Abstract
Obesity occurs when an excessive dietary fat intake leads to expansion of adipose tissue, which mainly consists of adipocytes that arise from proliferating and differentiating adipose stem cells, the preadipocytes. Obesity is a consequence of both adipocyte hypertrophy and hyperplasia. Knowledge about preadipocyte differentiation is relatively well established, whereas the mechanism responsible for preadipocyte proliferation is incompletely understood and only in the early stage of comprehension. In this regard, we have recently identified that Delta-like 1 homolog (Dlk1) (also known as Preadipocyte factor 1 [Pref-1]) inhibits preadipocyte proliferation by regulating their entry into G1/S-phase. This novel disclosure, adding to the previous published data on Dlk1 repression of preadipocyte differentiation, has given us the chance to firmly place Dlk1 as a master regulator of preadipocyte homeostasis and adipose tissue expansion. Dlk1 manipulation may, therefore, open new perspectives in obesity treatments.
Keywords: DLK1, preadipocyte proliferation, preadipocyte differentiation, adipose tissue expansion, obesity treatment
Obesity is one of the world’s fastest growing health hazards and among the leading risks for deaths globally. WHO estimates that in 2008, more than 1.4 billion adults were overweight and obesity rates among developed countries have increased substantially during the past 3 decades. The consequences of excess body weight are numerous and include type 2 diabetes, hypertension, coronary artery disease, and many types of cancer.1-5 With regard to this vast array of health implications, the need to develop new effective strategies for controlling obesity has become more and more crucial.
Obesity can be defined as the excessive accumulation of adipose tissue caused by a chronic imbalance between energy intake and energy expenditure.6 On the cellular level, adipose tissue enlargement has previously been suggested to occur merely as a result of adipocyte hypertrophy.7-9 However, by analyzing the integration of 14C derived from nuclear bomb tests in genomic DNA, Spalding et al. elegantly measured adipocyte turnover in humans, and found that adipocyte number is a major determinant of fat mass in adults.10 The number of mature adipocytes stays constant in adulthood in lean and obese individuals, even after marked weight loss, suggesting that the number of adipocytes is set during the childhood and adolescence.7 Similar results have been demonstrated in mice.8 Since the progenitor cells are constantly renewing depending on mechanisms of both proliferation and differentiation, it seems likely that a mechanism such as adipocyte apoptosis negatively balances the number of mature adipocytes. Yet the knowledge about adipose tissue apoptosis is still scarce. Nonetheless, it now seems well documented that obesity is a consequence of both adipocyte hypertrophy and the number of adipocytes.
Adipocytes originate from proliferating and differentiating adipose stem cells, the preadipocytes, located in the stromal vascular fraction (SVF) of adipose tissue. Whereas much is known for preadipocyte differentiation, the mechanisms regulating preadipocyte proliferation are poorly understood.11 In this regard, we have recently identified that Delta-like 1 homolog (Dlk1) (also known as Preadipocyte factor 1 [Pref-1]) inhibits preadipocyte proliferation by regulating their entry into the G1/S-phase of the cell cycle.12 Since Dlk1 has long been considered an important repressor of preadipocyte differentiation,13 this dual inhibitory function on preadipocytes places Dlk1 as a master regulator of preadipocyte homeostasis and adipose tissue expansion (Fig. 1). In this commentary, we therefore highlight our recent findings, and sum up on the dual inhibitory function of Dlk1 in preadipocytes and adipogenesis, knowledge that may not only allow us to understand better the cellular and molecular basis of adipose tissue growth in physiological and pathophysiological states, but also may provide means to develop therapeutic strategies for the treatment and prevention of obesity.
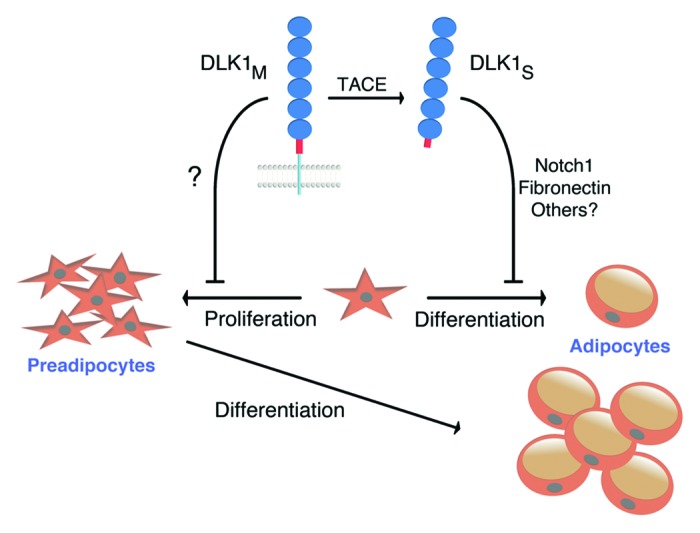
Figure 1. Schematic figure of DLK1’s dual function in fat. Whereas the soluble form of DLK1 inhibits preadipocyte differentiation, preadipocyte proliferation is repressed by the membrane tethered DLK1.
Delta-like 1 homolog (Dlk1) is a paternally expressed imprinted gene, encoding a transmembrane glycoprotein with epidermal growth factor (EGF)-like repeats in its extracellular domain.14 The membrane tethered DLK1 can be cleaved by tumor necrosis α converting enzyme (TACE) at an extracellular juxtamembrane protease recognition site, generating a biologically active soluble form.15 In the mouse, several isoforms, possessing the protease recognition site or not, are generated through alternative splicing of the Dlk1 gene.16 In humans, however, only one cleavable and one non-cleavable isoform is present.17 The Dlk1 gene is highly expressed during embryonic development and Dlk1 has been shown to be involved in the differentiation of various tissue types.18-21 Postnatally, however, Dlk1 expression is downregulated and becomes restricted to cells of neuroendocrine origin and preadipocytes or preadipocyte precursors found within the stromal vascular fraction (SVF) of adipose tissue.11,22,23 It is well established that Dlk1 acts as an inhibitor of in vitro preadipocyte differentiation. Dlk1 is highly expressed in proliferating preadipocytes, but its expression is abolished upon adipogenic differentiation.24 Several studies have demonstrated that overexpression of Dlk1 in preadipocytes, as well as treatment with soluble DLK1 inhibits preadipocyte differentiation into adipocytes.14,25,26 On the other hand, adipocyte differentiation is enhanced when Dlk1 levels are reduced.27 Likewise, Dlk1 is known to inhibit adipogenesis in vivo. Through targeted disruption of the Dlk1 gene, Moon et al. have demonstrated increased adiposity in mice lacking Dlk1. They reported that the increased fat mass in Dlk1-null mice was the result of both enhanced adipocyte differentiation and fat cell maturation, thus reflecting adipocyte hypertrophy rather than hyperplasia.28 In agreement, mice overexpressing the large soluble form of DLK1 in adipose tissue display decreased adiposity due to adipocyte hypotrophy.29
Although DLK1 is a member of the EGF-like homeotic family of proteins that include the NOTCH receptors and their ligands, DLK1 differs from the canonical NOTCH ligands by lacking the DSL (Delta/Serrate/LAG-2) domain, involved in receptor binding.30 Nevertheless, Dlk1 has been suggested to modulate Notch signaling through the DOS (Delta and OSM-11) domain, found in classical and putative Notch ligands.31 The molecular mechanism by which Dlk1 regulates adipocyte differentiation is controversial and an interaction partner/receptor has yet to be identified with convincing biological and functional evidence. Still, DLK1 has been proposed to inhibit adipocyte differentiation through interaction with either NOTCH1 or FIBRONECTIN (Fig. 1).32,33 However, the mechanism of DLK1 action is debated, and several other interaction partner(s) have been proposed as well.19,34-38
Likewise, with some controversies, the inhibitory effect of Dlk1 on adipogenesis is ascribed to occur only by the large soluble form. Mei et al. showed that only the large soluble form is active and sufficient to inhibit adipocyte differentiation, and that neither the small soluble form nor the membrane attached form of DLK1 affects adipogenesis.25 In disagreement, Garces et al. have suggested that membrane and secreted DLK1 protein variants likely play opposite roles in the control of adipogenesis.24
Our recent study12 aimed to take a step forward regarding the effect of Dlk1 on preadipocyte proliferation in vitro and in vivo, specifically studying whether differential roles apply for the large membrane tethered and soluble DLK1 isoforms in this process.
To accomplish this, we used a novel approach specifically reducing the endogenous level of different DLK1 variants in proliferating preadipocytes that naturally express the protein. We thus designed two different siRNAs, one specifically targeting the protease encoding site, only present in mRNA encoding for the cleavable DLK1 isoform, and another siRNA targeting a mRNA sequence common to all Dlk1 mRNA variants. By use of this strategy, treated preadipocytes expressed differential levels of the membrane and soluble DLK1 isoforms. Global expression profiling revealed substantial differential regulation of 4 cell cycle-signaling pathways by cleaved and non-cleaved Dlk1 isoforms. These data thus supports the idea, that cleavable and non-cleavable DLK1 isoforms act differently.24,25 Our data clearly showed that numerous genes in the cell cycle are affected at different time points in the same direction of having more preadipocyte proliferation, when specifically the level of membrane bound DLK1 is reduced. This was confirmed functionally, by a substantial increase in proliferation rate of the preadipocytes in vitro. We did not check adipogenic differentiation of the preadipocytes expressing different membrane and soluble DLK1 isoforms levels. Yet, since initiation of adipogenic differentiation depends on preadipocyte density, it seems likely that adipogenic differentiation will be enhanced in preadipocytes with low levels of membrane DLK1 as these cells will be higher in numbers.
Our study also revealed that in vivo preadipocyte proliferation is enhanced in Dlk1−/− mice. We unraveled this by use of EdU (5-ethynyl-2′-deoxyuridine), which is a novel alternative method to detect proliferating cells compared with conventional BrdU (5-bromo-2′-deoxyuridine) assays.39 The identity of in vivo preadipocytes is still unknown,11 but they have been demonstrated to be contained within the stromal vascular fraction (SVF). We have unpublished data (Fig. 2) showing that proliferating cells within developing adipose tissue mainly reside in proximity with the vasculature and clearly enriched in the adipose tissue mesenchyme (Fig. 2). We found that the SVF derived from Dlk1−/− mice comprised around 7.0% proliferating cells whereas 3.7% was seen in Dlk1+/+ SVF cells. In relation to this, Spalding et al. reported that 10% of adipocytes renew annually at adult ages and levels of body mass index. Neither adipocyte death nor generation time is altered in early onset obesity, suggesting a tight regulation of fat cell number in this condition during adulthood.7 Therefore, pathological disorders may indeed increase adipocyte renewal substantially resulting in obesity. By targeting the unknown “ligand/receptor” for DLK1 in the fat by specific mimicking drugs, we may thus slow down excess adipocyte renewal and inhibit obesity. Such a drug may be specific for either the soluble or the membrane DLK1 or both depending on whether hypertrophy or hyperplasia induced obesity is present. Yet, to obtain a suitable drug to fight hyperplasia induced obesity through DLK1 targeting, the mechanism by which DLK1 represses preadipocyte proliferation needs to be clarified. Our results suggest that membrane DLK1 regulates several components in the G1-S phase of the cell cycle, which is in agreement with membrane DLK1’s ability to repress S-phase entry of leukemic cells.40 Our observation, that soluble DLK1 has no effect on preadipocyte proliferation is in line with a previous study showing that DLK1 mutants encoding only the cleavable DLK1 isoform do not have an impact on hematopoietic cell proliferation.40 Thus, although the soluble DLK1 may be the only active isoform directly inhibiting the preadipocyte differentiation step of adipogenesis (Fig. 1),28,29 the membrane tethered DLK1 seems to repress adipose tissue expansion likely at an earlier developmental stage by lowering adipocyte numbers (Fig. 1).
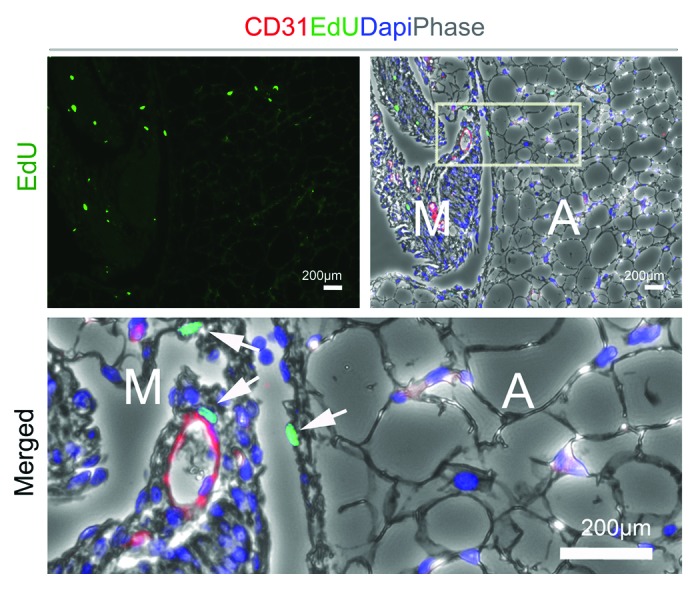
Figure 2. Identification of proliferating cells in developing fat of 6-weeks old C57Bl/6 mice. EdU (5-ethynyl-2′-deoxyuridine) was injected into mice and gonadal fat pads isolated and analyzed one week after. Double fluorescence of EdU, CD31, and Dapi was performed on sectioned fat. Proliferating cells mainly resides within the mesenchyme (M) close to blood vessels rather than in the mature adipose tissue (A).
Yet, several controversies exist on whether both DLK1 forms have an effect on proliferation as well as differentiation of cells in general, and these issues as well as the identification of the DLK1 interaction partner(s) therefore needs to be elucidated in more detail. However, the fact that Dlk1 has a dual role in preadipocytes (Fig. 1) firmly places this gene as a major regulator of adipogenesis, and further insights into Dlk1 signaling mechanism, may yield future means to develop therapeutic strategies for the treatment and prevention of obesity.
Acknowledgments
The authors acknowledge support from the Danish National Research Council (09-073648), Department of Clinical Biochemistry and Pharmacology at Odense University Hospital, University of Southern Denmark (Faculty of Health Science), and Odense University Hospital Research Fund.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/24994
References
- 1.Alpert MA, Lambert CR, Terry BE, Cohen MV, Mukerji V, Massey CV, et al. Influence of left ventricular mass on left ventricular diastolic filling in normotensive morbid obesity. Am Heart J. 1995;130:1068–73. doi: 10.1016/0002-8703(95)90210-4. [DOI] [PubMed] [Google Scholar]
- 2.Kuchta KF. Pathophysiologic changes of obesity. Anesthesiol Clin North America. 2005;23:421–9, vi. doi: 10.1016/j.atc.2005.03.004. [vi.] [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 4.Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–87. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 5.Satia-Abouta J, Patterson RE, Schiller RN, Kristal AR. Energy from fat is associated with obesity in U.S. men: results from the Prostate Cancer Prevention Trial. Prev Med. 2002;34:493–501. doi: 10.1006/pmed.2002.1018. [DOI] [PubMed] [Google Scholar]
- 6.Tataranni PA, Ravussin E. Effect of fat intake on energy balance. Ann N Y Acad Sci. 1997;819:37–43. doi: 10.1111/j.1749-6632.1997.tb51797.x. [DOI] [PubMed] [Google Scholar]
- 7.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–7. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 8.Rigamonti A, Brennand K, Lau F, Cowan CA. Rapid cellular turnover in adipose tissue. PLoS One. 2011;6:e17637. doi: 10.1371/journal.pone.0017637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arner E, Westermark PO, Spalding KL, Britton T, Rydén M, Frisén J, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–9. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–43. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Park KW, Halperin DS, Tontonoz P. Before they were fat: adipocyte progenitors. Cell Metab. 2008;8:454–7. doi: 10.1016/j.cmet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Mortensen SB, Jensen CH, Schneider M, Thomassen M, Kruse TA, Laborda J, et al. Membrane-tethered delta-like 1 homolog (DLK1) restricts adipose tissue size by inhibiting preadipocyte proliferation. Diabetes. 2012;61:2814–22. doi: 10.2337/db12-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 14.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–34. doi: 10.1016/0092-8674(93)90252-L. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Sul HS. Ectodomain shedding of preadipocyte factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol Cell Biol. 2006;26:5421–35. doi: 10.1128/MCB.02437-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smas CM, Green D, Sul HS. Structural characterization and alternate splicing of the gene encoding the preadipocyte EGF-like protein pref-1. Biochemistry. 1994;33:9257–65. doi: 10.1021/bi00197a029. [DOI] [PubMed] [Google Scholar]
- 17.Lee YL, Helman L, Hoffman T, Laborda J. dlk, pG2 and Pref-1 mRNAs encode similar proteins belonging to the EGF-like superfamily. Identification of polymorphic variants of this RNA. Biochim Biophys Acta. 1995;1261:223–32. doi: 10.1016/0167-4781(95)00007-4. [DOI] [PubMed] [Google Scholar]
- 18.Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res. 2004;19:841–52. doi: 10.1359/jbmr.040118. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Qanie D, Jafari A, Taipaleenmaki H, Jensen CH, Säämänen AM, et al. Delta-like 1/fetal antigen-1 (Dlk1/FA1) is a novel regulator of chondrogenic cell differentiation via inhibition of the Akt kinase-dependent pathway. J Biol Chem. 2011;286:32140–9. doi: 10.1074/jbc.M111.230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen CH, Meyer M, Schroder HD, Kliem A, Zimmer J, Teisner B. Neurons in the monoaminergic nuclei of the rat and human central nervous system express FA1/dlk. Neuroreport. 2001;12:3959–63. doi: 10.1097/00001756-200112210-00021. [DOI] [PubMed] [Google Scholar]
- 21.Jensen CH, Jauho EI, Santoni-Rugiu E, Holmskov U, Teisner B, Tygstrup N, et al. Transit-amplifying ductular (oval) cells and their hepatocytic progeny are characterized by a novel and distinctive expression of delta-like protein/preadipocyte factor 1/fetal antigen 1. Am J Pathol. 2004;164:1347–59. doi: 10.1016/S0002-9440(10)63221-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floridon C, Jensen CH, Thorsen P, Nielsen O, Sunde L, Westergaard JG, et al. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66:49–59. doi: 10.1046/j.1432-0436.2000.066001049.x. [DOI] [PubMed] [Google Scholar]
- 23.Andersen DC, Jensen L, Schrøder HD, Jensen CH. “The preadipocyte factor” DLK1 marks adult mouse adipose tissue residing vascular cells that lack in vitro adipogenic differentiation potential. FEBS Lett. 2009;583:2947–53. doi: 10.1016/j.febslet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Garcés C, Ruiz-Hidalgo MJ, Bonvini E, Goldstein J, Laborda J. Adipocyte differentiation is modulated by secreted delta-like (dlk) variants and requires the expression of membrane-associated dlk. Differentiation. 1999;64:103–14. doi: 10.1046/j.1432-0436.1999.6420103.x. [DOI] [PubMed] [Google Scholar]
- 25.Mei B, Zhao L, Chen L, Sul HS. Only the large soluble form of preadipocyte factor-1 (Pref-1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: role of alternative splicing. Biochem J. 2002;364:137–44. doi: 10.1042/bj3640137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smas CM, Chen L, Sul HS. Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol Cell Biol. 1997;17:977–88. doi: 10.1128/mcb.17.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smas CM, Chen L, Zhao L, Latasa MJ, Sul HS. Transcriptional repression of pref-1 by glucocorticoids promotes 3T3-L1 adipocyte differentiation. J Biol Chem. 1999;274:12632–41. doi: 10.1074/jbc.274.18.12632. [DOI] [PubMed] [Google Scholar]
- 28.Moon YS, Smas CM, Lee K, Villena JA, Kim KH, Yun EJ, et al. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol. 2002;22:5585–92. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, et al. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J Clin Invest. 2003;111:453–61. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tax FE, Yeargers JJ, Thomas JH. Sequence of C. elegans lag-2 reveals a cell-signalling domain shared with Delta and Serrate of Drosophila. Nature. 1994;368:150–4. doi: 10.1038/368150a0. [DOI] [PubMed] [Google Scholar]
- 31.Komatsu H, Chao MY, Larkins-Ford J, Corkins ME, Somers GA, Tucey T, et al. OSM-11 facilitates LIN-12 Notch signaling during Caenorhabditis elegans vulval development. PLoS Biol. 2008;6:e196. doi: 10.1371/journal.pbio.0060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baladrón V, Ruiz-Hidalgo MJ, Nueda ML, Díaz-Guerra MJ, García-Ramírez JJ, Bonvini E, et al. dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp Cell Res. 2005;303:343–59. doi: 10.1016/j.yexcr.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Zhao L, Smas C, Sul HS. Pref-1 interacts with fibronectin to inhibit adipocyte differentiation. Mol Cell Biol. 2010;30:3480–92. doi: 10.1128/MCB.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baladrón V, Ruiz-Hidalgo MJ, Bonvini E, Gubina E, Notario V, Laborda J. The EGF-like homeotic protein dlk affects cell growth and interacts with growth-modulating molecules in the yeast two-hybrid system. Biochem Biophys Res Commun. 2002;291:193–204. doi: 10.1006/bbrc.2002.6431. [DOI] [PubMed] [Google Scholar]
- 35.Baladrón V, Ruiz-Hidalgo MJ, Gubina E, Bonvini E, Laborda J. Specific regions of the extracellular domain of dlk, an EGF-like homeotic protein involved in differentiation, participate in intramolecular interactions. Front Biosci. 2001;6:A25–32. doi: 10.2741/baladron. [DOI] [PubMed] [Google Scholar]
- 36.Miyaoka Y, Tanaka M, Imamura T, Takada S, Miyajima A. A novel regulatory mechanism for Fgf18 signaling involving cysteine-rich FGF receptor (Cfr) and delta-like protein (Dlk) Development. 2010;137:159–67. doi: 10.1242/dev.041574. [DOI] [PubMed] [Google Scholar]
- 37.Nueda ML, García-Ramírez JJ, Laborda J, Baladrón V. dlk1 specifically interacts with insulin-like growth factor binding protein 1 to modulate adipogenesis of 3T3-L1 cells. J Mol Biol. 2008;379:428–42. doi: 10.1016/j.jmb.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 38.Yu F, Hao X, Zhao H, Ge C, Yao M, Yang S, et al. Delta-like 1 contributes to cell growth by increasing the interferon-inducible protein 16 expression in hepatocellular carcinoma. Liver Int. 2010;30:703–14. doi: 10.1111/j.1478-3231.2010.02214.x. [DOI] [PubMed] [Google Scholar]
- 39.Li K, Lee LA, Lu X, Wang Q. Fluorogenic “click” reaction for labeling and detection of DNA in proliferating cells. Biotechniques. 2010;49:525–7. doi: 10.2144/000113463. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Forman SJ, Bhatia R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene. 2005;24:4472–6. doi: 10.1038/sj.onc.1208637. [DOI] [PubMed] [Google Scholar]


