Abstract
The cells of the innate and adaptive immune systems have been implicated in the development of obesity-induced metaflammation and metabolic disorders including type 2 diabetes. Galectin-3, a β-galactoside-binding lectin, modulates immune/inflammatory responses and specifically binds to advanced glycation end products (AGE), modified lipoproteins, and endotoxin. In the recently published study we demonstrate proinflammatory changes in the visceral adipose tissue and pancreatic islets in galectin-3-deficient mice fed high-fat diet which also exhibited excess adiposity, hyperglycemia, insulin resistance and systemic inflammation compared with their diet matched wild-type controls. This was associated with the increased incidence of Type-1 T and NKT cells and pro-inflammatory CD11c+CD11b+ macrophages in the visceral adipose tissue. Severe insulitis, infiltration of macrophages expressing NLRP3 inflammasome and IL-1β, and enhanced accumulation of AGE were present within the pancreatic islets in obese LGALS3−/− mice. Moreover, increased caspase-1 dependent IL-1β secretion with increased expression of NLRP3 inflammasome and phospho-NFκBp65 were observed in LGALS3−/− peritoneal macrophages stimulated in vitro by lipopolysaccharide and/or saturated fatty acid palmitate. The amplified high-fat diet-induced obesity and hyperglycemia and exacerbated inflammation in adipose tissue and pancreatic islets in LGALS3−/− mice suggest an important role for galectin-3 in the regulation of adiposity, metaflammation and type 2 diabetes.
Keywords: IL-1β, NFκB, NLRP3 inflammasome, galectin-3, inflammation, insulin resistance, obesity, type 2 diabetes
Obesity and its strong association with insulin resistance and type 2 diabetes have initiated the investigations of the underlying mechanisms of these disorders. Obesity itself leads to an inflammatory response, termed metaflammation, in metabolic tissues including adipose tissue, pancreatic islets, liver, muscle, and brain. Metaflammation is a low-grade, chronic inflammatory response that is induced by excess nutrients and often leads to the development of insulin resistance and metabolic disorders.1 The pathophysiology, inflammatory triggers, and molecular pathways that associate metaflammation, diet, and type 2 diabetes are incompletely understood. It has been postulated that the degree of adiposity, the nature of immune/inflammatory response, and the composition of gut microbiota are the most important factors associated with the obesity-related pathologies.2
Obesity-Induced Inflammation in Adipose Tissue
Obesity is associated with the increased infiltration of immune cells into the adipose tissue and enhanced tissue expression of pro-inflammatory cytokines. Although the signals involved in the activation and attraction of immune cells into metabolic tissues are not fully elucidated, it is believed that adipocytes initiate inflammation in response to metabolic triggers related to excess nutritients.3 Obese adipose tissue infiltration with type 1 T helper lymphocytes and NKT cells, and classically activated M1 macrophages with decreased immunosuppressive regulatory T cells (Tregs) precedes metabolic dysfunctions.4-8 In obesity, increased expression of TNF-α, IL-1β, and IL-6 in adipose tissue, but also liver, pancreas, and brain mediate the development of insulin resistance.9 The protective roles on the instigation of nutrient excess-induced inflammation have been attributed to adipose tissue-associated tolerogenic Tregs, type 2 T helper cells, and alternatively activated M2 macrophages10,11 and anti-inflammatory IL-10.12 Besides the roles for Th1/Th2 cells in maintaining metabolic homeostasis, the most recent data demonstrate the direct role of IL-13/STAT3 pathway in glucose metabolism, which might be the novel therapeutic approach in the treatment of insulin resistance and type 2 diabetes.13 Toll-like receptor (TLR) and Nod-like receptor (NLR) family of pattern recognition receptors (PRRs) sense obesity-related metabolic “danger” molecules.14,15 Of these PRRs, TLR4 receptor can be activated by high glucose and free fatty acids (FFAs) to activate NFκB family of transcription factors that upregulate the expression of pro-inflammatory genes, which is the critical molecular mechanism in the development of insulin resistance.16,17 In macrophages, NLR activation by obesity-induced signals stimulates NLRP3 inflammasome that consists of NLRP3 molecules, adaptor protein ASC and procaspase-1 that catalytically activates caspase-1 causing the release of IL-1β and IL-18. This pro-inflammatory pathway has been recently shown to have a crucial role in obesity-induced inflammation and insulin resistance.18 Important roles and increased activation of the intracellular kinases c-jun N-terminal kinase (JNK), inhibitor of κ kinase (IKK), and more recently protein kinase R (PKR) in the induction of metaflammation have been demonstrated in adipose tissue and liver in obese animals.19,20 Taken together, multiple types of immune cells and signaling pathways may operate in diet-induced metaflammation in adipose tissue.
Obesity-Induced Inflammation in Pancreatic Islets
The pancreas has a central role in glucose homeostasis by insulin and glucagon production. Obesity-induced inflammation in pancreatic islets in type 2 diabetes leads to reduced insulin secretion and β cells apoptosis during diabetes progression.21 Insulin resistance often precedes the development of type 2 diabetes where the islets first enhance their insulin secretion and the subsequent failure of β cells to compensate impaired insulin sensitivity results in insulin deficiency. High-fat diet induces accumulation of macrophages in islets which express IL-1β that could activate NFκB pathway and promote apoptosis of β cells. The factors that could instigate an inflammatory response within pancreatic islets in the context of obesity and overnutrition are cellular stress mechanisms such as lipotoxicity and glucotoxicity, oxidative stress, endoplasmic reticulum stress, amyloid deposition in the pancreas, and perturbed autophagy.22
Galectin-3
Galectins are a family of 15 animal lectins that bind β-galactoside by conserved carbohydrate-recognition domains (CRD) and also interact with other ligands. Galectins are located intracellularly, on the cell surface and in the extracellular spaces and exert a variety of cellular functions important in adipogenesis and atherosclerosis including cell-extracellular matrix interactions, cell activation and growth, and apoptosis.23 While the majority of galectins are anti-inflammatory, galectin-3, appears to have both pro- and anti-inflammatory roles depending on the cell type, cellular localization and pathophysiological condition. Galectin-3 deficiency results in the suppressed inflammation in models of peritonitis,24 acetaminophen induced hepatotoxicity associated with increased activation of alternative macrophages,25 autoimmune/inflammatory diseases,26-28 and malignancy29 (Table 1). On the other hand, LGALS3−/− mice have exacerbated sensitivity to endotoxin.30 Moreover, Gal-3 ablation led to accelerated diabetes-associated kidney damage and enhanced diet-induced atherogenesis.31,32 These effects in Gal-3 deficiency may be attributed to the ability of Gal-3 to act as a scavenger molecule for glucose adducts which are elevated in animals on lipid-rich diet. Advanced glycation end products (AGEs) and the receptor for AGEs (RAGE) have been linked to the pathogenesis of diabetic complications and also in enhanced apoptosis and dysfunction of pancreatic β cells. Gal-3 protects β cells from the cytotoxic effect of IL-1β in rats and its increased expression in islet endothelial cells in obesity-induced diabetes in mice was demonstrated.33,34 In agreement, the elevated circulating levels of Gal-3 in patients with type 2 diabetes positively correlate with body mass index and negatively with glycated hemoglobin (HbA1c), suggesting a possible protective role for Gal-3 in deregulated glycemia. Gal-3 deficiency in models of hepatic steatosis/inflammation are either protective or increases disease severity.35,36 Gal-3 is expressed by both adipocytes and adipose tissue macrophages and its serum levels are elevated during obesity in both humans and experimental animals. LGALS3−/− mice have increased hepatic expression of peroxisome proliferator-activated receptor γ (PPARγ), suggesting the participation of Gal-3 in the regulation of fatty acid and glucose metabolism in the liver37 (Table 2).
Table 1. Immunoregulatory role of galectin-3 in autoimmune, inflammatory, and malignant diseases.
| Models | Strategies used | Disease outcome | Mechanisms | References |
|---|---|---|---|---|
| EAE |
Gal-3-deficient mice immunization with myelin oligodendrocyte glycoprotein (MOG)35–55 peptide |
Decreased severity |
Reduced leukocyte infiltration and higher frequency of Foxp3+ T regs in the CNS Decreased IFN-γ and IL-17 production by LN cells Higher serum levels of IL-10, IL-5, and IL-13 |
26 |
| Type 1 diabetes |
Diabetes induction by multiple low doses of streptozotocin in Gal-3-deficient mice |
Decreased severity |
Decreased mononuclear cell infiltration in pancreatic islets Absence of TNF-α and IL-17 in draining pancreatic LN Decreased TNF-α and NO production by macrophages |
27 |
| Experimental hepatitis |
Con A-induced hepatitis in Gal-3-deficient mice |
Decreased severity |
Decreased influx of mononuclear cells and CD4+ cells expressing proinflammatory cytokines, while increased IL-10 expressing CD4+ T cells and F4/80+ macrophages in liver |
28 |
| Con A-induced hepatitis in mice pre-treated with Gal-3 inhibitor TD139 |
Decreased severity |
Decreased influx of mononuclear cells and CD4+ cell expressing proinflammatory cytokines, while increased IL-10 expressing CD4+ T cells and F4/80+ macrophages in liver |
||
| Murine melanoma | Intravenous inoculation of B16F1 malignant melanoma in Gal-3-deficient mice | Resistance to lung metastases | Decreased adhesion of B16F1 cells to lung tissue Increased NK-cell cytotoxicity to B16F1 cells in vitro Higher percentage of effective cytotoxic CD27highCD11bhigh, and reduced NK cells expressing inhibitory KLRG1 receptor in spleens |
29 |
Table 2. Immunoregulatory effects of galectin-3 in lipid-rich diet-induced metabolic disorders.
| Models | Strategies used | Disease outcome | Mechanisms | References | |
|---|---|---|---|---|---|
| Obesity |
Diet-induced obesity in ob/ob mice |
Increased expression of Gal-3 in VAT and SAT |
Age and diet modulation of galectins expression |
37 |
|
| Type 2 diabetes |
High-fat diet-induced obesity in Gal-3-deficient mice |
Accelerated obesity and type 2 diabetes |
Enhanced inflammation in adipose tissue and pancreatic islets |
38 |
|
| High-fat diet-induced obesity in Gal-3-deficient mice |
Increased adiposity, hyperglycemia, systemic inflammation |
Reduced adipose tissue expression of adiponectin, Gal-12, ATLG and PPRγ, the role of gut microbiota |
44 |
||
| Diabetic glomerulopathy |
Injection of CML-MSA in Gal-3-deficient mice |
Increased severity |
Higher circulating AGE, increased expression of AGE receptor |
31 |
|
| Non-alcoholic steatohepatitis |
High-fat diet-induced NASH in Gal-3-deficient mice |
Decreased severity |
Scavenging role of Gal-3 |
35 |
|
| Age-related NASH in Gal-3-deficient mice |
Increased severity |
Increased hepatic protein levels of AGE, RAGE, PPARγ |
36 |
||
| Atherosclerosis | High-cholesterol diet-induced atherosclerosis in Gal-3-deficient mice |
Increased severity |
Th1 cells, increased ox-LDL and upregulation of RAGE in atheroma |
32 |
|
| High-cholesterol diet-induced atherosclerosis in Gal-3-ApoE-deficient mice | Decreased severity | Reduced lipid core and collagen in atheroma, low iNOS | 46 | ||
The Role of Gal-3 in Obesity-Related Metabolic Disorders
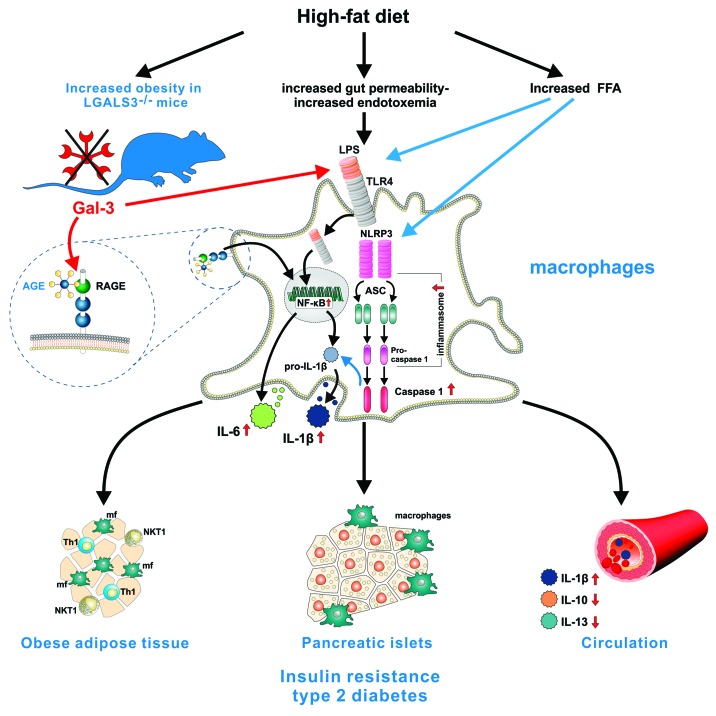
The role of Gal-3 in obesity-related metabolic disorders has not been fully clarified. We used LGALS3−/− mice to investigate the role of this galectin in the model of high fat diet (HFD)-induced obesity. Our work identifies Gal-3 as an important regulator of metaflammation.38 Namely, LGALS3−/− mice had enhanced adiposity, hyperglycemia, hyperinsulinemia, and insulin resistance in comparison with their diet-matched wild-type controls. LGALS3+/+ and LGALS3−/− mice exhibited different growth curves when they were fed a high-fat diet for up to 18 weeks, the body weights and visceral fat weights were significantly higher in Gal-3-deficient mice, while the food intake was indistinguishable between the two genotypes. Visceral adipose tissue of obese LGALS3−/− mice was infiltrated with Type 1 CD3+ T and CD3+NK1.1+ NKT lymphocytes expressing IFN-γ, while Tregs were reduced. The ongoing obesity-induced inflammation was most likely due to facilitated production of IFN-γ following T-cell receptor engagement in Gal-3-deficient T/NKT cells.39 The greater presence of proinflammatory F4/80+CD11c+CD206+ macrophages and F4/80+CD11b+CD11c+ bone-marrow-derived cells was found in adipose tissue of obese LGALS3−/− mice, with marked reduction of the alternatively activated M2 macrophages, the findings that are consistent with the role of Gal-3 in promoting the M2 macrophages polarization.40 Inflammation in pancreatic islets in obese LGALS3−/− mice was reflected by the presence of severe insulitis, which was also observed in lean LGALS3−/− mice, the findings that suggest an important role for Gal-3 in islets homeostasis during obesity. Moreover, increased accumulation of macrophages that expressed NLRP3 inflammasome and IL-1β in the visceral adipose tissue and pancreas was found in obese LGALS3−/− mice, with the similar trend in islets in lean LGALS3−/− mice. LGALS3−/− islets in obese animals had increased deposition of advanced glycation end products (AGE) and receptor for AGE (RAGE) expression. Type 2 diabetes is currently considered as an auto-inflammatory disease with the central role for NLRP3-ASC inflammasome-mediated IL-1β production.41 We demonstrate the trend toward increased NLRP3 inflammasome and ASC proteins in pancreatic and visceral adipose whole tissue lysates in obese LGALS3−/− mice and significantly increased mature caspase-1 protein expression. LGALS3−/− peritoneal macrophages produced larger amounts of caspase-1-dependent IL-1β, had increased NLRP3 inflammasome expression and caspase-1 activity in response to in vitro stimulation with lipopolysaccharide (LPS) and/or saturated fatty acid palmitate compared with wild-type cells. In addition, silencing of NLRP3 inflammasome by siRNA attenuated IL-1β production by LGALS3−/− macrophages, the results that indicate the enhanced NLRP3 inflammasome activation in Gal-3-deficient cells. TLR4 agonists including LPS might increase NLRP3 inflammasome expression in murine macrophages in a NFκB-dependent manner.42 We show that LGALS3−/− macrophages stimulated by LPS in vitro produced increased amounts of IL-1β and IL-6, due to the known ability of Gal-3 to bind LPS thus preventing its activity30 and exhibited increased expression of phospho-NFκBp65. High-fat diet strongly increases intestinal permeability and endotoxemia.43 Thus, elevated levels of endotoxin and free fatty acids could increase phospho-NFκBp65 and NLRP3 inflammasome expression in macrophages in obese LGALS3−/− mice. Additionally, AGE, through binding to RAGE could activate NFκB signaling pathway and increase IL-1β and TNF-α production. We show increased pancreatic and visceral adipose tissue phospho-NFκBp65 expression, which could be related to increased accumulation of AGE and upregulated RAGE in obese LGALS3−/− mice. These findings indicate that NFκB-mediated pro-inflammatory pathway operates in enhanced metaflammation observed in LGALS3−/− mice. Similar findings of Gal-3 as an important modulator of adiposity and metaflammation are provided in the most recent study by Pang et al.44 This study demonstrates the development of excess adiposity and systemic inflammation in Gal-3 deficiency that was associated with impaired fasting glucose levels and reduced adipose tissue expression of adiponectin, Gal-12, ATGL, and PPARγ. Also, the role for the gut microflora in mediating the fasting hyperglycemia in Gal-3-deficient mice was revealed by the administration of antibiotics in this study. In our study we show that obese LGALS3−/− mice had increased systemic inflammation, having elevated serum levels of IL-6 and IL-1β. Low serum levels of IL-13 and IL-10 in obese LGALS3−/− mice might contribute to amplified obesity-induced inflammation. At the same time, LGALS3−/− mice fed low-fat diet for 18 weeks had significantly increased serum levels of IL-1β, while decreased IL-6 and IL-10, indicating the important influence of Gal-3 deficiency on systemic IL-1β levels. Collectively, the amplified obesity-induced inflammation in adipose tissue and pancreatic islets in LGALS3−/− mice suggest a protective role for Gal-3 in obesity and type 2 diabetes which could be of therapeutic relevance (Fig. 1).
Figure 1. Gal-3 is an important regulator of adiposity, metaflammation, and type 2 diabetes. Fat-enriched diets increase plasma concentration of endotoxin (metabolic endotoxemia) and saturated fatty acids such as palmitate, that stress macrophages in metabolic tissues including adipose tissue and pancreatic islets leading to increased production of inflammatory cytokines IL-1β, TNF-α, IL-6, and chemokines which further attract immune cells into tissues and promote systemic inflammation. Gal-3 binds endotoxin and acts as a scavenger molecule for advanced glycation and lipoxidation end products. TLR4, the receptor for lipopolysaccharide (LPS), mediates the innate immune response to bacterial pathogens, but also triggers inflammatory processes upon exposure to certain dietary fats by activating NFκB pathway and NLRP3 inflammasome in macrophages leading to IL-1β secretion that causes insulin resistance. Ablation of Gal-3 leads to accelerated obesity and systemic inflammation, enhanced inflammation in adipose tissue and pancreatic islets, as indicated by increased type-1 T and NKT cells and pro-inflammatory macrophages in visceral adipose tissue and NLRP3 inflammasome and IL-1β expressing macrophages in islets in high-fat diet-fed mice. This is associated with the increased activation of NFκB signaling pathway through binding of AGE to upregulated receptor for AGE (RAGE) and activation of NLRP3 inflammasome by LPS and/or palmitate resulting in increased caspase-1-dependent IL-1β production and IL-6 by LGALS3−/− macrophages. The increase in circulating IL-1β and the decrease of anti-inflammatory IL-10 and IL-13 contribute to the development of insulin resistance and type 2 diabetes in Gal-3-deficient mice.
Future Perspectives
We anticipate that this study will lead to further investigation of the role of galectin-3 in the regulation of intracellular signaling that controls many important cellular processes, including energy metabolism. Future studies will help to elucidate the role of galectin-3 in other metabolic tissues in the course of diet-induced obesity or aging. In particular, elucidating the mechanisms of the protective role of galectin-3 in the pancreatic islets in the course of obesity would be of great importance. Further studies would address the issues relevant for glucose and lipid metabolism such as the role of Gal-3 in the crosstalk between the NLRP3 inflammasome and autophagy that critically mediates cytoplasmic receptor NLRP3-dependent activation of the inflammasome by the saturated fatty acids contained in a high-fat diet. Also, the role of galectin-3 in functioning of brown adipose tissue, most recently shown to have beneficial effects in glucose metabolism and obesity,45 would be of importance. Finally, the mechanisms underlying the development of the innate lymphoid cells (ILC) subsets and their in vivo functions in Gal-3 deficiency would contribute to further elucidation of the role of these cells in obesity-related metabolic disorders.
Acknowledgments
We would like to thank Milan Milojevic for technical assistance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/24881
References
- 1.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–74. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 2.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 3.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 2010;18:1918–25. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–9. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 6.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 9.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389:610–4. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 10.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107:9765–70. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Yu TY, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–35. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, et al. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest. 2013;123:261–71. doi: 10.1172/JCI64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739–45. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 15.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 16.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-kappaB. Nat Med. 2005;11:183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–25. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–9. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solinas G, Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596–611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- 20.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 21.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1β in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–21. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 23.Liu F-T, Rabinovich GA. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010;1183:158–82. doi: 10.1111/j.1749-6632.2009.05131.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsu DK, Yang RY, Pan Z, Yu L, Salomon DR, Fung-Leung WP, et al. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am J Pathol. 2000;156:1073–83. doi: 10.1016/S0002-9440(10)64975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dragomir AC, Sun R, Choi H, Laskin JD, Laskin DL. Role of galectin-3 in classical and alternative macrophage activation in the liver following acetaminophen intoxication. J Immunol. 2012;189:5934–41. doi: 10.4049/jimmunol.1201851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H-R, Al Rasebi Z, Mensah-Brown E, Shahin A, Xu D, Goodyear CS, et al. Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J Immunol. 2009;182:1167–73. doi: 10.4049/jimmunol.182.2.1167. [DOI] [PubMed] [Google Scholar]
- 27.Mensah-Brown EP, Al Rabesi Z, Shahin A, Al Shamsi M, Arsenijevic N, Hsu DK, et al. Targeted disruption of the galectin-3 gene results in decreased susceptibility to multiple low dose streptozotocin-induced diabetes in mice. Clin Immunol. 2009;130:83–8. doi: 10.1016/j.clim.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Volarevic V, Milovanovic M, Ljujic B, Pejnovic N, Arsenijevic N, Nilsson U, et al. Galectin-3 deficiency prevents concanavalin A-induced hepatitis in mice. Hepatology. 2012;55:1954–64. doi: 10.1002/hep.25542. [DOI] [PubMed] [Google Scholar]
- 29.Radosavljevic G, Jovanovic I, Majstorovic I, Mitrovic M, Lisnic VJ, Arsenijevic N, et al. Deletion of galectin-3 in the host attenuates metastasis of murine melanoma by modulating tumor adhesion and NK cell activity. Clin Exp Metastasis. 2011;28:451–62. doi: 10.1007/s10585-011-9383-y. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Komai-Koma M, Gilchrist DS, Hsu DK, Liu F-T, Springall T, et al. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J Immunol. 2008;181:2781–9. doi: 10.4049/jimmunol.181.4.2781. [DOI] [PubMed] [Google Scholar]
- 31.Pugliese G, Pricci F, Iacobini C, Leto G, Amadio L, Barsotti P, et al. Accelerated diabetic glomerulopathy in galectin-3/AGE receptor 3 knockout mice. FASEB J. 2001;15:2471–9. doi: 10.1096/fj.01-0006com. [DOI] [PubMed] [Google Scholar]
- 32.Iacobini C, Menini S, Ricci C, Scipioni A, Sansoni V, Cordone S, et al. Accelerated lipid-induced atherogenesis in galectin-3-deficient mice: role of lipoxidation via receptor-mediated mechanisms. Arterioscler Thromb Vasc Biol. 2009;29:831–6. doi: 10.1161/ATVBAHA.109.186791. [DOI] [PubMed] [Google Scholar]
- 33.Karlsen AE, Størling ZM, Sparre T, Larsen MR, Mahmood A, Størling J, et al. Immune-mediated beta-cell destruction in vitro and in vivo-A pivotal role for galectin-3. Biochem Biophys Res Commun. 2006;344:406–15. doi: 10.1016/j.bbrc.2006.03.105. [DOI] [PubMed] [Google Scholar]
- 34.Darrow AL, Shohet RV, Maresh JG. Transcriptional analysis of the endothelial response to diabetes reveals a role for galectin-3. Physiol Genomics. 2011;43:1144–52. doi: 10.1152/physiolgenomics.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iacobini C, Menini S, Ricci C, Blasetti Fantauzzi C, Scipioni A, Salvi L, et al. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J Hepatol. 2011;54:975–83. doi: 10.1016/j.jhep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Nomoto K, Tsuneyama K, Abdel Aziz HO, Takahashi H, Murai Y, Cui ZG, et al. Disrupted galectin-3 causes non-alcoholic fatty liver disease in male mice. J Pathol. 2006;210:469–77. doi: 10.1002/path.2065. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes DH, Pini M, Castellanos KJ, Montero-Melendez T, Cooper D, Perretti M, et al. Adipose tissue specific modulation of galectin expression in lean and obese mice: Evidence for regulatory function. Obesity (Silver Spring) 2013;21:310–9. doi: 10.1002/oby.20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pejnovic N, Pantic J, Jovanovic I, Radosavljevic G, Milovanovic M, Nikolic I, et al. Galectin-3 deficiency accelerates high-fat diet induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes. 2013;62:1932–44. doi: 10.2337/db12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen HY, Fermin A, Vardhana S, Weng IC, Lo KF, Chang EY, et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci U S A. 2009;106:14496–501. doi: 10.1073/pnas.0903497106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, et al. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–8. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 41.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiao Y, Wang P, Qi J, Zhang L, Gao C. TLR-induced NF-κB activation regulates NLRP3 expression in murine macrophages. FEBS Lett. 2012;586:1022–6. doi: 10.1016/j.febslet.2012.02.045. [DOI] [PubMed] [Google Scholar]
- 43.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 44.Pang J, Rhodes DH, Pini M, Akasheh RT, Castellanos KJ, Cabay RJ, et al. Increased adiposity, dysregulated glucose metabolism and systemic inflammation in Galectin-3 KO mice. PLoS One. 2013;8:e57915. doi: 10.1371/journal.pone.0057915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–23. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackinnon AC, Liu X, Hadoke PW, Miller MR, Newby DE, Sethi T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology. 2013;23:654–63. doi: 10.1093/glycob/cwt006. [DOI] [PMC free article] [PubMed] [Google Scholar]