to the previous reports (Yoshida et al. 2006a, 2009b). Action potential waveforms were observed with respect to the following parameters: time of peak–peak, peak amplitude/antipeak amplitude ratio, antipeak amplitude, and peak amplitude. The mean spontaneous impulse discharge for each unit was calculated by averaging the number of spikes over the 10 s period that distilled water flowed over the taste pore prior to each stimulation. The final criteria for the occurrence of a response were the following: 1) the number of spikes was larger than the mean + 2SDs of the spontaneous discharge in two repeated trials and 2) more than three spikes were evoked by taste stimulation. Taste response magnitude was obtained by counting the total number of impulses for 30 s after the onset of stimulus application and subtracting the spontaneous impulse discharge.
RESULTS
Quantitative determination of ATP
We set out to examine the parameters affecting ATP release from type II taste cells. We anticipated that apical stimulation of taste cells would lead to basolateral secretion of ATP, measurable by collecting solution within the recording electrode (see methods for details). Gustducin, a G protein involved in sweet, bitter, and umami transduction is found in most type II cells and not in other subtypes of taste cells (Boughter Jr et al. 1997; Yang et al. 2000). In our experiments, gustducin-expressing type II taste cells from Gust-GFP transgenic mice were identified by their green fluorescence under confocal microscopy (Fig. 1A). We measured action potentials from a single taste cell (Yoshida et al. 2006a, 2009b). At the end of the recording we measured the ATP concentration within the collected recording electrode solution by a luciferase assay. Figure 1C shows representative records from GFP-positive and GFP-negative taste cells. Application of the artificial sweetener saccharin to the GFP-positive cell increased the firing rate and measurable ATP (153 pM) was detected in the recording electrode solution collected from this cell. In contrast, saccharin did not elicit action potentials in GFP-negative taste cells and ATP collected from these cells was below the detection limit (n = 9, Table 1).
Fig. 1.

Extracellular recording system to trap adenosine triphosphate (ATP) released from a taste bud cell of α-gustducin–green fluorescence protein (Gust-GFP) mice. A: merged confocal and differential interference contrast images around the stimulating pipette tip with a fungiform taste bud in the experimental setup. B: sample records from a GFP-positive taste bud cell. NaCl, 300 mM; saccharin (Sac), 20 mM; HCl, 10 mM; cyclohexamide (Chx), 500 μM; monosodium glutamate (MSG), 300 mM. C: representative ATP release from a GFP-positive (a) and -negative (b) taste bud cell by application of 20 mM Sac. Left: extracellular recording of electrical activity (arrowheads indicate offset of bath perfusion and onset of taste stimulation). Right: ATP concentration in the recording electrode solution measured by luciferase assay (N.D., not detectable; detection limit, 40 pM).
Table 1.
Taste responsiveness of fungiform taste bud cells tested
| GFP |
||||
|---|---|---|---|---|
| Factor | Positive Cell (n = 33) | Negative Cell (n = 9) | ||
| Taste response | Sweet | Bitter | Umami | No response to sweet |
| Number of cells | 13 | 16 | 4 | 9 |
Relationship between firing rates and released ATP
We next examined the relationship between firing rates and released ATP in sweet-sensitive gustducin-expressing type II cells stimulated with the sweetener saccharin. We found a positive dependence of ATP concentrations on firing rates (r = 0.67, Fig. 2A). A similar positive correlation was observed in bitter-sensitive gustducin-expressing type II cells responding to bitter quinine or cyclohexamide (r = 0.76, Fig. 2B). We also observed umami-sensitive gustducin-expressing cells that responded to monosodium glutamate with action potentials and released ATP at levels detectable in the recording electrode (Fig. 3A). There were no significant differences in the ATP concentrations per spike among sweet-, bitter-, and umami-sensitive gustducin-expressing cells [one-way ANOVA, F(2,30) = 0.98, P = 0.39; Fig. 3B]. The results indicate that type II cells release ATP in response to a sweet, bitter, or umami compound in a firing rate–dependent manner. Next we examined whether tastant-stimulated type III cells release ATP. We used GAD67-GFP mice to identify type III taste cells: a subset of type III cells are known to express GAD67 (DeFazio et al. 2006). Previous studies have shown that GAD67-expressing taste cells responded to sour or multiple-taste stimuli (Tomchik et al. 2007; Yoshida et al. 2009b). We observed many GFP-positive taste bud cells from GAD67-GFP mice that increased their firing rate on application of HCl or NaCl. However, in all cases the ATP collected from these cells was below the detection limit (n = 6, Fig. 3A). To determine whether ATP release from type II cells is dependent on action potentials we examined the effect of spontaneous activity of type II cells on ATP release. In gustducin-expressing taste cells in which the firing rate was at least five spikes per 30 s we detected released ATP (Fig. 3C). There was a strong positive correlation between firing rates and the concentration of ATP released from gustducin-expressing taste cells with firing rates at least five spikes per 30 s (r = 0.71). These results indicate that action potentials in type II cells play a crucial role in eliciting release of ATP from these cells.
Fig. 2.
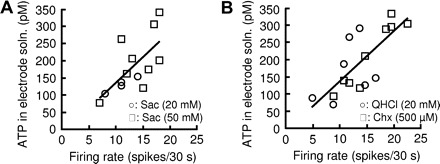
Firing rate–dependent ATP release from sweet- or bitter-sensitive type II cells. Plot of ATP concentrations in recording electrode solutions against firing rates of type II cells in response to a sweet (A) or bitter (B) compound. Taste stimulants were saccharin (Sac; 20 mM, ○; 50 mM, □) for sweet taste, and quinine (QHCl; 20 mM, ○) or cyclohexamide (Chx; 500 μM, □) for bitter taste.
Fig. 3.
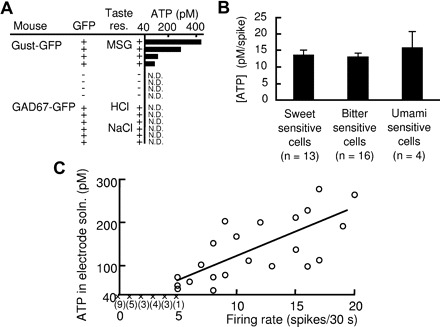
Tastants elicited ATP release from type II cells but not from type III cells. A: ATP concentrations in solutions of the recording electrode attached to type II cells in response to an umami compound (MSG, 300 mM) and in type III cells in response to sour (HCl, 10 mM) or salty (NaCl, 300 mM) compounds. + under “Taste res.,” responsive to a taste compound indicated; −, irresponsive; N.D., not detectable (detection limit, 40 pM). B: ATP concentrations per spike released from type II cells in response to sweet, bitter, or umami compounds. C: correlation between spontaneous firing activity and ATP release from type II cells: ○, ATP detected and quantified; ×, ATP not detectable (detection limit, 40 pM). The numbers of sampled cells in which ATP was not detectable are shown in parentheses. The fitted line was drawn with the ATP-detected samples (r = 0.71).
Effects of hemichannel blockers on ATP release
Previous reports have indicated that type II cells release ATP through hemichannels (Huang et al. 2007; Romanov et al. 2007, 2008). We examined the effects of hemichannel blockers on ATP release from type II cells. The application of 5 or 10 μM carbenoxolone, a blocker relatively sensitive to pannexin 1 (e.g., Bruzzone et al. 2005), in the bath and the recording electrode solutions led to undetectable levels of ATP in the recording electrode solution, even when type II cells responded to cyclohexamide (Fig. 4). However, the mixture of connexin mimetic peptide blockers GAP26 and GAP27 did not affect the amount of ATP released from type II cells (Fig. 4B). These results support the hypothesis that pannexin 1 participates in the mechanisms of action potential–involved ATP release from type II cells.
Fig. 4.

The hemichannel blocker carbenoxolone (CBX) blocked ATP release from type II cells. A, left: a representative response to cyclohexamide (Chx 500 μM). Arrowhead, offset of bath perfusion and onset of taste stimulation; CBX (+), application of 10 μM CBX in both bath and recording electrode solutions; N.D., not detectable. B: ATP release from type II cells in response to Chx was blocked by application of CBX to both bath and recording electrode solutions. ATP release was not affected by application of lower concentrations of CBX (0.01 μM, P = 0.41; 0.1 μM, P = 0.33; 1 μM, P = 0.087; vs. “no blockers” by t-test) or mixtures of GAP26 + GAP27 (500 μM, respectively, P = 0.74; vs. “no blockers” by t-test).
A role of action potentials for ATP release
To examine a role of action potentials for ATP release from type II cells through hemichannels, we used a voltage-gated Na+ channel blocker tetrodotoxin (TTX). Figure 5A shows two measurements of ATP release from the same gustducin-expressing cell in response to a bitter compound, cyclohexamide. After recording the response to cyclohexamide, we removed the recording electrode from the gustducin-expressing taste cell to measure ATP levels in the recording electrode solution by luciferase assay. We subsequently attached the newly prepared recording electrode to the gustducin-expressing taste cell, which again enabled us to determine the ATP release in response to cyclohexamide. There were no significant differences in ATP levels detected between the first and the second measurements in gustducin-expressing cells responding to cyclohexamide: first, 259 ± 44 pM; second, 230 ± 40 pM (means ± SE, n = 4, P = 0.39, paired t-test, Fig. 5B). According to these procedures, we examined the effects of TTX on ATP release from gustducin-expressing taste cells that had been confirmed to secrete ATP in response to cyclohexamide. The application of 50 nM TTX in the bath and the recording electrode solutions blocked the generation of action potentials and decreased ATP levels, which remained detectable (Fig. 6). The results suggest that action potentials enhance ATP release through hemichannels in type II cells.
Fig. 6.
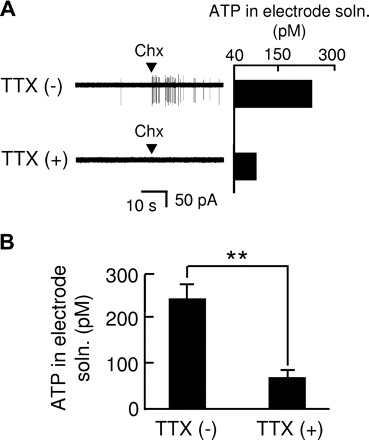
Voltage-gated Na+ channel blocker tetrodotoxin (TTX) suppressed ATP release from type II cells. A: representative records from a type II cell by application of cyclohexamide (Chx, 1 mM). TTX (−), TTX-free; TTX (+), 50 nM TTX in the bath and the recording electrode solutions. B: effects of TTX (50 nM) on ATP release from type II cells. TTX (−), n = 7; TTX (+), n = 5; **P < 0.01. ATP release was significantly suppressed by TTX (P = 0.002, t-test).
DISCUSSION
To monitor ATP released from taste cells with action potentials we used a modification of the loose patch configuration. Apical application of sweet, bitter, or umami compounds to gustducin-expressing type II taste bud cells elicited action potentials and ATP release detectable in the recording electrode solution. In our experimental setup for recording from type II cells we confirmed by confocal microscopy that the recording electrode was attached to a single GFP-labeled gustducin-expressing taste cell from Gust-GFP transgenic mice. ATP release was not detected when the recording electrode was attached to GFP-negative taste bud cells from Gust-GFP mice. In addition, these GFP-negative cells did not respond to the sweet compound saccharin. These results suggest that ATP detected in the recording electrode solution is derived from the gustducin-expressing type II cells themselves. Our results are consistent with the previous report of sweet/bitter taste mixtures eliciting ATP release from type II cells by Ca2+ mobilization (Huang et al. 2007). Additionally, our method enabled us to show that each gustducin-expressing type II cell releases ATP in response to a single stimulant of sweet, bitter, or umami taste (Figs. 2 and 3). This indicates that a single taste compound is able to trigger ATP release.
Several lines of evidence suggest that firing of action potentials is involved in ATP release from type II cells. A positive correlation was observed between the concentration of ATP detected in the recording electrode solution and firing rates of type II cells: the slopes of the concentration versus firing rate curves did not differ between sweet- and bitter-sensitive type II cells (Fig. 2). Moreover, there was no significant difference in mean values of ATP concentrations per spike among sweet-, bitter-, and umami-sensitive type II cells (Fig. 3B). Concentrations of ATP released increased depending on the spontaneous firing rate (Fig. 2C); the slope of the fitted curve was not different from those in response to sweet or bitter taste. Our results appear consistent with a previous report in which it was found that serial depolarization induced ATP release from type II cells (Romanov et al. 2007). It is noteworthy that action potential–independent ATP release was detectable: application of TTX reduced but did not abolish ATP release from type II cells in response to a bitter compound. Taste transductions of sweet, bitter, and umami compounds have been shown to elicit an increase in intracellular Ca2+ and membrane depolarization though TRPM5 prior to firing of action potentials (e.g., Zhang et al. 2003). Huang et al. (2007) previously showed that a rise in intracellular Ca2+ of type II cells by taste stimulation induced the ATP release; Huang and Roper (2010) recently reported that membrane depolarization by TRPM5 also opens ATP-permeable hemichannels in mouse vallate taste receptor cells. Our results support the hypothesis that action potentials enhance ATP release triggered by an intracellular Ca2+ rise and subsequent membrane depolarization by TRPM5 in type II cells.
The exocytosis-independent machinery for transmitter release from type II cells may likely involve hemichannels. ATP release from type II cells was blocked by low concentrations (10 μM; e.g., Bruzzone et al. 2005) of carbenoxolone, indicating a role for pannexin 1 in ATP release. Pannexin 1 hemichannels are activated not only by membrane depolarization but also by intracellular Ca2+ (e.g., Bruzzone et al. 2003). Action potentials of type II cells may make hemichannels, including pannexin 1, easier to open. Our results are consistent with those using taste stimulation (Dando and Roper 2009; Huang et al. 2007), but not electrical stimulation (Romanov et al. 2007, 2008). Differences in stimulation of type II cells may contribute to the distinct effects of hemichannel blockers on ATP release, although the mechanisms remain uncertain.
Our findings shed light on the importance of ATP in gustatory information transmission from taste cells with action potentials to afferent fibers. Yoshida et al. (2006a,b) previously showed that mouse fungiform taste cells with action potentials and innervating afferent fibers share similar response profiles in selectivity to basic taste modalities, suggesting that taste cells with action potentials contribute to the major component of taste information transmitted to gustatory afferents. Other reports have shown the expression of P2X2 and P2X3 in innervating fibers (Bo et al. 1999) and the ablation of fiber responses to taste compounds by knocking out these ATP receptors (Finger et al. 2005). We showed here that type II cells with action potentials are able to release ATP in response to a single taste compound, thus confirming that ATP plays the role of a critical transmitter of peripheral information in sweet, bitter, and umami taste to gustatory afferents. Unfortunately, we could not examine ATP release from taste cells that do not express gustducin and may respond to a sweet, bitter, or umami compound.
Our findings support the hypothesis that ATP released from type II cells functions as an autocrine and paracrine transmitter among taste bud cells. Hayato et al. (2007) found the expression of P2X7 and P2Y1 in type II cells and P2X2, P2X7, and P2Y1 in type III cells. They also reported that basolateral application of ATP increased intracellular Ca2+ concentrations of a subset of taste bud cells as well as depolarized or hyperpolarized taste bud cells with action potentials. Huang et al. (2009) demonstrated that ATP and its degradation product adenosine diphosphate (ADP) provide positive autocrine feedback onto type II cells via P2Y1 receptors. In addition, Huang et al. (2005) showed that ATP induced Ca2+ mobilization of isolated type III cells to release serotonin. Taste information perceived by type II cells would partly be conveyed through type III cells to CNS; a part of type III cells exhibits transient rise in intracellular Ca2+ in response to sweet, bitter, and/or umami compounds (Tomchik et al. 2007).
Type III cells are known to exhibit Ca2+ mobilization by sour taste (Huang et al. 2008b) and firing rate increase by sour or salt taste (Yoshida et al. 2009b). However, we detected no ATP release from type III cells with action potentials in response to a sour or salt taste compound. This is consistent with previous reports that ATP release from isolated type III cells is undetectable by ATP biosensors (Huang et al. 2007; Romanov et al. 2008), but appears to be at odds with another report that transgenic mice lacking P2X2 and P2X3 had deficiencies in afferent nerve response to sweet, bitter, umami, sour, and salt taste compounds (Finger et al. 2005). It is possible that the concentration of ATP released from type III cells may have been below the detection limit (40 pM) of our method. Another possibility is that type III cells require another transmitter. Previous reports using biosensor cells showed release of serotonin and noradrenalin from type III cells (Huang et al. 2005, 2008a). Thus the relative contributions of transmitters to taste information may depend on taste cell types, although functional roles of other transmitter candidates such as serotonin on gustatory afferents are to be elucidated. There possibly exists another pathway where type III cells do not directly participate in perception of salt taste compounds. For salt taste, there is a major class of taste cells, with amiloride sensitivity and epithelial Na+ channel expression, in specific response to sodium salt (Chandrashekar et al. 2010; Yoshida et al. 2009a). This class of taste cells is not found in gustducin-expressing cells and GAD67-expressing cells (Yoshida et al. 2009b); one possibility is in a subset of type II cells without expression of gustducin or a subset of type III cells without expression of GAD67. Another possibility is that salt-sensitive taste cells may be type I cells with amiloride-sensitive Na+ current (Vandenbeuch et al. 2008). Further observations—including precise characterization of taste response profiles and transmitters released in every type of taste cells—are required to elucidate coding and transmission mechanisms from taste cells to gustatory afferent fibers.
GRANTS
This work was supported by Grants-in-Aid for Scientific Research 18077004 and 18109013 to Y. Ninomiya and 20791355 to Y. Murata.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
Present addresses: Y. Murata: Department of Physiology, Kochi Medical School, Nankoku, Kochi 783–8585, Japan.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Bo X, Alavi A, Xiang Z, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport 10: 1107–1111, 1999 [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr, Pumplin DW, Yu C, Christy RC, Smith DV. Differential expression of alpha-gustducin in taste bud populations of the rat and hamster. J Neurosci 17: 2852–2858, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92: 1033–1043, 2005 [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA 100: 13644–13649, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJP, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of phospholipase C signaling pathway. J Comp Neurol 468: 311–321, 2004 [DOI] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol 587: 5899–5906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci 26: 3971–3980, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499, 2005 [DOI] [PubMed] [Google Scholar]
- Gao N, Lu M, Echeverri F, Laita B, Kalabat D, Williams ME, Hevezi P, Zlotnik A, Moyer BD. Voltage-gated sodium channels in taste bud cells. BMC Neurosci 10: 20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayato R, Ohtubo Y, Yoshii K. Functional expression of ionotropic purinergic receptors on mouse taste bud cells. J Physiol 584: 473–488, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Gγ13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 2: 1055–1062, 1999 [DOI] [PubMed] [Google Scholar]
- Huang YJ, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci 29: 13909–13918, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci USA 104: 6436–6441, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci 25: 843–847, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci 28: 13088–13093, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Stimac R, Roper SD. Presynaptic (type III) cells in mouse taste buds sense sour (acid) taste. J Physiol 15: 2903–2912, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Roper SD. Intracellular Ca2+ and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol (May 24, 2010). doi:10.1113/jphysiol.2010.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RG. Mammalian taste bud type III cell: a critical analysis. J Ultrastruct Mol Struct Res 95: 175–188, 1986 [DOI] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J 26: 657–667, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol 132: 731–744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD. Regenerative impulses in taste cells. Science 220: 1311–1312, 1983 [DOI] [PubMed] [Google Scholar]
- Roper SD. Cell communication in taste buds. Cell Mol Life Sci 63: 1494–1500, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomiaoka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467: 60–79, 2003 [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci 27: 10840–10848, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci 9: 1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Tabata S, Crowley HH, Margolskee RF, Kinnamon JC. Ultrastructual localization of gustducin immunoreactivity in microvilli of type II taste cells in the rat. J Comp Neurol 425: 139–151, 2000 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Horio N, Murata Y, Yasumatsu K, Shigemura N, Ninomiya Y. NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience 159: 795–803, 2009a [DOI] [PubMed] [Google Scholar]
- Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, Margolskee RF, Ninomiya Y. Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol 587: 4425–4439, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, Ishizuka S, Ninomiya Y. Taste responsiveness of fungiform taste cells with action potentials. J Neurophysiol 96: 3088–3095, 2006a [DOI] [PubMed] [Google Scholar]
- Yoshida R, Yasumatsu K, Shigemura N, Ninomiya Y. Coding channels for taste perception: information transmission from taste cells to gustatory nerve fibers. Arch Histol Cytol 69: 233–242, 2006b [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


