Abstract
FNDC5 (fibronectin domain-containing [protein] 5) was initially discovered and characterized by two groups in 2002. In 2011 FNDC5 burst into prominence as the parent of irisin, a small protein containing the fibronectin type III domain. Irisin was proposed to be secreted by skeletal muscle cells in response to exercise, and to circulate to fat tissue where it induced a transition to brown fat. Since brown fat results in dissipation of energy, this pathway is of considerable interest for metabolism and obesity. Here I review the original discoveries of FNDC5 and the more recent discovery of irisin. I note in particular three problems in the characterization of irisin: the antibodies used to detect irisin in plasma lack validity; the recombinant protein used to demonstrate activity in cell culture was severely truncated; and the degree of shedding of soluble irisin from the cell surface has not been quantitated. The original discovery proposing that FNDC5 may be a transmembrane receptor may deserve a new look.
Keywords: irisin, FNDC5, brown adipocyte, exercise, fibronectin
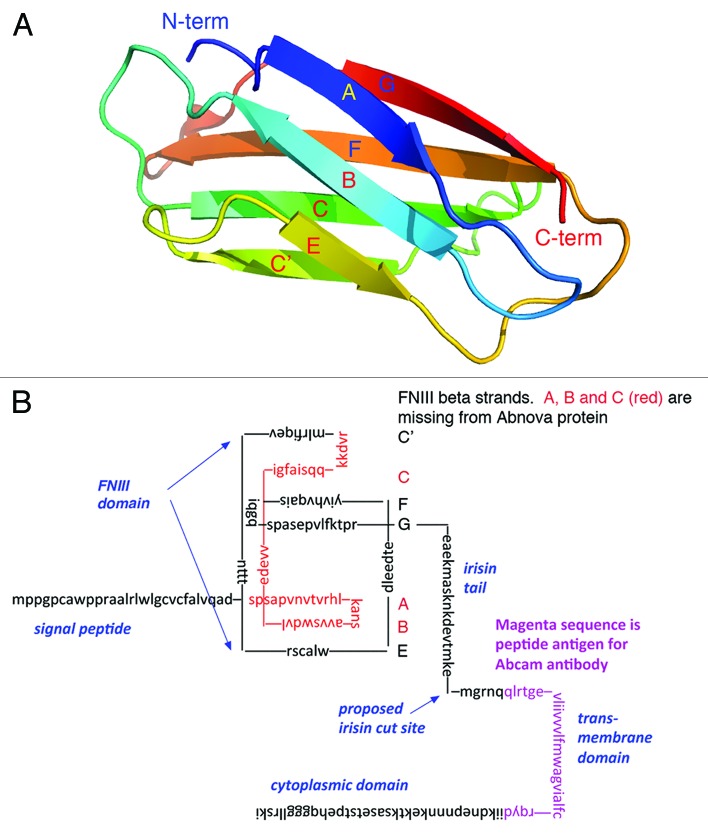
The fibronectin type 3 (FNIII) protein domain is one of biology’s favorite building blocks. It is found in tandem arrays in extracellular matrix proteins such as fibronectin and tenascin, and it forms the ligand-binding ectodomain of many receptors.1 FNIII domains are about 90 amino acids (aas) long, and share only 15–20% sequence identity. In spite of this limited aa sequence identity, all FNIII domains have an identical protein fold. The domain is a small globule, with 3 β strands on one side and 4 on the other (Fig. 1A). This fold is similar to that of the immunoglobulin domain, but one strand is switched to the opposite side.
Figure 1. Structure of an FNIII domain, and diagram of FNDC5 sequence showing domains. (A) A ribbon diagram of an FNIII domain from tenascin, from pdb file 1ten,17 generated with PyMol (http://pymol.org/sites/default/files/pymol_0.xml). All FNIII domains have this same folding structure. (B) The sequence of mouse FNDC5 (NP_081678) showing domains. The FNIII domain is separated into proposed β strands, with the ABE sheet on the bottom and the C’CFG sheet on top.
Early Discoveries of FNDC5
FNDC5 (fibronectin [type 3]-domain containing [protein] 5) was initially discovered in a genomic search, with a focus on FNIII domains.2 Figure 1B shows the aa sequence of FNDC5 with important features indicated. The first 29 aas of the mouse FNDC5 are a signal peptide, followed immediately by the single FNIII domain of 94 aas. The next 28 aas are of unknown structure and function, and contain the putative cleavage site for irisin (see below). This is followed by a 19 aa transmembrane domain and a 39 aa cytoplasmic domain. FNDC5 is thus a type I transmembrane protein with its FNIII domain extracellular, similar to some cytokine receptors.1 Based on this structural information, the authors of this initial discovery speculated that FNDC5, and the closely related FNDC4, “are likely receptors of an as yet to be identified ligand”.2
FNDC5 was discovered independently by Ferrer-Martinez et al.,3 in a search for peroxisomal proteins. About half of mammalian peroxisomal proteins are targeted for transport by a “conserved COOH-terminal tripeptide (SKL and its functional variants)”.4 Mouse FNDC5 has a C-terminal SKI. To test its localization, the authors expressed FNDC5 with a green fluorescent protein (GFP) fused to its N terminus, and found a punctate localization to peroxisomes. However, this fusion is probably invalid because the GFP would block the signal peptide, forcing FNDC5 to be a cytoplasmic protein. This cytoplasmic protein, with its C-terminal SKI, should transport into peroxisomes. However, the native protein would be transported across the membrane and anchored as a transmembrane protein, eliminating any possible transport into peroxisomes. Moreover, FNDC5 sequences from several vertebrate species have short peptide segments following the SKI/V/F. “Addition of even one aa to the C terminus of SKL kills the peroxisomal transport sequence” (S. Subramanian,4 personal communication). I would conclude that FNDC5 is not a peroxisomal protein.
The Discovery of Irisin, an Exercise Hormone That Stimulates “Browning of Adipocytes”
In 2012, Boström et al. rediscovered FNDC5 in a new context—as the precursor of “irisin”, a proposed exercise hormone.5 They were investigating the possibility that skeletal muscle, in response to exercise, might secrete a factor that circulated in the blood stream to fat tissue, where it could cause the transformation of white or beige adipocytes into brown, known as the browning response. The potential to induce brown adipocyte tissue is of considerable interest for research on obesity, diabetes, and general metabolism. See Vosselman et al.6 for a recent review, Herzig and Wolfrum7 for an introduction to a special issue devoted to pathways in brown adipocyte tissue activation, and Raschke and Eckel8 for a review of myokines, signaling proteins secreted by muscle.
The top candidate from the screen of Boström et al. was FNDC5.5 They found that FNDC5 mRNA was upregulated in skeletal muscle of both mouse and human following exercise. They later used adenovirus to overexpress full length FNDC5 in the liver of mice. They found a dramatic increase in brown fat in the FNDC5-expressing mice (Fig. 6A of Boström et al.5). These two results remain as intriguing support for the exercise hormone hypothesis.
How could FNDC5, expressed as a transmembrane protein in skeletal muscle, cause an increase in brown adipocyte tissue? Boström et al. proposed that the ectodomain of FNDC5 was cleaved by an unknown protease, producing the soluble irisin protein (111 aas comprising the FNIII domain and a short C-terminal tail: spsa…tmke in Fig. 1B). Boström et al proposed that irisin traveled through the blood to fat tissue, where it interacted with unknown receptors to induce the browning response. However, I believe that 3 key experiments in the study of Boström et al. have serious flaws.
Problem 1: The Irisin Antibodies Are Not Valid
A key point in the study of Boström et al.5 was to show that irisin protein was present in plasma of human and mouse, and that it was upregulated following exercise (their Fig. 5). This was demonstrated by western blots, which used a polyclonal antibody obtained from the company Abcam. This antibody is described in the Abcam catalog (ab117436) as being made against a peptide corresponding to C-terminal aa 149–178 of the human FNDC5 (aa 146–175 in mouse, magenta in Fig. 1B). This peptide contains primarily the transmembrane segment, and does not include any sequence from the irisin peptide. The Q&A section of the Abcam website has a clear statement that “I do not expect this antibody to bind to the cleaved Irisin protein”. The western blots in Figures 4 and 5 of Boström et al.5 therefore have to be discounted; and that means there is no evidence that irisin or FNDC5 protein actually exists in plasma.
Following the initial discovery of irisin,5 there have been about 20 papers from various groups pursuing its study. Many of these studies have examined mRNA levels of FNDC5 and other genes that might be related to the proposed exercise-hormone pathway. These assays of gene expression are not questioned here, although they have given mixed results. However, a number of the studies have also claimed to quantitate the level of irisin protein in plasma, as a function of exercise or other potential metabolic correlates. Regrettably these studies have all relied on commercially available antibodies or ELISA kits that have questionable validation. Several studies used the Abcam antibody to aa 149–178, which is irrelevant to irisin; an antibody sold by Acris and one from USCN Life Sciences are described as “FNDC5 C-term” and may be the same as the Abcam antibody. An antibody from Aviscera was listed for a while as “no longer available”, and is now removed from their catalog. None of these antibodies has been validated by quantitative western blot analysis.
An antibody and ELISA kit from Phoenix Pharmaceuticals, developed initially for immunohistology,9 seemed promising. However, a recent study by Roca-Rivada et al.10 has published complete (10–60 kDa) western blots directly comparing the antibodies from Abcam and Phoenix Pharmaceuticals. The results are not encouraging. This study did not look at plasma, but examined the proteins secreted by excised fat and skeletal muscle tissue. The Abcam antibody stained a prominent band of 25 kDa secreted from both tissues, and a weak band of 50 kDa in some lanes. The text mentioned additional high molecular weight bands. The Phoenix antibody also stained a sharp 25 kDa band, even though the antigens for the Abcam and Phoenix antibodies do not share any sequence. The Phoenix antibody also stained prominent bands of 45–50 kDa, and in some cases these were much stronger than the 25 kDa band. Unlike the original study of Boström et al.,5 where the stained bands were diffuse as expected for heavy glycosylation, the 25 kDa bands in Roca-Rivada et al.10 are sharp.
Any polyclonal antiserum contains antibodies to cross-reacting proteins. To provide a quantitative measure of antigen levels in plasma, an antibody needs to be tested by quantitative western blotting for cross-reacting proteins in plasma, and for its sensitivity to the target antigen added to plasma. Since none of the commercial antibodies have been so validated, I suggest that all published assays of irisin protein levels need to be reconsidered.
Problem 2: The Recombinant Irisin/FNDC5 Used to Demonstrate Browning in Fat Cell Culture Was a Severely Truncated Protein
After identifying FNDC5 as a top myokine candidate, Boström et al.5 tested a recombinant FNDC5 protein for activity in adipocyte cell culture. Their Figure 3 shows that when cells cultured from inguinal fat pads were treated with 200 ng/ml recombinant FNDC5 protein, there was a dramatic increase in Ucp1, the marker for brown adipocytes.
The problem is that the recombinant FNDC5 used in this experiment was a severely truncated protein. The protein was obtained commercially from the company Abnova (H00252995-P01), and is described in the catalog as “Human FNDC5 full-length ORF (NP_715637.1, 1 aa–137 aa) recombinant protein with GST-tag at N-terminal”. Unfortunately, this protein is not full-length FNDC5, but is missing the first 70 aas. This missing segment includes the signal peptide and, most importantly, the first three β strands of the FNIII domain. These missing β strands, almost half of the FNIII domain, are colored red in the sequence diagramed in Figure 1B. (It turns out that the database entry NP_715637.1 missed the unusual AUA start codon of the human sequence, and chose the next methionine, 70 aas later, as the start. There is now an updated entry NP_715637.1, which contains the full-length protein of 212 aas.) The truncated protein has only the E strand remaining on the 3-strand side; on the 4-strand side it is missing the C strand (Fig. 1A and B), so strands C′ and F will be missing their hydrogen-bonding partners. One should wonder how the truncated protein could fold into any structure resembling the native FNIII domain, and therefore be suspicious of any biological activity attributed to the truncated FNIII peptide.
The truncation of the FNDC5 protein used in Boström et al. has never been noted in the literature. However, in a separate study from the same group, the experiment was partially repeated with a protein that contained the full length FNIII domain fused at its C terminus to the Fc fragment of human immunoglobulin G.11 Figure 4C of Wu et al.11 shows that irisin-Fc produced a strong increase in Ucp1 when applied to cultured fat cells; however, it required a 5-fold higher concentration to match the activity of the truncated Abnova FNDC5 protein, which was tested in parallel. Restoring confidence in this assay will require extended exploration of these and related protein constructs to map the site(s) of the activity.
Problem 3: What Fraction of FNDC5 is Cleaved to Produce Soluble Irisin?
To act as a hormone, the ecto-domain of FNDC5 would need to be cleaved from its transmembrane attachment. To test for this Boström et al.5 used mass spectrometry (MS) to analyze FNDC5 collected from culture medium. The MS found some FNDC5 in the medium, and indicated that it terminated in …TMKE-140 of the mouse sequence. Boström et al. concluded that an unidentified protease cleaved the protein at this point, releasing a 111 aa segment SPSAP…TMKE, which they named irisin. A problem with this experiment is that there was no quantitation of shed protein relative to protein remaining on the cell surface. MS can be very sensitive, so the protein shed into the media might have been only a small fraction of the total. It is essential to know what fraction of FNDC5 is cleaved, to determine if cleavage and shedding is a major or minor pathway.
Tissues That Express FNDC5
The pattern of tissue expression was analyzed in the two original discoveries of FNDC5.2,3 Each group probed a commercial (from Clontech) northern blot of adult mouse tissues, and the results were largely in agreement. Expression was very strong in heart, strong in brain, weak in testis and lung, and absent in spleen and kidney. Results were ambiguous for skeletal muscle, the tissue of primary interest here. However, expression in skeletal muscle was confirmed by a more extensive northern analysis, which showed a fairly constant expression of FNDC5 mRNA in skeletal muscle from embryonic day 15.5 to adult.3
A recent survey of adult human tissues using quantitative PCR gave a pattern partially confirming these early northern blots.12 Strong expression of FNDC5 was reported in skeletal muscle, pericardium, and rectum; moderate expression was found in heart, intracranial artery, tongue, and optic nerve. Very low expression was found in brain, contradicting the previous mouse studies.2,3 Interestingly, expression of FNDC5 in skeletal muscle across a range of human subjects was strongly correlated with expression of PGC1α,12 consistent with the proposed link of both proteins to exercise.5 Finally, the website BioGPS.org shows a gene expression pattern for FNDC5 in adult mouse: expression was very high in heart, and moderately high in brain and skeletal muscle.
Timmons et al.13 have questioned whether upregulation of FNDC5 in skeletal muscle is actually related to exercise. They found, using an Affymetrix screen, that FNDC5 was upregulated in muscle following exercise only in a subset of elderly subjects (and they noted that the 8 subjects in the Boström et al. study were also elderly). They concluded that FNDC5 is likely not a primary pathway in the response to exercise, since the benefits of exercise to health apply across all ages. In their reply to this critique,13 Boström et al. noted that the earlier comprehensive Timmons study also failed to identify PGC1α as a gene upregulated by exercise, questioning the general validity of their screen.
FNDC5 Activity in Neural Development
The two original discoveries of FNDC5 found that FNDC5 mRNA was highly expressed in adult brain.2,3 Pursuing the potential role in brain, the laboratory of Baharvand found that FNDC5 expression was upregulated when mouse embryonic stem cells were induced to differentiate into neurons by application of retinoic acid.14 They later showed that differentiation of stem cells into neuroblasts was decreased when FNDC5 was knocked down by short hairpin RNA.15 In a separate study Moon et al.16 applied full length recombinant irisin (not glycosylated) to neural cells, and observed an enhancement of cell proliferation. These results complement the original discoveries that FNDC5 is highly expressed in brain.2,3
Conclusions
This leaves us with unresolved issues. Is irisin really an exercise hormone, released from skeletal muscle to act on an unknown receptor? Or might it actually remain as the ectodomain of a transmembrane FNDC5, perhaps a receptor with an unknown ligand, as suggested by Teufel et al.?2 This field really needs a validated antibody that can quantitate levels of FNDC5 protein in plasma and on the cell surface. One could then address the key question—is a substantial fraction of the ectodomain cleaved and released as a circulating hormone, or does it remain in its tissue of origin as a transmembrane protein with an unknown function?
Disclosure of Potential Conflicts of Interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Acknowledgments
Supported by NIH grant CA47056.
Glossary
Abbreviations:
- FNIII
fibronectin type 3
- aa
amino acid
Footnotes
Previously published online: www.landesbioscience.com/journals/adipocyte/article/26082
References
- 1.Bork P, Doolittle RF. Proposed acquisition of an animal protein domain by bacteria. Proc Natl Acad Sci U S A. 1992;89:8990–4. doi: 10.1073/pnas.89.19.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teufel A, Malik N, Mukhopadhyay M, Westphal H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene. 2002;297:79–83. doi: 10.1016/S0378-1119(02)00828-4. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer-Martínez A, Ruiz-Lozano P, Chien KR. Mouse PeP: a novel peroxisomal protein linked to myoblast differentiation and development. Dev Dyn. 2002;224:154–67. doi: 10.1002/dvdy.10099. [DOI] [PubMed] [Google Scholar]
- 4.Subramani S. Components involved in peroxisome import, biogenesis, proliferation, turnover, and movement. Physiol Rev. 1998;78:171–88. doi: 10.1152/physrev.1998.78.1.171. [DOI] [PubMed] [Google Scholar]
- 5.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vosselman MJ, van Marken Lichtenbelt WD, Schrauwen P. Energy dissipation in brown adipose tissue: From mice to men. Mol Cell Endocrinol. 2013 doi: 10.1016/j.mce.2013.04.017. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 7.Herzig S, Wolfrum C. Brown and white fat: from signaling to disease. Biochim Biophys Acta. 2013;1831:895. doi: 10.1016/j.bbalip.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Raschke S, Eckel J. Adipo-myokines: two sides of the same coin-mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724. doi: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–62. doi: 10.1016/j.neuroscience.2013.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belén Crujeiras A, Seoane LM, Casanueva FF, Pardo M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8:e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–38. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostadsharif M, Ghaedi K, Hossein Nasr-Esfahani M, Mojbafan M, Tanhaie S, Karbalaie K, Baharvand H. The expression of peroxisomal protein transcripts increased by retinoic acid during neural differentiation. Differentiation. 2011;81:127–32. doi: 10.1016/j.diff.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Ostadsharif M, Ghaedi K, Hossein Nasr-Esfahani M, Mojbafan M, Tanhaie S, Karbalaie K, Baharvand H. The expression of peroxisomal protein transcripts increased by retinoic acid during neural differentiation. Differentiation. 2011;81:127–32. doi: 10.1016/j.diff.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Hashemi MS, Ghaedi K, Salamian A, Karbalaie K, Emadi-Baygi M, Tanhaei S, Nasr-Esfahani MH, Baharvand H. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience. 2013;231:296–304. doi: 10.1016/j.neuroscience.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 16.Moon HS, Dincer F, Mantzoros CS. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism. 2013;62:1131–6. doi: 10.1016/j.metabol.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leahy DJ, Hendrickson WA, Aukhil I, Erickson HP. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992;258:987–91. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]