Abstract
Gold(I)-catalyzed allene-vinylcyclopropane cycloisomerization leads to the tricyclic framework of the protoilludanes in a single step by a reaction that involves a cyclopropane ring expansion and a Prins cyclization.
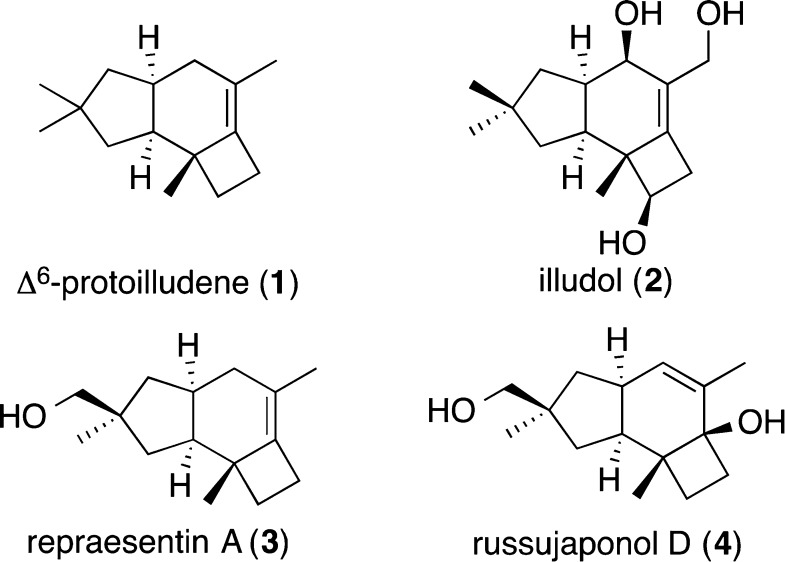
Illudanes and protoilludanes have attracted much attention from the perspective of their biosynthesis,1 biology,2 and organic synthesis.3 Representative members of this numerous family of sesquiterpenes are Δ6-protoilludene (1),4,5 illudol,6 repraesentin A (3),7 and russujaponol D (4)8 (Figure 1).
Figure 1.
Representative protoilludane sesquiterpenes.
Access to this class of compounds still poses important synthetic challenges due to their structural complexity. As part of a program on the development of new gold(I)-catalyzed cascade reactions for the synthesis of complex sesquiterpenes,9 we now report a new approach for the construction of the skeleton of this family of natural products in one single step through a gold-catalyzed cycloisomerization.
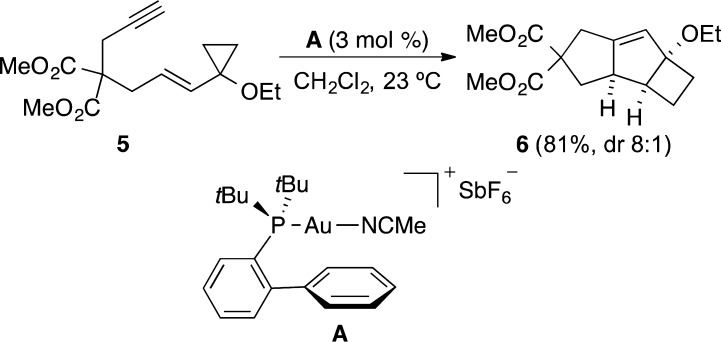
Cycloisomerization reactions catalyzed by gold(I) complexes have been intensively investigated and represent one of the most powerful methods for the construction of complex molecules in a single step.10 Among these various transformations, we demonstrated that complex tricyclic compounds such as 6 with an octahydrocyclobuta[a]pentalene skeleton can be obtained by a sequence involving a ring expansion and a Prins cyclization from cyclopropylenyne 5 (Scheme 1).11,12
Scheme 1. Gold(I)-Catalyzed Cycloisomerization of Cyclopropylenyne 5(11).
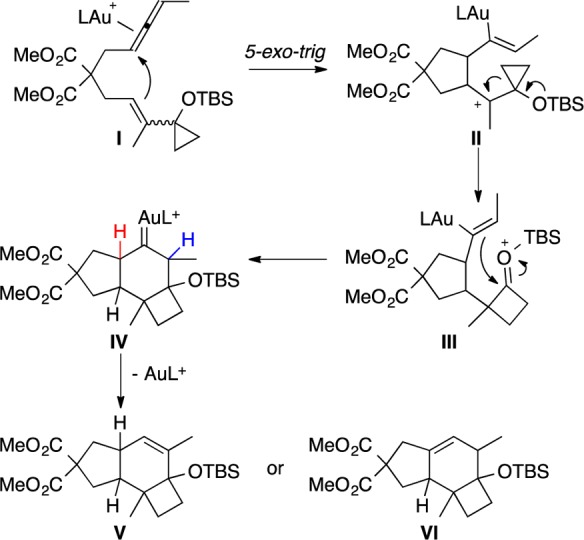
Based on this work, we decided to prepare the related cyclization of allenes with vinylcyclopropanes to access the carbon skeleton of the illudanes from a common intermediate. A plausible mechanism for this reaction is depicted in Scheme 2. Coordination of AuL+ to the allene (I) could trigger a 5-exo-trig cyclization to furnish cationic intermediate II, which could undergo a ring expansion to generate III. A Prins cyclization of the vinyl gold with the oxonium cation could give gold(I) carbene intermediate IV. Finally, proton elimination followed by demetalation would provide V or VI. Regarding the relative configuration of II, precedents exist for the formation of related intermediates with both the cis or trans configuration in gold(I)-catalyzed cycloisomerizations of allenenes.13
Scheme 2. Gold-Catalyzed Allene-vinylcyclopropane Cycloisomerization.
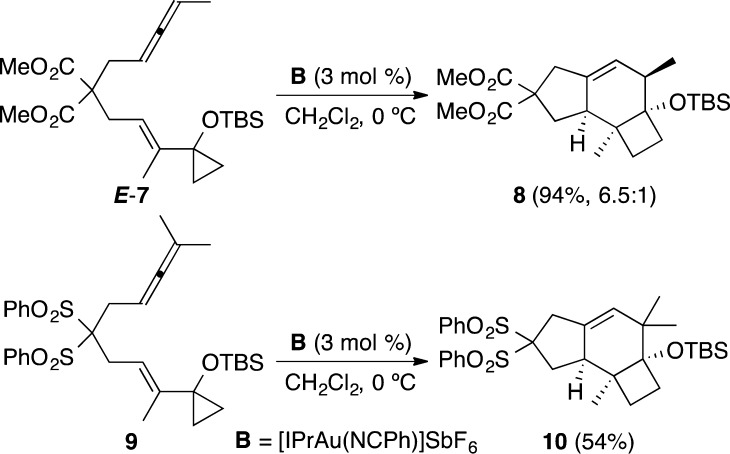
We first studied the reaction of substrate E-7. The cyclization was carried out satisfactorily using [IPrAu(NCPh)]SbF6 (B) (3 mol %) in CH2Cl2 at 0 °C to give 8 in 94% yield as a 6.5:1 mixture of two alkene regioisomers (Scheme 3).14 On the other hand, cyclization of substrate 9 with two methyl groups at the allene terminus furnished tricyclic compound 10 in 54% yield as a single isomer.
Scheme 3. Gold(I)-Catalyzed Cyclization of Allene-E-vinylcyclopropanes E-7 and 9.
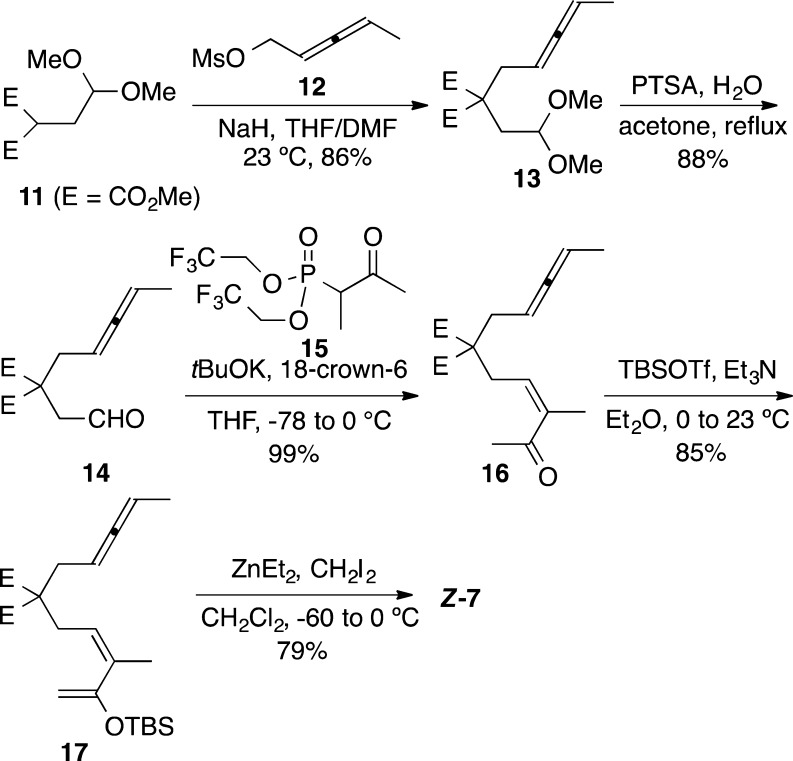
Although the allene-vinylcyclopropane cyclization provided the desired tricyclic system as originally planned, the relative configuration of 8 and 10 was the opposite to that of the natural protoilludenes. In keeping with the stereospecificity demonstrated in gold(I)-catalyzed cyclization of related enynes,10 we prepared substrate Z-7 from known malonate 11(15) (Scheme 4). Thus, the anion of malonate 11 was alkylated with mesylate 12 to give allenyl malonate 13 in 86% yield. The acetal was then hydrolyzed to furnish aldehyde 14 (88%), which was alkenylated with phosphonate 15 to yield ketone 16 in almost quantitative yield with the desired Z configuration.12b,16 Finally, formation of silylenol ether with TBSOTf and Et3N, followed by Simmons–Smith cyclopropanation, led to Z-7 (67% over two steps).
Scheme 4. Synthesis of Substrate Z-7.
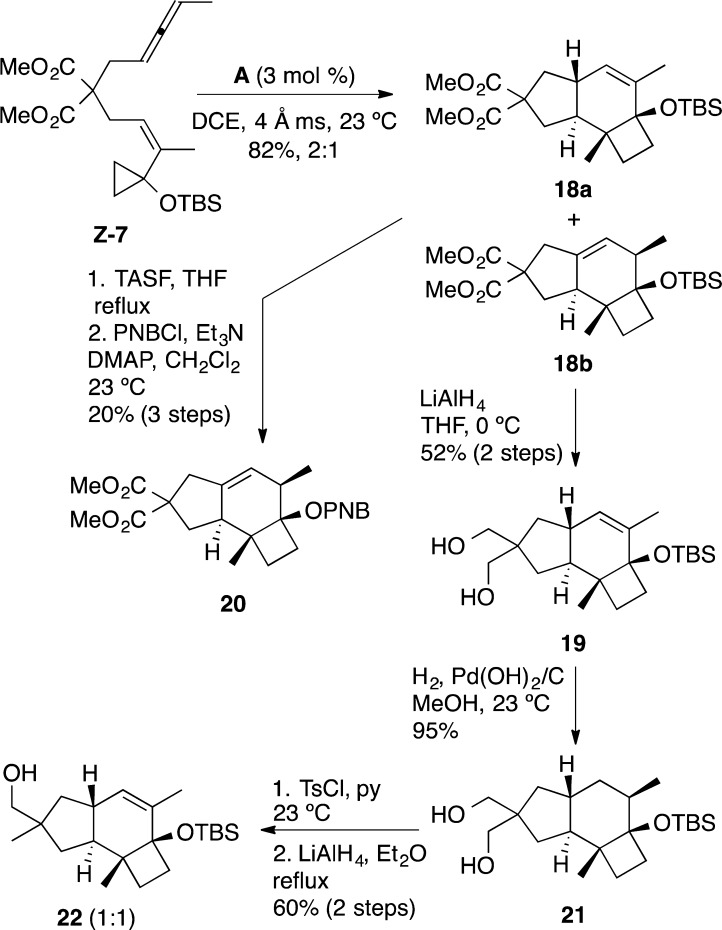
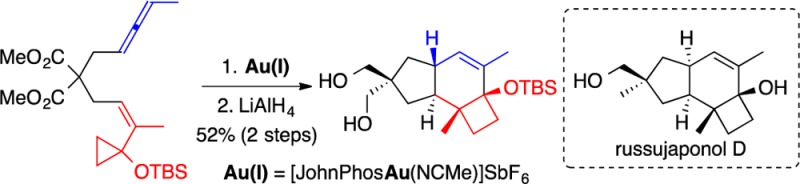
Cyclization of Z-7 was best carried out in 1,2-dichloroethane at 23 °C in the presence of catalyst A (3 mol %).17 The reaction led to the formation of two major products 18a and 18b in a 2:1 ratio (82%, NMR yield), which could not be separated by chromatography (Scheme 5). Their structures were assigned by transformation into crystalline derivatives. First, reduction of the malonate with LiAlH4 led to the isolation of crystalline diol 19 in 52% yield (2 steps from Z-7), whose structure was determined by X-ray diffraction, which confirmed the trans configuration at the junction between the five- and six-membered rings (Figure 2).18a On the other hand, desilylation with tris(dimethylamino)sulfonium difluorotrimethylsilicate (TASF), followed by esterification with p-nitrobenzoyl chloride and recrystallization, furnished ester 20 in 20% yield (2 steps from Z-7), whose structure was again assigned by X-ray diffraction (Figure 2).18b Hydrogenation of 19 in an autoclave under 50 bar of H2 using a Pearlman catalyst furnished 21 in 95% yield as a single diastereoisomer. Monotosylation of the diol followed by LiAlH4 reduction gave alcohol 22 in 60% yield as a 1:1 mixture of diastereoisomers at the newly formed stereocenter, which is structurally related to russujaponol D (4).
Scheme 5. Gold(I)-Catalyzed Cyclization of Z-7.
Figure 2.
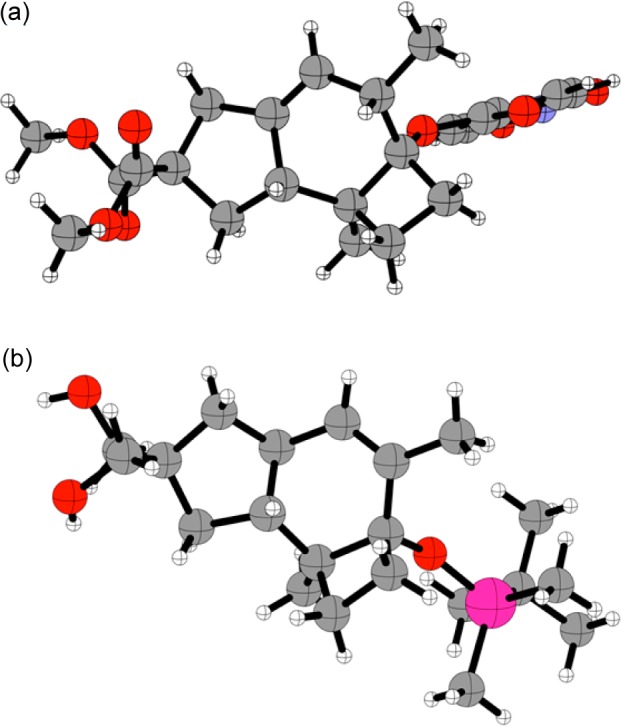
X-ray structures of 19 (a) and 20 (b).
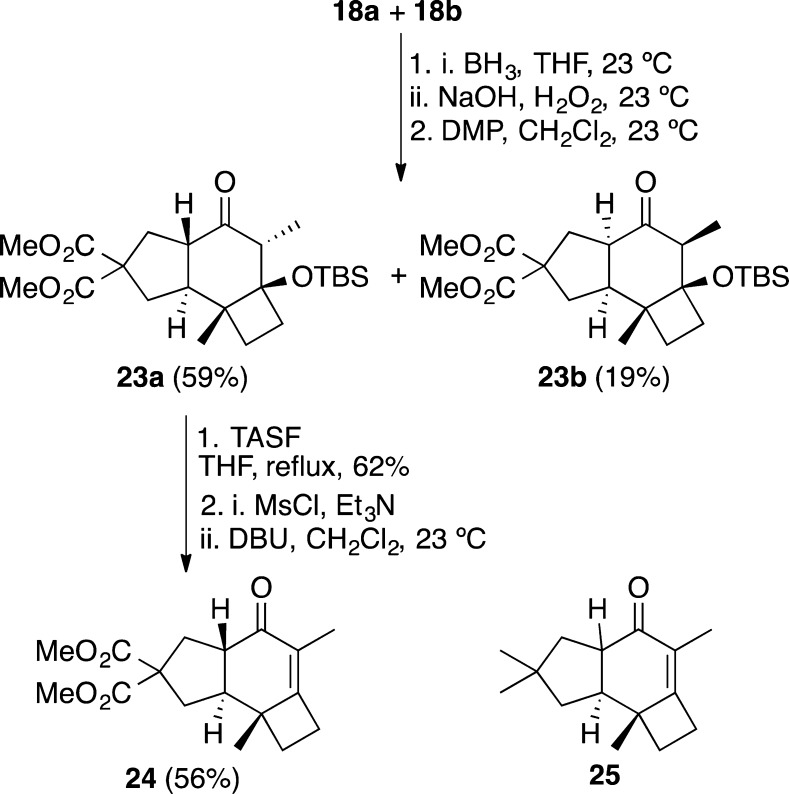
We also explored the introduction of the oxygen functionality on the six-membered ring by hydroboration/oxidation (Scheme 6). The reaction led to a mixture of several diastereomeric alcohols, which could not be separated by chromatography. However, treatment of this mixture with Dess-Martin periodinane gave two ketones 23a and 23b in 59% and 19% yields respectively, whose relative configurations were determined by NOE analysis. The TBS group of the major compound 23a was removed with TASF, and the resulting alcohol was converted into an unstable mesylate intermediate, which was eliminated with DBU to furnish enone 24. No epimerization was observed under these conditions to give the corresponding cis-stereoisomer of 24.19
Scheme 6. Transformations of Compounds 18a and 18b.
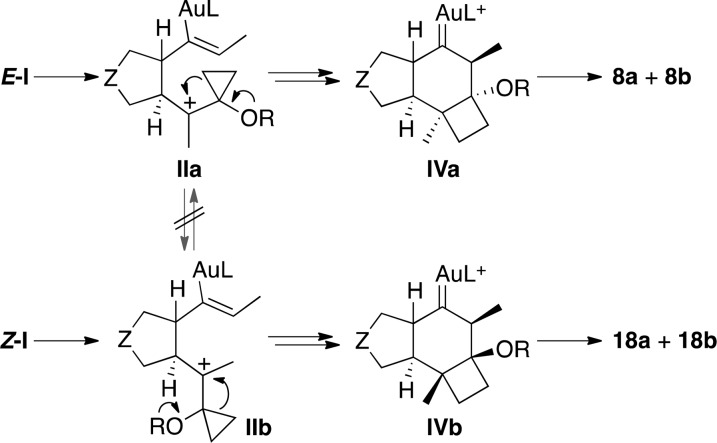
Our results demonstrate that intermediates IIa and IIb do not undergo equilibration and that the cyclopropylcarbenium to cyclobutane ring expansions occurs stereospecifically to form IVa or IVb, by intramolecular Prins reaction (Scheme 7). Regarding the configuration of cyclopentanes IIa and IIb, formation of 18a as the major product in the cyclization of Z-7 suggests that the gold(I)-catalyzed allene-vinylcyclopropane cycloisomerization leads to intermediates II with the trans-relative configuration. However, compounds 8a and 18b could arise from either trans- or cis-configured intermediates.
Scheme 7. Stereospecific Cyclizations of E- and Z-I.
In summary, we have shown that the gold-catalyzed allene-vinylcyclopropane cycloisomerization leads directly to complex tricyclic compounds with the skeleton of the protoilludanes. Ongoing work is focused on exploring new routes and catalysts to access the desired cis-fusion as well as on developing asymmetric syntheses of these natural compounds.
Acknowledgments
We thank MICINN (Project CTQ2010-16088/BQU), the European Research Council (Advanced Grant No. 321066), the Spanish Ministry of Education and Science (predoctoral FPU fellowship to A.P.), the AGAUR (Project 2009 SGR 47 and Beatriu de Pinós postdoctoral fellowship to D.L.), and the ICIQ Foundation for financial support. We are grateful to the ICIQ X-ray diffraction unit for the X-ray crystal structures.
Supporting Information Available
Experimental procedures and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Zu L.; Xu M.; Lodewyk M. W.; Cane D. E.; Peters R. J.; Tantillo D. J. J. Am. Chem. Soc. 2012, 134, 11369–11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasova M.; Sturla S. J. Chem. Rev. 2012, 112, 3578–3610. [DOI] [PubMed] [Google Scholar]
- For a review, see: ; a Siengalewicz P.; Mulzer J.; Rinner U. Eur. J. Org. Chem. 2011, 7041–7055. [Google Scholar]; b See also: Schwartz B. D.; Matoušová E.; White R.; Banwell M. G.; Willis A. C. Org. Lett. 2013, 15, 1934–1937. [DOI] [PubMed] [Google Scholar]
- Hansen T. V.; Stenstrøm Y. Naturally Occuring Cyclobutanes. Organic Synthesis: Theory and Applications; Hudlicky J. A. I.Elsevier Science Ltd.: Oxford, 2001; pp 1–39. [Google Scholar]
- For total synthesis, see: ; a Furukawa J.; Morisaki N.; Kobayashi H.; Iwasaki S.; Nozoe S.; Okuda S. Chem. Pharm. Bull. 1985, 33, 440–443. [Google Scholar]; b Oppolzer W.; Nakao A. Tetrahedron Lett. 1986, 27, 5471–5474. [Google Scholar]
- McMorris T. C.; Nair M. S. R.; Anchel M. J. Am. Chem. Soc. 1967, 89, 4562–4563. [DOI] [PubMed] [Google Scholar]
- Hirota M.; Shimizu Y.; Kamo T.; Makabe H.; Shibata H. Biosci. Biotechnol. Biochem. 2003, 67, 1597–1600. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K.; Kaneko A.; Matsumoto Y.; Hama H.; Arihara S. J. Nat. Prod. 2006, 69, 1267–1270. [DOI] [PubMed] [Google Scholar]
- a Jiménez-Núñez E.; Molawi K.; Echavarren A. M. Chem. Commun. 2009, 7327–7329. [DOI] [PubMed] [Google Scholar]; b Molawi K.; Delpont N.; Echavarren A. M. Angew. Chem., Int. Ed. 2010, 49, 3517–3519. [DOI] [PubMed] [Google Scholar]; c Gaydou M.; Miller R. E.; Delpont N.; Ceccon J.; Echavarren A. M. Angew. Chem., Int. Ed. 2013, 52, 6396–6399. [DOI] [PubMed] [Google Scholar]
- a Zhang L.; Sun J.; Kozmin S. A. Adv. Synth. Catal. 2006, 348, 2271–2296. [Google Scholar]; b Fürstner A.; Davies P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. [DOI] [PubMed] [Google Scholar]; c Hashmi A. S. K. Chem. Rev. 2007, 107, 3180–3211. [DOI] [PubMed] [Google Scholar]; d Li Z.; Brouwer C.; He C. Chem. Rev. 2008, 108, 3239–3265. [DOI] [PubMed] [Google Scholar]; e Arcadi A. Chem. Rev. 2008, 108, 3266–3325. [DOI] [PubMed] [Google Scholar]; f Jiménez-Núñez E.; Echavarren A. M. Chem. Rev. 2008, 108, 3326–3350. [DOI] [PubMed] [Google Scholar]; g Gorin D. J.; Sherry B. D.; Toste F. D. Chem. Rev. 2008, 108, 3351–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Michelet V.; Toullec P. Y.; Genêt J.-P. Angew. Chem., Int. Ed. 2008, 47, 4268–4315. [DOI] [PubMed] [Google Scholar]; i Aubert C.; Fensterbank L.; Garcia P.; Malacria M.; Simonneau A. Chem. Rev. 2011, 111, 1954–1993. [DOI] [PubMed] [Google Scholar]; j Krause N.; Winter C. Chem. Rev. 2011, 111, 1994–2009. [DOI] [PubMed] [Google Scholar]
- Jiménez-Núñez E.; Claverie C. K.; Nieto-Oberhuber C.; Echavarren A. M. Angew. Chem., Int. Ed. 2006, 45, 5452–5455. [DOI] [PubMed] [Google Scholar]
- See also: ; a Sethofer S. G.; Staben S. T.; Hung O.; Toste F. D. Org. Lett. 2008, 10, 4315–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jiao L.; Yuan C.; Yu Z.-X. J. Am. Chem. Soc. 2008, 130, 4421–4430. [DOI] [PubMed] [Google Scholar]; c Jiao L.; Lin M.; Zhuo L.-G.; Yu Z.-X. Org. Lett. 2010, 12, 2528–2531. [DOI] [PubMed] [Google Scholar]; d Jiao L.; Yu Z.-X. J. Org. Chem. 2013, 78, 6842–6848. [DOI] [PubMed] [Google Scholar]
- a Luzung M. R.; Mauleón P.; Toste F. D. J. Am. Chem. Soc. 2007, 129, 12402–12403. [DOI] [PubMed] [Google Scholar]; b Mauleón P.; Zeldin R. M.; González A. Z.; Toste F. D. J. Am. Chem. Soc. 2009, 131, 6348–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Alonso I.; Trillo B.; López F.; Montserrat S.; Ujaque G.; Castedo L.; Lledós A.; Mascareñas J. L. J. Am. Chem. Soc. 2009, 131, 13020–13030. [DOI] [PubMed] [Google Scholar]; d González A. Z.; Benitez D.; Tkatchouk E.; Goddard W. A. III; Toste F. D. J. Am. Chem. Soc. 2011, 133, 5500–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The minor isomer 8b could not be isolated, and its structure was tentatively assigned as a derivative of 3,7b-dimethyl-1,2,4a,5,6,7,7a,7b-octahydro-2aH-cyclobuta[e]inden-2a-ol (stereoisomer of 18a, Scheme 5), with the trans configuration between the five- and six-membered rings.
- Shitani R.; Okamoto K.; Otomaru Y.; Ueyama K.; Hayashi T. J. Am. Chem. Soc. 2005, 127, 54–55. [DOI] [PubMed] [Google Scholar]
- Yu W.; Su M.; Jin Z. Tetrahedron Lett. 1999, 40, 6725–6728. [Google Scholar]
- See Supporting Information for a screening of catalysts.
- a X-Ray crystal structure of 19: CCDC 953503.; b X-Ray crystal structure of 20: CCDC 953502.
- DFT calculations (B3LYP, 6-31G*) on model cis-and trans-25 show that the trans isomer is the most stable (ΔΔH° = 2.5 kcal· mol–1).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.