Abstract
Adiponectin is an insulin sensitizing fat cell (FC) hormone whose levels are related to adipose tissue (AT) mass and depot distribution. We hypothesized that the nature of AT expansion (hypertrophy vs. hyperplasia) contributes to obesity-related reductions in serum adiponectin and that this effect is influenced by the regional distribution of AT to subcutaneous (S) and visceral (V) depots. Thirteen obese subjects provided paired AT biopsies. Serum total and high molecular weight (HMW) adiponectin levels were determined by ELISA. Secretion was quantified following 24-h explant culture. FC size, number, % large, and % small FC were determined by microscopic analysis. Secretion of total adiponectin was highest by SAT (P = 0.008) and correlated more strongly with serum adiponectin (total: P = 0.015, r = 0.77; HMW: P = 0.005, r = 0.83) than did secretion by VAT (P = 0.05, r = 0.66 for both). FC size was greatest in SAT and correlated negatively with both serum (total: P = 0.01, r = −0.74; HMW: P = 0.03, r = −0.69) and secreted (total: P = 0.05, r = −0.72; HMW: P = 0.02, r = −0.87) adiponectin. The % small FC in SAT correlated positively with both serum (total: P = 0.006, r = 0.87; HMW: P = 0.009, r = 0.79) and secreted (total: P = 0.03, r = 0.75; HMW: P = 0.01, r = 0.92) adiponectin. VAT FC size correlated negatively with serum HMW adiponectin (P = 0.01, r = −0.76) but not with any measure of secretion. VAT had the greatest % small FC, which related positively to serum HMW (P = 0.004, r = 0.81) and to secreted total adiponectin (P = 0.02, r = 0.78). These studies indicate that differences in fat cell size and depot distribution of AT expansion are important influences on adiponectin in obesity.
Keywords: adipocyte, fat cell size, adiponectin, high molecular weight, insulin resistance, adipose tissue depots, subcutaneous adipose tissue, visceral adipose tissue
Introduction
Obesity is a significant public health concern. Greater than 30% of the United States population is obese and at risk to develop insulin resistance and associated metabolic disease, including hypertension, hyperlipidemia, fatty liver disease, atherosclerosis, and T2DM.1
AT is distributed to a variety of locations, and in humans the contributions of SAT and VAT can be distinguished.2 Individuals with increased VAT are at greater risk of developing insulin resistance and cardiometabolic disease2,3 than are those with similar amounts of AT as SAT.4 These findings lend support to the idea that different AT depots are functionally distinct metabolic units.
The variation in FC size that occurs in obesity may have an important impact on AT function.5,6 During states of nutrient excess AT may expand by a variable balance between increases in FC size (hypertrophy) and increases in FC number (hyperplasia). Increases in FC size are associated with FC dysfunction,7,8 increases in pro-inflammatory cytokine secretion,9 and an increased risk of developing T2DM.6
AT depots differ in the distribution of FC sizes,10 suggesting that this difference might in part underlie the functional differences between SAT and VAT. In support of this view, Veilleux et al. reported that hypertrophy of VAT but not SAT FCs is associated with a more atherogenic lipid profile.11 Others have found that a number of functional aspects of FC activity relate to FC size.8,10
Adiponectin is a potent insulin-sensitizing, anti-inflammatory, and anti-atherogenic FC hormone that may link obesity to the development of insulin resistance.12 Adiponectin levels are low in obesity and in conditions associated with insulin resistance, including hyperlipidemia, T2DM, and NASH.12 In vivo, regional AT distribution appears to contribute to the regulation of adiponectin. An increased VAT mass is an independent predictor of reduced serum adiponectin levels.13 Depot differences are evident in the secretion of adiponectin by cultured human VAT and SAT explants.14,15 Adiponectin circulates in three major forms: a low molecular weight (LMW) trimer, a medium molecular weight (MMW) hexamer, and a high molecular weight (HMW) multimer.16 Increases in the SA, the ratio of HMW adiponectin to total adiponectin and an indicator of insulin sensitivity, as well as the absolute amount of HMW adiponectin correlate with improvements in insulin sensitivity and lowering of hepatic glucose production.17 Circulating levels of these multimeric complexes appear to be regulated at the level of the FC in response to changes in total body fat mass18 and regional AT distribution.19,20
The manner by which AT expands, through hyperplasia and/or hypertrophy, could represent another potential regulator of adiponectin synthesis and secretion. Reductions in mean FC size and increases in adiponectin both occur in response to weight loss.21,22 In support of this view, Drolet et al. demonstrated an inverse relationship between mean FC diameter and total adiponectin secretion—a relationship evident only in VAT.15 We postulate that FC size contributes to obesity related reductions in adiponectin and that this effect is influenced by the regional distribution of excess AT. To address this hypothesis, we have examined the relationship between AT depots, FC size, and secretion of total and multimeric adiponectin in human obesity.
Results
Clinical features and adiponectin
To examine the relationship between serum adiponectin and anthropometric and cellular measures of obesity, we recruited 13 obese weight stable subjects undergoing laparoscopic Roux-en-Y gastric bypass for weight reduction. Subject characteristics are presented in Table 1. Participants were euglycemic with a predominantly central distribution of excess AT (waist circumference 149.2 ± 10.1 cm male vs. 123.2 ± 3.2 cm female). There was a tendency (P = 0.075) for central adiposity to be positively associated with an increased index of insulin resistance23 (HOMA-IR).
Table 1. Subject characteristics.
| Age (y) |
46 ± 2 |
| Gender |
9 F | 4 M |
| BMI (kg/m2) |
46.1 ± 2.3 |
| Waist circumference (cm) |
131.2 ± 5.0 |
| WHR |
0.91 ± 0.02 |
| Fasting glucose (mM) |
5.35 ± 0.21 |
| Fasting insulin (pM) |
26.0 ± 3.9 |
| HOMA-IR |
6.30 ± 1.03 |
| Fasting triglycerides (mg/dL) |
142 ± 77 |
| HDL (mg/dL) |
45 ± 11 |
| Serum total adiponectin (µg/mL) |
15.33 ± 2.14 |
| Serum HMW adiponectin (µg/mL) | 3.76 ± 0.82 |
Results are provided as mean measures ± SD. BMI, body mass index; WHR, waist:hip ratio; HOMA-IR, homeostasis model assessment of insulin sensitivity, calculated multiplying fasting plasma insulin by fasting plasma glucose, then dividing by the constant 22.5, i.e., HOMA-IR = (FPI × FPG)/22.5.
Serum total and HMW adiponectin levels did not differ significantly between male and female subjects, although there were tendencies for both total (17.24 ± 2.8 vs. 11.03 ± 1.6 μg/ml, P = 0.14) and HMW adiponectin (4.2 ± 0.99 vs. 1.8 ± 0.52, P = 0.15) levels to be higher in female subjects. SA, the ratio of HMW adiponectin to total adiponectin and an indicator of insulin sensitivity, did not differ significantly between male17 and female subjects, although there was again a tendency for higher SA in females (0.25 ± 0.04 vs. 0.15 ± 0.02, P = 0.12). While levels of total adiponectin in the serum were not significantly associated with measures of adiposity, insulin action, or lipidemia (Table 2), both the SA and serum HMW adiponectin levels were significantly related to the HOMA-IR.
Table 2. Relationships between clinical characteristics and circulating adiponectin.
| Variable | Serum total Ad | Serum HMW Ad | SA |
|---|---|---|---|
| BMI |
−0.30 |
−0.40 |
−0.39 |
| WHR |
−0.39 |
−0.49 |
−0.52 |
| Serum Insulin |
−0.56 |
−0.71*,† |
−0.67*,† |
| HOMA-IR |
−0.44 |
−0.59*,† |
−0.64*,† |
| Triglycerides |
−0.66† |
−0.66*,† |
−0.53† |
| HDL | 0.48 | 0.66* | 0.41 |
Results are provided on 13 subjects as Pearson r values. SA, insulin sensitivity index was determined as the ratio of HMW to total adiponectin. *P < 0.05, †x, y log-transformed.
FC size
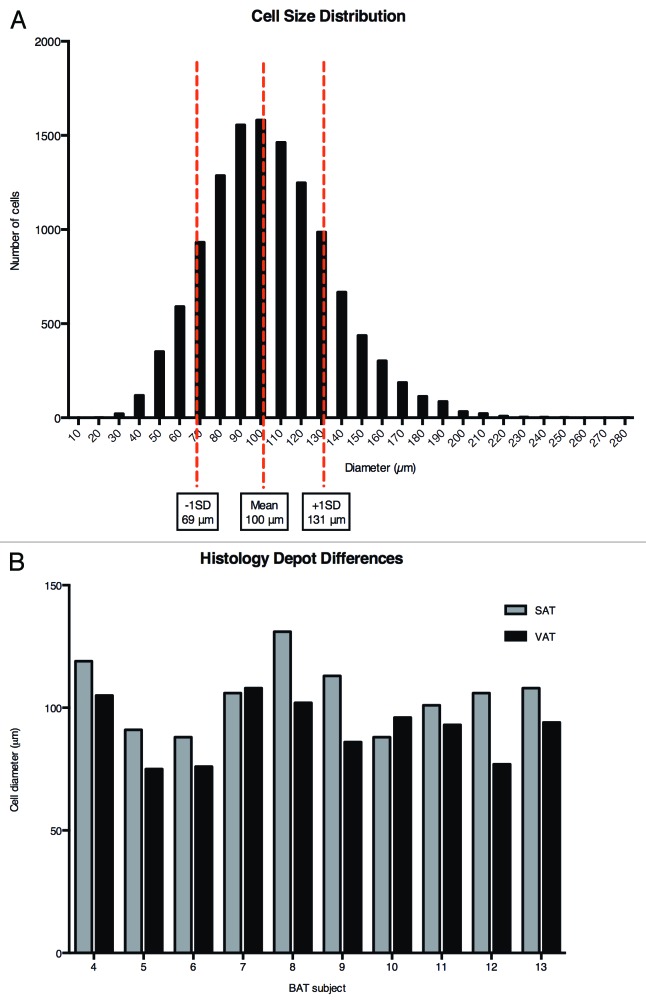
Figure 1A presents a histogram of FC diameters determined on intact SAT and VAT from all subjects. While FC diameters displayed a near Gaussian distribution, considerable heterogeneity was observed. For subsequent consideration, “small” FCs are defined as those with a diameter < 69 µm, one SD below the mean, while “large” FCs are taken as those with diameters > 131 µm, one SD above the mean. Differences between the SAT and VAT depots of the same subject were observed (Fig. 1B). FCs from VAT were routinely smaller than those from the SAT depot (87 ± 4% of paired SAT, P = 0.011), in agreement with previous findings.24 Besides differences in the average diameter, the % small FCs was greater in VAT compared with SAT (20.0 ± 11.3% vs. 11.7 ± 6.4%, P = 0.02). Conversely, SAT contained a greater % large FCs (19.5 ± 14.6 vs. 10.5 ± 13.7%, P = 0.01). Gender differences were evident in both FC size and FC size distribution, with males having a larger average VAT diameter (P = 0.03) and a smaller % small FCs (P = 0.03) compared with females.
Figure 1. FC size distribution: depot differences. Top panel, histogram of FC diameters determined on intact tissue. Results pooled for 10 subjects and both depots. Bottom panel, comparison of average FC diameter between paired depots in 10 subjects.
There were no relationships between a measure of total adiposity (BMI) and FC diameter for either depot (Table 3). However, as might be expected, VAT FC size (P = 0.007, r = 0.79) and the % large VAT FCs (P = 0.04, r = 0.48) were positively associated with WHR, an indicator of visceral adiposity. SAT FC size was a strong predictor of insulin action, displaying significant associations with both fasting insulin levels and the HOMA-IR. Increases in the % small VAT FCs related positively to the SA index of insulin sensitivity (P = 0.02, r = 0.75), and to a smaller waist (P = 0.01, r = −0.74) and WHR (P = 0.001, r = −0.86).
Table 3. Relationship between FC size and clinical characteristics.
| Variable | SAT FC diameter | VAT FC diameter |
|---|---|---|
| BMI |
−0.11 |
0.05 |
| WHR |
0.53 |
0.79* |
| Serum Insulin |
0.86* |
0.39 |
| HOMA-IR |
0.70* |
0.61† |
| Triglycerides |
0.76*†† |
0.60 |
| HDL | −0.33 | −0.55 |
Results are provided on 10 subjects as Pearson r values. *P < 0.05, †x and y log transformed, ††x log transformed.
FC size and adiponectin secretion
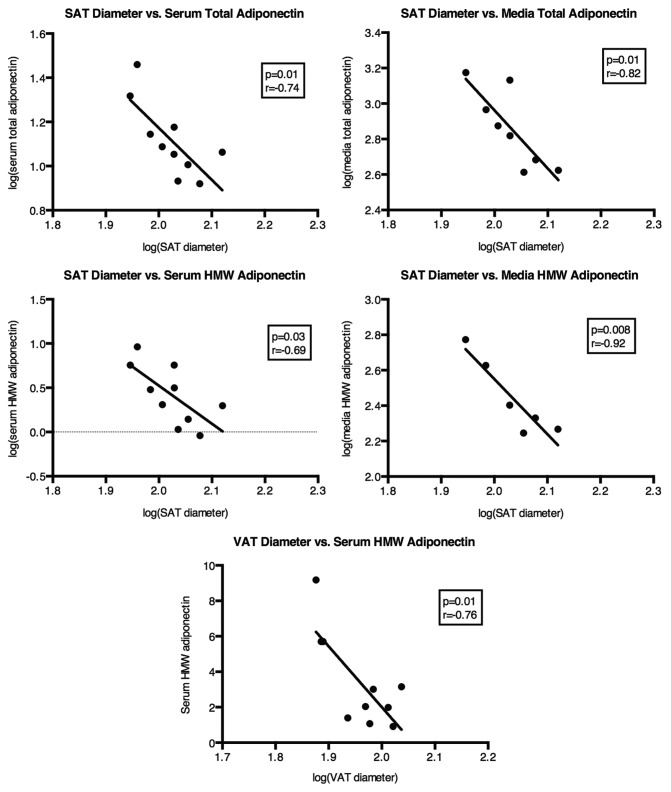
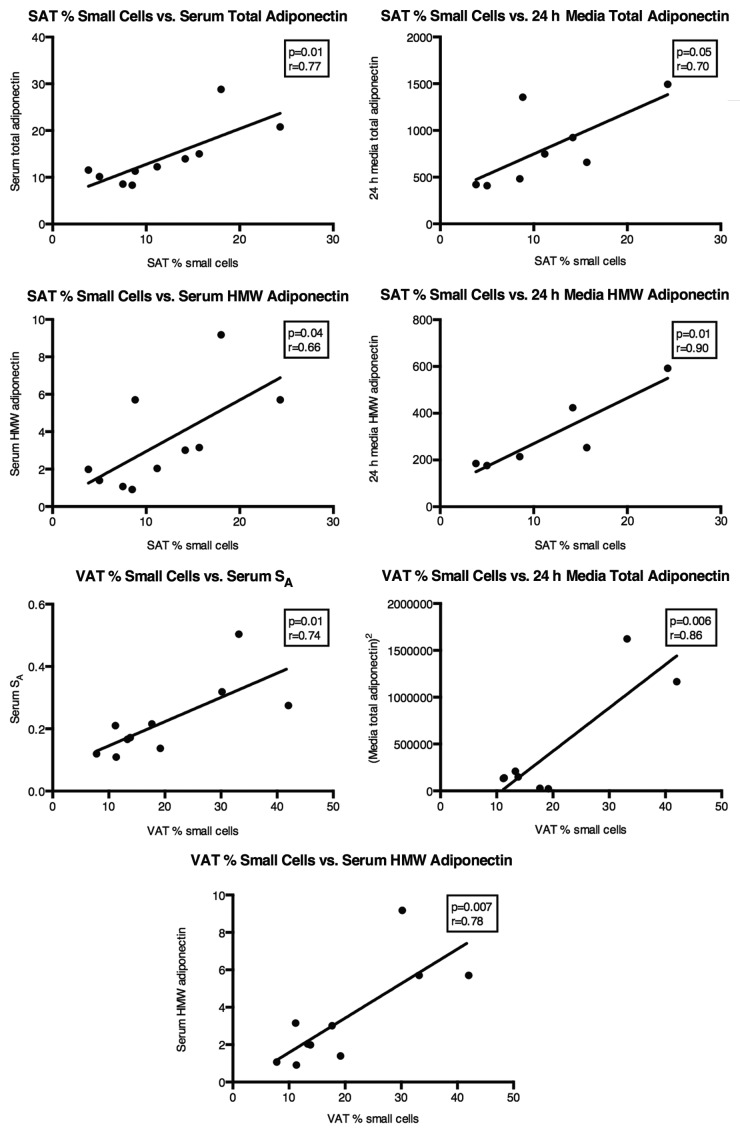
Reflective of the relationship between SAT FC size and insulin action, SAT FC diameter was also strongly associated, in a negative manner, with total and HMW adiponectin in the circulation (Fig. 2). The same was true for circulating SA (r = −0.74, P = 0.03). Conversely, there was no correlation between VAT FC diameter and circulating total adiponectin (r = −0.37, P = 0.36), yet VAT FC size was significantly associated with circulating HMW adiponectin (Fig. 2) and nearly so with SA (r = −0.65, P = 0.08). Average FC diameter describes a property of an entire population. To understand more about how adiponectin secretion and assembly into HMW may be influenced by FC size, the results were analyzed in light of the % of FCs in each depot that could be classified as small, with a greater % small FCs suggesting expansion of that depot through hyperplasia. In SAT the % small FCs was positively associated with both total and HMW adiponectin in the circulation (Fig. 3). A depot difference was apparent in the fact that the % small FCs in VAT was not related to total circulating adiponectin, though it was correlated with HMW in the serum, similar to the situation with SAT (Fig. 3).
Figure 2. Relationships between averaged SAT and VAT FC diameter and features of circulating and 24 h secreted total and HMW adiponectin. n = 10 for all serum adiponectin measures. n = 8 for media total adiponectin and n = 6 for media HMW adiponectin measures.
Figure 3. Relationships between % small (diameter < 69 µm) FCs in SAT and VAT and features of circulating and 24 h secreted total and HMW adiponectin. n = 10 for all serum adiponectin measures. n = 8 for media total adiponectin and n = 6 for media HMW adiponectin measures.
Ex vivo adiponectin secretion
In order to understand more about differences between AT depots with regard to the multimerization and secretion of adiponectin, SAT and VAT were maintained in culture under controlled conditions and the release of adiponectin into the media evaluated. We have reported previously that over the initial period, 24–48 h, AT maintained in this manner reflects many of the properties of freshly obtained AT.14 In agreement with previous reports,15,25 SAT routinely released more total adiponectin into the media than the paired VAT (Fig. 4, P = 0.011), a difference that was observed in all of the subjects (data not shown). Of the two depots, SAT represents the major contributor to adiponectin in the circulation, as total adiponectin secreted from SAT over 24 h in culture was strongly and significantly correlated with circulating adiponectin levels in the same subjects (r = 0.77, P = 0.015). The same is true for HMW adiponectin released from SAT and that in the circulation (r = 0.83, P = 0.005). The role of VAT appears to be of lesser importance compared with SAT, as neither the association between secreted and circulating total adiponectin (r = 0.63, P = 0.069) nor secreted and circulating HMW adiponectin (r = 0.62, P = 0. 076) reached statistical significance. In generalized linear models with both SAT and VAT depots predicting serum levels, SAT alone was significant. Adding SAT to VAT improved model fit, while adding VAT to SAT did not. Beyond the contributions of the individual depots to the pool of circulating adiponectin, the possibility exists for crosstalk between the depots. Even when studied separately in explant culture, there were strong associations between adiponectin secretion from paired SAT and VAT for both total (r = 0.83, P = 0.0016) and HMW (r = 0.75, P = 0.020) adiponectin.
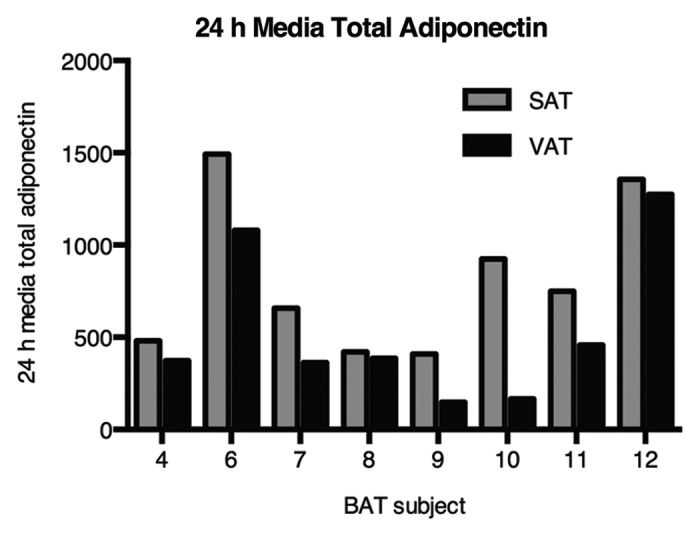
Figure 4. Ex vivo release of total adiponectin from AT from 8 subjects over 24 h. in culture: Comparison of SAT and VAT. Equal weights of tissue were maintained in 30 mL of defined media (see Methods).
FC size had a direct impact on adiponectin secretion from intact SAT, as the amount of both total and HMW adiponectin released into the media over a 24-h period fell with increasing FC diameter (Fig. 2). Consistent with that behavior, adiponectin secretion was positively associated with the % small FCs in SAT (Fig. 3), suggesting that small FCs in SAT autonomously secrete more adiponectin and assemble a higher proportion into HMW multimers. Unlike the situation with regard to total adiponectin in the serum, VAT FC size (diameter) was significantly associated with total adiponectin released into the media (r = −0.82, P = 0.04). Any such relationship with secreted HMW did not attain significance (r = −0.41, P = 0.27). In vitro, VAT behaved similarly to SAT in that the % small FCs was positively associated with total adiponectin released into the media (Fig. 3).
Discussion
This study demonstrates several major findings. First, we confirm earlier findings by ourselves and others that depot differences exist in the secretion of adiponectin, with greater release by SAT.14,15 In this report we extend these earlier findings to demonstrate a primary role of SAT in the determination of circulating levels of both total and HMW adiponectin. We also report the existence of depot differences in the relative degree of hypertrophic vs. hyperplastic expansion of AT in obesity. Because increases in FC size have been shown to impair FC function26 and correlate with insulin resistance27 and risk for T2DM,6 these findings may have important implications for the relationship between obesity and the occurrence of metabolic disease.
One possible contributor to obesity-related reductions in adiponectin may be dysfunctional SAT.28 Our group has previously shown that secretion of adiponectin from SAT is reduced in obese subjects.14 Our current study shows that despite an obesity-associated reduction in adiponectin secretion, SAT still secretes and contributes more significantly to serum adiponectin than does VAT (Table 4). Because of its larger mass and greater secretion rate, even small reductions in adiponectin production by SAT have the potential to significantly lower circulating levels of adiponectin. In support of this regulatory role of SAT, pharmacologic agents or conditions that act to alter the expansion and/or activity of SAT exert significant effects on circulating levels of adiponectin.29-32
Table 4. Intradepot and interdepot influence of FC size distribution (% small FC) on secreted adiponectin.
| |
% small FC SAT |
% small FC VAT |
||
|---|---|---|---|---|
| r | P value | r | P value | |
| 24 h media total adiponectin (same depot) |
0.70 |
0.05* |
0.83 |
0.006* |
| 24 h media HMW adiponectin (same depot) |
0.93 |
0.007* |
0.96 |
0.003* |
| SAT - 24 h media total adiponectin |
|
|
0.94 |
0.0005* |
| SAT - 24 h media HMW adiponectin | 0.91 | 0.01* | ||
Results are provided as Pearson r values. *P < 0.05. n = 8 for media total, n = 6 for media HMW.
Our novel findings regarding the relationship between FC size and adiponectin secretion suggest that depot morphology may in part underlie the reductions in adiponectin that are associated with visceral obesity. In agreement with previous work,24 hypertrophy appears to be the predominant mechanism for expansion of SAT, as surrogate measures of hypertrophic expansion, mean FC diameter and % large FCs, were greatest in SAT. In contrast, hyperplastic expansion appears predominant in VAT, which contained a significantly greater % small FCs and a significantly smaller mean FC diameter. Interestingly, in males, VAT contained a greater % small FCs as well as a greater overall mean FC diameter, suggesting preferential expansion of this depot vs. SAT in males by a combination of both cellular hypertrophy and hyperplasia.
During states of nutrient excess AT expands via a combination of hypertrophy and hyperplasia. The relative balance between these processes has metabolic consequences for a given individual. While hypertrophic obesity is strongly associated with insulin resistance and development of the metabolic syndrome,33 little is known regarding the effects of FC hyperplasia on metabolic dysfunction. A recent report by Arner et al. suggests that a genetic predisposition to limit SAT hyperplasia is associated with an increased risk for T2DM.34 Consistent with these findings, we report that reductions in the % small FCs in SAT, our surrogate measure of AT hyperplasia, are associated with reductions in both secreted and circulating levels of total and HMW adiponectin. These changes in adiponectin may underlie the increased risk for T2DM identified by Arner et al.34
Alterations in FC size have metabolic effects.35 For example, enlargement of FCs has been associated with altered lipid and energy metabolism,36 increased inflammatory cytokine production,9 and insulin resistance.35 In agreement with Hoffstedt et al., we found significant relationships between average SAT FC size and multiple measures of insulin action, including fasting insulin, triglycerides, and HOMA-IR.37 Although no association was identified between average VAT FC size and these measures of insulin sensitivity, we identified a significant relationship between the % large VAT FCs and HOMA-IR, suggesting that hypertrophy of VAT FCs may adversely affect insulin sensitivity.
These results give rise to the following question: do depot differences in AT morphology underlie obesity-related reductions in circulating and secreted adiponectin? Studies by some investigators suggest that in SAT, FC size is inversely correlated with serum adiponectin.38,39 For example, the reductions in FC size that occur with weight loss are associated with increases in circulating adiponectin.21,22 In the current report we examine the relationship between FC size and the secretion of adiponectin by SAT and VAT, as well as the relative contributions of each of these depots to circulating levels of adiponectin. Our findings of an inverse relationship between SAT FC size and serum adiponectin are in agreement with those of Drolet et al., and extend their findings with our inclusion of important inverse relationships between FC size and serum HMW adiponectin in both depots.15 Our findings support the postulate that the hypertrophic expansion of SAT, more so than VAT, may underlie obesity-related reductions in serum total and HMW adiponectin. The inclusion of data on HMW adiponectin, the most biologically potent form,40 and measurements of large numbers of individual FCs in situ further strengthen our findings.
The occurrence of metabolic disease has long been linked to the accumulation of VAT,41 with expansion of VAT, more so than SAT, strongly associated with reductions in serum adiponectin.42 These observations suggest that adiponectin secretion may be impaired in obese VAT. In support of this view, we confirm reduced adiponectin secretion by the VAT of obese subjects, a finding reported previously by our group and others.14,15,43 However, the relationship between VAT and serum adiponectin is likely to be more complicated, due in part to the fact that VAT makes up only 20% of total body fat. Even assuming that the adiponectin secretion rates by VAT and SAT are similar, a complete loss of adiponectin secretion by VAT is unlikely to account for more than a 20% reduction in serum adiponectin, suggesting that other factors likely contribute to the lower adiponectin levels associated with visceral obesity. Although our study cannot determine the reason for the relationship between VAT and adiponectin in vivo, it is clear that reductions in SAT adiponectin secretion must play a role. To better understand the regulation of adiponectin in obesity, we sought to determine if our data suggests the presence of cross-depot communication. Intriguingly, the % large VAT FC, our surrogate measure of hypertrophic expansion, related significantly to reductions in the secretion of both total and HMW adiponectin by SAT. In addition, we identified a significant relationship between the % small FC in VAT and the secretion of total adiponectin by SAT (Table 4). Although these findings do not establish causality, one potential explanation for these observations is the existence of cross-depot communication.
Several limitations of our study need to be acknowledged. First, although we report a strong relationship between FC size and adiponectin, this study was not designed to establish causality. Indeed, others have reported that adiponectin itself may exert effects on AT expansion.44 Second, results were normalized by tissue weight and not by FC number. We note that the greater mean FC size in SAT would predict fewer FCs per unit weight of AT in this depot and hence a greater per cell adiponectin secretion rate. Accordingly, we would predict that this analysis would increase the significance of our findings regarding depot differences. We further acknowledge that with a small data set it is difficult to contrast the relative contributions of the SAT and VAT depots to circulating adiponectin and replication in a larger data set is indicated. Third, although it is generally accepted that adiponectin levels vary inversely with BMI, we did not observe a significant relationship between circulating adiponectin and BMI in our study subjects. Instead, individuals with nearly identical BMIs had widely discrepant adiponectin levels, suggesting that in the severely obese, other factors such as the relative tendency for hyperplastic vs. hypertrophic expansion may contribute to the observed differences in serum adiponectin. Lastly, we acknowledge that our observations are restricted to a single point in time. Thus, we have not made dynamic measures of AT expansion but have instead used the % small and the % large cells as surrogate measures of hyperplastic and hypertrophic expansion. Future studies will assess these relationships as a dynamic process.
In summary, while the FC hormone adiponectin represents a potential molecular mediator of obesity-related disease, the role of AT expansion in the regulation of adiponectin remains uncertain. These results suggest that even within a cohort of similarly obese individuals, FC size differs and that this distribution of FC size relates meaningfully to the metabolic and hormonal function of AT. We postulate that the predominant mechanism by which AT expands under conditions of nutrient excess contributes importantly to the metabolic consequences of obesity, leading us to propose that interventions promoting hyperplastic expansion of AT, particularly of SAT, may mitigate the severity of obesity-related metabolic disease.
Materials and Methods
Human subjects
A total of 13 obese subjects, 9 females and 4 males, undergoing elective laparoscopic gastric bypass via Roux-en-Y for the treatment of obesity were recruited for this study. The institutional review boards of Scripps Memorial Hospital and the University of California, San Diego approved this study and all subjects gave informed consent. All subjects were weight stable at the time of surgery. Characteristics of study subjects are shown in Table 1. Waist circumference was measured at the level of the iliac crest and hip circumference was measured at the widest circumference.
Assays
All participants fasted for at least 12 h prior to blood collection and surgery. Fasting serum lipids and glucose were determined using standard assays. Serum insulin concentrations were determined by RIA (intraassay cv = 3.2%, interassay cv = 3.9%) (EMD Millipore, HI-11K). HOMA-IR was calculated as the product of the fasting glucose (mmol/L) and fasting insulin (μU/mL) divided by 22.5. Serum and conditioned media total and serum HMW adiponectin was determined by total (intraassay cv = 3.3%, interassay cv = 5.7%) and HMW adiponectin (intraassay cv = 2.5%, interassay cv = 5.6%) ELISA (EMD Millipore, EZHADP-61K and EZHMWA-64K). Insulin sensitivity index (SA) was determined as the ratio of HMW to total adiponectin.
Media HMW adiponectin, below the limit of detection by ELISA, was determined by semi-quantitative western blotting. Multimeric adiponectin was analyzed using 3–8% tris-acetate gels (Invitrogen, EA0378BOX). Samples of conditioned media (30 μl) were diluted 1:5 in Laemmli sample buffer under non-reducing, non-heat denaturing conditions. Adiponectin standards equal to 2.5, 5.0, and 15 ng of total recombinant human adiponectin (EMD Millipore, EZHADP-61K) were run for semi-quantitative analysis on each gel. Electrophoresis and western blot analysis was performed by standard techniques using primary mouse anti-human adiponectin (1:500) (BD Transduction Laboratories, 611644) and secondary goat anti-mouse-IR-800 (1:20 000) (LICOR, 926–32210) antibodies. Band detection and signal intensity were determined using the LICOR Odyssey imager and software. Quantitation of total and HMW adiponectin was completed for each standard and each unknown. To determine the amount of HMW adiponectin in conditioned media, the percentage of HMW in the samples as determined by quantitation was multiplied by the total adiponectin in the media as determined by ELISA. This value was then normalized between gels using a factor determined by quantitation of the adiponectin standards. Reproducibility of this method was established by running adiponectin standards in triplicate on eight gels. The standard deviations of the calculated values of HMW and % HMW adiponectin were 1.53 ng/mL and 0.41% respectively.
Adipose tissue biopsy
Paired SAT and VAT biopsies were obtained from the superficial abdominal subcutaneous adipose tissue and from visceral adipose tissue of the greater omentum. Biopsy tissue was placed in a sterile HEPES salts solution, as previously described,14 and transported to the lab for immediate processing.
Adipose tissue explant culture
AT explants were cultured (2 g AT/30 ml) in defined medium as previously described.14 Conditioned media was collected at 24 h and analyzed as described above for total and HMW adiponectin.
Adipose tissue histology and imaging
Single pieces (0.5–0.8 g) of adipose tissue obtained from each depot were placed in 70% ethanol immediately following the biopsy. Preparation of samples for histologic analysis consisted of progressive dehydration in ethanol and citrisolv (Fisherbrand, 22-143-975) and paraffin embedding in cassette molds, which were then stored at −20 °C for 24 h prior to further processing. Multiple slides were prepared from each biopsy sample using a microtome with a slice thickness of 5 μm. Prepared slides were H&E stained according to standard methods. Images of the H&E stained adipose tissue slides were obtained at a 4× magnification together with imaging of a 2000 μm calibration instrument and FC diameter was determined on 600 cells per depot. Data obtained from the Image J protocol was then analyzed using statistical techniques. To extrapolate to cell volume from the diameter, the formula for the volume of a sphere was used.
Statistical analysis
All statistical analysis was performed using Graph Pad Prism 5.0b except the multivariate analysis, which was performed using SPSS (IBM SPSS 19 statistical software). Interdepot differences were determined by paired t test analysis and all correlational data are reported based on Pearson correlation analysis with log transformation of non-normally distributed data when required. We used Pearson correlation to test the association of adiponectin secretion by each depot with serum levels of adiponectin. Generalized linear models were utilized to evaluate the relative contributions of the two depots to the serum levels. The significance of the two depots in the multivariate model and the change in goodness of fit according to Akaike’s Information Criterion (AIC) for each variable if added last were evaluated in each case. Due to limitations in tissue availability, not all analysis was completed on all subjects. Numbers of subjects studied are given in table and figure legends. In all cases, statistical significance was taken as a P value less than or equal to 0.05.
Acknowledgments
We thank Paula Sicurello of the Core Microscope Facility (Veterans Medical Research Foundation) for assistance with tissue microscopy and Leslie Carter for assistance with insulin RIA. This work was supported by a pilot and feasibility study in endocrinology and diabetes granted by the UCLA/UCSD DERC (P30 DK063491) (to S.P.), the Department of Veterans Affairs Medical Research Service (P30 DK063491) (to R.R.H.) and a Distinguished Clinical Scientist Award from the American Diabetes Association (to R.R.H.). Note: Due to space limitations, we are unable to cite all of the fundamentally important studies that have been published in this field. Where possible we have selected review articles as an informational resource.
Glossary
Abbreviations:
- AT
adipose tissue
- FC
fat cell
- HMW
high molecular weight
- HOMA-IR
homeostasis model assessment of insulin sensitivity
- NASH
non-alcoholic steatohepatitis
- S
subcutaneous
- SA
insulin sensitivity index
- T2DM
type 2 diabetes mellitus
- V
visceral (omental)
- WHR
waist/hip ratio
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/24953
References
- 1.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162:2074–9. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 2.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/er.21.6.697. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Cupples LA, Ramaswami R, Stokes J, 3rd, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease; the Framingham Study. J Clin Epidemiol. 1991;44:183–90. doi: 10.1016/0895-4356(91)90265-B. [DOI] [PubMed] [Google Scholar]
- 4.Krotkiewski M, Björntorp P, Sjöström L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–62. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arner E, Westermark PO, Spalding KL, Britton T, Rydén M, Frisén J, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–9. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 7.Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res Suppl. 1988;19:26–9. [PubMed] [Google Scholar]
- 8.Despres JP, Fong BS, Julien P, Jimenez J, Angel A. Regional variation in HDL metabolism in human fat cells: effect of cell size. Am J Physiol. 1987;252:E654–9. doi: 10.1152/ajpendo.1987.252.5.E654. [DOI] [PubMed] [Google Scholar]
- 9.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 10.Fried SK, Kral JG. Sex differences in regional distribution of fat cell size and lipoprotein lipase activity in morbidly obese patients. Int J Obes. 1987;11:129–40. [PubMed] [Google Scholar]
- 11.Veilleux A, Caron-Jobin M, Noël S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60:1504–11. doi: 10.2337/db10-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–88. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 13.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips SA, Ciaraldi TP, Oh DK, Savu MK, Henry RR. Adiponectin secretion and response to pioglitazone is depot dependent in cultured human adipose tissue. Am J Physiol Endocrinol Metab. 2008;295:E842–50. doi: 10.1152/ajpendo.90359.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drolet R, Bélanger C, Fortier M, Huot C, Mailloux J, Légaré D, et al. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity (Silver Spring) 2009;17:424–30. doi: 10.1038/oby.2008.555. [DOI] [PubMed] [Google Scholar]
- 16.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–63. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 17.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–62. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 18.Swarbrick MM, Austrheim-Smith IT, Stanhope KL, Van Loan MD, Ali MR, Wolfe BM, et al. Circulating concentrations of high-molecular-weight adiponectin are increased following Roux-en-Y gastric bypass surgery. Diabetologia. 2006;49:2552–8. doi: 10.1007/s00125-006-0452-8. [DOI] [PubMed] [Google Scholar]
- 19.Tamei N, Ogawa T, Ishida H, Ando Y, Nitta K. Relationship of high-molecular-weight adiponectin levels to visceral fat accumulation in hemodialysis patients. Intern Med. 2010;49:299–305. doi: 10.2169/internalmedicine.49.2905. [DOI] [PubMed] [Google Scholar]
- 20.Kishida K, Kim KK, Funahashi T, Matsuzawa Y, Kang HC, Shimomura I. Relationships between circulating adiponectin levels and fat distribution in obese subjects. J Atheroscler Thromb. 2011;18:592–5. doi: 10.5551/jat.7625. [DOI] [PubMed] [Google Scholar]
- 21.Varady KA, Tussing L, Bhutani S, Braunschweig CL. Degree of weight loss required to improve adipokine concentrations and decrease fat cell size in severely obese women. Metabolism. 2009;58:1096–101. doi: 10.1016/j.metabol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Pasarica M, Tchoukalova YD, Heilbronn LK, Fang X, Albu JB, Kelley DE, et al. Look AHEAD Adipose Research Group Differential effect of weight loss on adipocyte size subfractions in patients with type 2 diabetes. Obesity (Silver Spring) 2009;17:1976–8. doi: 10.1038/oby.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 24.Goldrick RB. Morphological changes in the adipocyte during fat deposition and mobilization. Am J Physiol. 1967;212:777–82. doi: 10.1152/ajplegacy.1967.212.4.777. [DOI] [PubMed] [Google Scholar]
- 25.Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L, et al. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes. 2003;52:667–74. doi: 10.2337/diabetes.52.3.667. [DOI] [PubMed] [Google Scholar]
- 26.Laurencikiene J, Skurk T, Kulyté A, Hedén P, Aström G, Sjölin E, et al. Regulation of lipolysis in small and large fat cells of the same subject. J Clin Endocrinol Metab. 2011;96:E2045–9. doi: 10.1210/jc.2011-1702. [DOI] [PubMed] [Google Scholar]
- 27.Lundgren M, Svensson M, Lindmark S, Renström F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50:625–33. doi: 10.1007/s00125-006-0572-1. [DOI] [PubMed] [Google Scholar]
- 28.Arioglu E, Duncan-Morin J, Sebring N, Rother KI, Gottlieb N, Lieberman J, et al. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med. 2000;133:263–74. doi: 10.7326/0003-4819-133-4-200008150-00009. [DOI] [PubMed] [Google Scholar]
- 29.Wagstaff AJ, Goa KL. Rosiglitazone: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2002;62:1805–37. doi: 10.2165/00003495-200262120-00007. [DOI] [PubMed] [Google Scholar]
- 30.Caso G, Mileva I, McNurlan MA, Mynarcik DC, Darras F, Gelato MC. Effect of ritonavir and atazanavir on human subcutaneous preadipocyte proliferation and differentiation. Antiviral Res. 2010;86:137–43. doi: 10.1016/j.antiviral.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87:2395. doi: 10.1210/jc.87.5.2395. [DOI] [PubMed] [Google Scholar]
- 32.Garg A. Adipose tissue dysfunction in obesity and lipodystrophy. Clin Cornerstone. 2006;8(Suppl 4):S7–13. doi: 10.1016/S1098-3597(06)80039-6. [DOI] [PubMed] [Google Scholar]
- 33.Björntorp P, Bengtsson C, Blohmé G, Jonsson A, Sjöström L, Tibblin E, et al. Adipose tissue fat cell size and number in relation to metabolism in randomly selected middle-aged men and women. Metabolism. 1971;20:927–35. doi: 10.1016/0026-0495(71)90013-8. [DOI] [PubMed] [Google Scholar]
- 34.Arner P, Arner E, Hammarstedt A, Smith U. Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS One. 2011;6:e18284. doi: 10.1371/journal.pone.0018284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salans LB, Knittle JL, Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968;47:153–65. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blüher M, Wilson-Fritch L, Leszyk J, Laustsen PG, Corvera S, Kahn CR. Role of insulin action and cell size on protein expression patterns in adipocytes. J Biol Chem. 2004;279:31902–9. doi: 10.1074/jbc.M404570200. [DOI] [PubMed] [Google Scholar]
- 37.Hoffstedt J, Arvidsson E, Sjölin E, Wåhlén K, Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J Clin Endocrinol Metab. 2004;89:1391–6. doi: 10.1210/jc.2003-031458. [DOI] [PubMed] [Google Scholar]
- 38.Bahceci M, Gokalp D, Bahceci S, Tuzcu A, Atmaca S, Arikan S. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest. 2007;30:210–4. doi: 10.1007/BF03347427. [DOI] [PubMed] [Google Scholar]
- 39.Florant GL, Porst H, Peiffer A, Hudachek SF, Pittman C, Summers SA, et al. Fat-cell mass, serum leptin and adiponectin changes during weight gain and loss in yellow-bellied marmots (Marmota flaviventris) J Comp Physiol B. 2004;174:633–9. doi: 10.1007/s00360-004-0454-0. [DOI] [PubMed] [Google Scholar]
- 40.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–62. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 41.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 42.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 43.Perrini S, Laviola L, Cignarelli A, Melchiorre M, De Stefano F, Caccioppoli C, et al. Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia. 2008;51:155–64. doi: 10.1007/s00125-007-0841-7. [DOI] [PubMed] [Google Scholar]
- 44.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–37. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]