Abstract
Vibrio cholerae contains multiple copies of chemotaxis response regulator (VcCheY1–VcCheY4) whose functions are elusive yet. Although previous studies suggested that only VcCheY3 directly switches the flagellar rotation, the involvement of VcCheY4 in chemotaxis could not be ruled out. None of these studies, however, focused on the structure, mechanism of activation or molecular basis of FliM binding of the VcCheYs. From the crystal structures of Ca2+ and Mg2+ bound VcCheY3 we proposed the presence of a conformational barrier composed of the hydrophobic packing of W61, M88 and V106 and a unique hydrogen bond between T90 and Q97 in VcCheY3. Lesser fluorescence quenching and higher Km value of VcCheY3, compared to its mutants VcCheY3-Q97A and VcCheY3-Q97A/E100A supported our proposition. Furthermore, aforesaid biochemical data, in conjunction with the structure of VcCheY3-Q97A, indicated that the coupling of T90 and Q97 restricts the movement of T90 toward the active site reducing the stabilization of the bound phosphate and effectively promoting autodephosphorylation of VcCheY3. The structure of BeF3 − activated VcCheY3 insisted us to argue that elevated temperature and/or adequacy of phosphate pool might break the barrier of the free-state VcCheY3 and the conformational changes, required for FliM binding, occur upon phosphorylation. Structure of VcCheY4 has been solved in the free and sulfated states. VcCheY4sulf, containing a bound sulfate at the active site, appears to be more compact and stable with a longer α4 helix, shorter β4α4 loop and hydrogen bond between T82 and the sulfate compared to VcCheY4free. While pull down assay of VcCheYs with VcFliMNM showed that only activated VcCheY3 can interact with VcFliMNM and VcCheY4 cannot, a knowledge based docking explained the molecular mechanism of the interactions between VcCheY3 and VcFliM and identified the limitations of VcCheY4 to interact with VcFliM even in its phosphorylated state.
Introduction
Vibrio cholerae, the highly motile gram-negative bacterial pathogen that causes cholera, uses chemotaxis and motility to travel to its preferred intestinal niche to colonize [1]. Extensive studies on chemotaxis of Escherichia coli or Salmonella typhimurium showed that the ligand induced conformational change in methyl accepting chemotaxis protein (MCP) is sensed by the CheA–CheW complex eventually resulting autophosphorylation of the kinase CheA. Autophosphorylated CheA then donates phosphate to the response regulator CheY. Phosphorylated CheY interacts with the flagellar motor protein FliM and influence the direction of flagellar rotation from counter clock wise (CCW) to clock wise (CW) [2], [3]. CCW rotation results smooth swimming and CW rotation causes the cell to tumble [4]. Because of the presence of a single polar flagellum, V. cholerae does not tumble as such but reverses direction briefly, allowing the bacterium to randomly reorient itself and swim in a new direction.
The genomes of a large number of bacterial species, including Vibrio cholerae, Pseudomonas aeruginosa, Rhodobacter spaeroides, Myxococcus xanthus, Borrelia burgdorferi, and Yersinia pestis, encode for multiple paralogues of the various chemotaxis genes and chemotaxis in these bacteria is more complex [5], [6]. A recent genomic and bioinformatic analysis of over 450 bacteria indicates that more than 50% of the chemotaxis gene homologs have more than one copy of chemotaxis genes [5] and these genes are involved not only in flagellum-mediated chemotaxis but also in type-4 pilus-based motility [7], [8], polysaccharide biosynthesis associated with pilus-based gliding motility [9] and flagellar morphogenesis [10]. In many cases, however, genetic analysis has not been successful in deciphering the function of these chemotaxis gene homologs [5], [11].
The genome sequence of V. cholerae has three sets of Che protein and 45 MCP-like proteins [12]. Each set of che genes forms clusters where che cluster I (located on chromosome I) contains cheY1, cheA1, cheY2, cheR1, cheB1 and the putative gene cheW; cluster II of chromosome I contains cheW1, cheB2, cheA2, cheZ and cheY3, while cluster III of chromosome II contains cheB3, cheD, cheR3, cheW2, cheW3, cheA3 and cheY4.
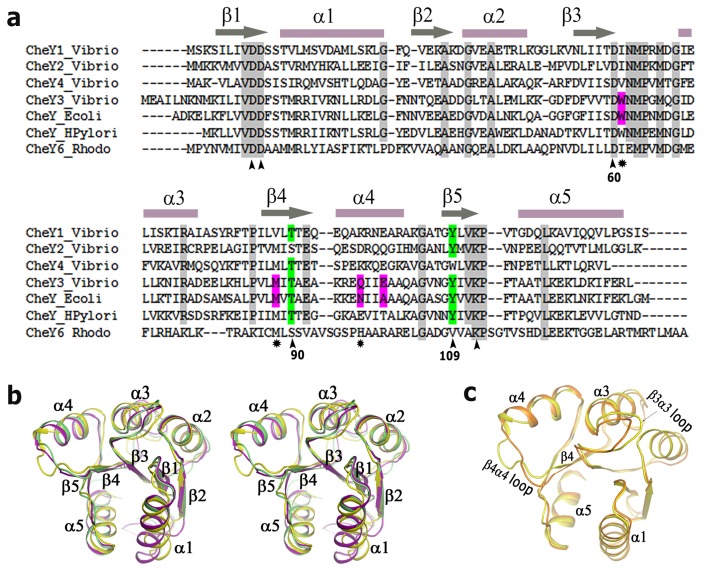
So far, the molecular characterization of all four CheYs of V. cholerae (namely, VcCheY1–VcCheY4) is restricted to a few in vivo studies where some of the chemotaxis related genes are found to be involved in the virulence of V. Cholerae [13]–[15] [3]. Attempts to identify the V. cholerae cheY responsible for the flagellar motion showed that a deletion of cheY3 impairs chemotaxis [1] while insertional disruption and duplication of the cheY4 gene result in decreased and increased motility respectively [13]. Swarming assay and assessment of the swimming behaviour indicated that only VcCheY3 directly switches flagellar rotation, although this study could not rule out the involvement of VcCheY4 in the motor action [14]. Later, Bandyopadhaya and Chaudhuri (2009) showed that inactivation of cheY3 or cheY4 generates a less motile and less adherent mutant [15]. Sequence analysis of VcCheYs indicate that only 17% residues are identical among them which comprise the residues involved in binding of the divalent metal ion and stabilization of the phosphorylated intermediate (Figure 1a). This implies that the basic machinery for the phosphorylation is intact for all four VcCheYs. Available literature, however, suggest that deletion of the cheY1 and cheY2 genes does not cause any defect in chemotaxis [14] and motility or adherence remains unaffected for the insertional mutants of cheY1 or cheY2 [15]. All these observations point to the fact that VcCheY3 and VcCheY4 are the key response regulators to control chemotaxis in V. cholerae.
Figure 1. Sequence alignment and overall structure comparison of CheYs.
(a) Amino acid sequences of VcCheY1–VcCheY4 are aligned with CheY6 of Rhodobacter spaeroides, CheY of Escherichia coli and CheY1 of Helicobacter pylori. Secondary structural elements are marked and labelled at the top. At the bottom important conserved residues implicated in activation/metal binding are marked as (∧) whereas other important residues are indicated as (*); as EcChey and StCheY possess 99% sequence identity only EcCheY was shown in the alignment file. (b) Stereo representation showing the comparison of the overall structures of VcCheY3 (violet), StCheY (green), each in free state, with VcCheY4free (yellow); (c) Superposition of the overall structure of VcCheY4free (yellow) on VcCheY4sulf (orange) showing the significant differences in helix α4 and β4α4 loop.
Structures of CheY from different bacterial sources suggest that although all of these response regulators possess an overall (β/α)5 fold, small differences in the amino acid sequence or point mutations lead to the subtle conformational variations that make each of these proteins unique in terms of their function [16]–[18]. Also, T87I and T87I/Y106W mutants of EcCheY were found to be phosphorylatable although these mutants were unable to generate clockwise rotation of the flagella [19]. In addition, both of these mutants had ∼5-fold lower autodephosphorylation rates and the mutants were completely resistant to CheZ activity, indicating that an isoleucyl side-chain at position 87 renders EcCheY unable to perform its chemotactic functions [20].
VcCheY3 bears only 37% sequence identity with that of VcCheY4 (Figure 1a) and so far, nothing is known about the structure, mechanism of activation or molecular basis of FliM binding for these two key response regulators, implicated in chemotaxis and virulence of V. cholerae. Here we report, the structures of VcCheY3 in Ca2+ and Mg2+ bound states, BeF3 − activated VcCheY3 (VcCheY3-BeF3 −) and of the mutant VcCheY3-Q97A. Our structural observations identified a unique conformational barrier in VcCheY3 that controls its phosphorylation event. Implication of this barrier is established by fluorescence spectroscopic study on VcCheY3 and its mutants VcCheY3-Q97A, VcCheY3-Q97A/E100A and VcCheY3-D60A, comparison of their Km values and pull down assay with VcFliMNM. We have also reported the structures of VcCheY4 in free and sulfate bound states here and comparison of these structures helped us to argue that VcCheY4 has a strong tendency to be phosphorylated and the phosphorylated state would be more stable compared to its free state. While our pull down assay showed that only activated VcCheY3 can interact with VcFliMNM and VcCheY4 cannot, structure based docking explained the molecular mechanism of the interactions between VcCheY3 and VcFliM and identified the structural limitations of VcCheY4 to interact with VcFliM even in its phosphorylated state.
Materials and Methods
Cloning, Overexpression and Purification
VcCheY3 and VcCheY4 were purified according to the previously described protocols [21], [22]. Briefly, the genes encoding VcCheY3 and VcCheY4 were amplified from V. cholerae O395 genomic DNA and cloned into pET28a+ vector. After transformation, cells were grown at 37°C until the optical density at 600 nm (OD600) reached 0.4 to 0.6. Protein expression was induced by the addition of IPTG (isopropyl-D-thiogalactopyranoside) to a final concentration of 0.1 mM. The cells were harvested by centrifugation and the resuspended pellet was lysed by sonication in presence of PMSF. The cell lysate was then centrifuged (12000 g for 50 mins) at 4°C. The 6×His tagged protein was isolated from the supernatant using Ni2+–NTA affinity chromatography (Qiagen) and were eluted with lysis buffer containing 150 mM imidazole. The eluted fractions were checked by 15% SDS–PAGE, pooled and dialyzed overnight against the thrombin clevage buffer (0.05 M Tris–HCl pH 8.0, 150 mM NaCl) and the 6×His tag was cleaved with 1 U thrombin by overnight incubation at 4°C. The proteins were further purified by gel filtration chromatography using a Sephacryl S-100 (GE-Healthcare) column (78×1.4 cm) pre-equilibrated with thrombin cleavage buffer containing 0.02% sodium azide at 4°C.
The gene encoding FliMNM (residue 1–250) was amplified from V. cholerae O395 genomic DNA and cloned into pET21b+ vector with a C-terminal 6×His-tag to get optimal expression level and solubility. The FliMNM protein was purified by growing cells in LB media to an optimal density 0.6–0.8 at 600 nm and induced with 1 mM IPTG. The cells were harvested after induction at 37°C for 3 h. Cell pellet was resuspended in lysis buffer containing 50 mM Tris-HCl pH 7.5, 250 mM NaCl, 1 mM PMSF and 10 mg lysozyme and lysed by sonication. After centrifugation (14000×g, for 45 mins and at 4°C) FliMNM with C-terminal 6×His-tag was isolated from the supernatant by using Ni2+-NTA agarose (Qiagen) and the protein was eluted with lysis buffer containing 200 mM Imidazole. After checking in 12% SDS-PAGE the eluted fractions were dialyzed against the lysis buffer.
Mutagenesis
VcCheY3-D60A, VcCheY3-Q97A and VcCheY3-Q97A/E100A were prepared by two-step PCR and verified by commercial sequencing. All the mutant proteins were purified using the same protocol described for the wild type protein.
FliMNM-CheY Interaction through Nickel Pull-down Assay
50 µl of Ni2+-NTA slurry (Qiagen) was washed three times with binding buffer containing 10 mM imidazole, 150 mM NaCl, 5 mM MgCl2, 0.15% Tween 20 and 50 mM Tris-Cl (pH 7.5) and the resin was then incubated with 0.1 ml purified FliMNM-His protein in a concentration of 0.2 mg/ml at 25°C for 20 mins with gentle shaking. The beads were then washed for three times with the binding buffer before adding VcCheY3, VcCheY3-Q97A, VcCheY3-Q97A/E100A, VcCheY3-D60A or VcCheY4. For activation, respective protein was pre-incubated for 20 mins with BeF3- (100 mM). The mixture was then added in the FliMNM-His bound Ni2+-NTA resin maintaining 1∶1 molar ratio and incubated for another 10 mins at 25°C. The beads were washed three times with the buffer and then resuspended in 25 µl of 4×SDS-PAGE gel loading buffer and were subjected to SDS-PAGE analysis and Coomassie blue staining.
Fluorescence Spectroscopy
Fluorescence measurement was carried out using a spectrofluorometer, Hitachi F-7000. Fluorescence was measured at an excitation wavelength of 295 nm and an emission wavelength of 340 nm with slit widths of 2.5 nm and 5 nm for excitation and emission, respectively. All reactions were carried out at 25°C. Equilibrium titrations of VcCheY3, VcCheY3-Q97A/E100A, VcCheY3-Q97A and VcCheY3-D60A were carried out with acetyl phosphate (acP) and beryllium fluoride (BeF3 −). The reactions in presence of acP were performed in a buffer containing 20 mM sodium phosphate (pH 7.5), 50 mM NaCl, and 2 mM MgCl2 and the same were 50 mM Tris-Cl (pH 7.5), 150 mM NaCl and 5 mM MgCl2 for BeF3 −. For all proteins the final concentrations were 1 µM, BeF3 − concentrations varies from 0 to 400 µM and the concentrations varies from 0 to 6 mM for acP. The fluorescence values were corrected for dilution. Km was determined as described previously by Lukat et al (1992) [23]. Acetyl phosphate and BeF3 − concentrations were plotted versus (Io − I)/(I − I inf), where Io is initial fluorescence intensity, I is the intensity at the corresponding acetyl phosphate concentration, and I inf is the intensity at the saturating concentration. From the plot, the reciprocal of the slope of the line corresponds to the Km value. According to proposed reaction scheme [23], [24], shown as follows, Km = Ks. k3/k2.
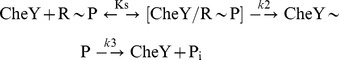 |
(1) |
Where Ks is the equilibrium dissociation constant between CheY and acetyl phosphate (the phosphor-donor, R∼P) and k2 and k3 are the phosphorylation and dephosphorylation rate constants, respectively.
If it is assumed that the observed quenching is a direct effect of the reduced quantum yield of phospho-CheY relative to that of CheY, the steady-state fluorescence at a given concentration of phospho-donor may be related to the kinetic parameters of the reaction scheme (Eq.1), where (Io−I)/(I−Iinf) = ([R∼P]k2)/(k3KS). All experimental data points were fitted by linear fit analysis using Microsoft EXCEL and Origin 8.
Crystallization and Data Collection
Crystallization data of VcCheY3 [21] and VcCheY4 [22] have been published earlier. Briefly, crystals of VcCheY3 that grew in low-salt condition using 5% (w/v) PEG 6000 in 0.1 M Tris–HCl pH 8.0 as precipitant, belong to space group R3 and diffracted to a resolution of 1.67 Å. Crystals of VcCheY3 were also grown in the presence of Mg2+ in a similar condition which diffracted up to 2.2 Å. VcCheY4 crystals grew in AMS at two different pH conditions. In the high-pH condition, hexagonal-shaped crystals were obtained using 0.8 M ammonium sulfate, 0.1 M Bicine pH 9.0, 4% glycerol as precipitant. In the low-pH condition, cube-shaped crystals were obtained using 0.8 M ammonium sulfate, 0.1 M citrate, 4% glycerol as precipitant. The low-pH and high-pH condition crystals were diffracted upto 1.67 Å and 1.9 Å with the space group C2 and P3221 respectively.
Crystals of VcCheY3-Q97A mutant grew in a drop consisting of 2 µl protein (6 mg/ml) solution and an equal volume of precipitant containing 1.6 M ammonium sulfate, 0.1 M Tris, pH 8.0. Cube-shaped crystals of VcCheY3-Q97A belonging to space group R3 diffracted to a resolution of 2.4 Å.
Activated VcCheY3 were prepared by mixing 20 µl of protein (6 mg/ml) solution with 5 µM of BeF3 solution and incubated for 5 minutes on ice. Crystals of activated VcCheY3 were grown in a drop contains 2 µl of above mixture and equal volume of precipitant solution consisting of 10% (w/v) PEG 6000 in 0.1 M Tris–HCl pH 8.0 and equilibrated for 7 day against 20% (w/v) PEG 6000 in 0.1 M Tris–HCl pH 8.0. Activated VcCheY3 crystals, after brief soaking in cryoprotectant solution containing 1 µM of BeF3−, diffracted upto 2.1 Å with the space group R3.
For data collection, crystals were fished out from the crystallization drops using nylon loop, briefly soaked in cryoprotectant solution and flash-cooled in a stream of nitrogen (Oxford Cryosystems) at 100 K. The diffraction data sets were collected using a MAR Research image-plate detector of diameter 345 mm and Cu Kα radiation generated by a Bruker–Nonius FR591 rotating-anode generator equipped with Osmic Max Flux confocal optics and operated at 50 kV and 70 mA. Data were processed and scaled using AUTOMAR (http://www.marresearch.com/automar/run.html). Data-collection and processing statistics are given in Table 1.
Table 1. Data collection and processing statistics.
| VcCheY3 Mg2+ bound | VcCheY3-Q97A | VcCheY3-BeF3 − | |
| Space group | R3 | R3 | R3 |
| Unit-cell parameters (Å ) | a = b = 67.48, c = 74.46 | a = b = 65.858, c = 65.039 | a = b = 67.320, c = 72.660 |
| Oscillation range (°) | 0.5 | 0.5 | 0.5 |
| Number of images | 92 | 138 | 88 |
| Maximum resolution (Å ) | 30.0–2.2 | 30.0–2.8 | 30.0–2.1 |
| No. of molecules per ASU | 1 | 1 | 1 |
| Mathews coefficient (VM; Å3 Da−1) | 2.23 | 1.86 | 2.19 |
| Solvent content (%) | 44.9 | 33.77 | 43.86 |
| No. of observations | 16597 | 9361 | 10428 |
| No. of unique reflections | 6341 | 4141 | 7391 |
| Mosaicity (°) | 1.59 | 0.5 | 0.35 |
| Completeness (%) | 98.9(100) | 97.9(98.8) | 94.3(92.2) |
| Rmerge† (%) | 8.45(44.3) | 7.39 (27.80) | 3.12(22.47) |
| Average I/σ(I) | 7.5(2.7) | 5.3(2.0) | 6.5(2.0) |
Structure Determination and Refinement
The structures of wild type VcCheY3, VcCheY4, VcCheY3-Q97A and activated VcCheY3 (VcCheY3-BeF3 −) were solved by molecular replacement using MOLREP of CCP4 suite [25]. Packing considerations indicated the presence of one molecule in the asymmetric unit for all the structures.
The wild type VcCheY3 structure in its Ca2+ bound form was solved by using the coordinates of the Salmonella CheY (PDBID: 2 CHE) as template. The structure was refined by alternating cycles of model building and refinement using ‘O’ and CNS [26], [27] to a final R cryst and Rfree values of 20.2% and 22.9% respectively. The poly-ala model of VcCheY3 was used as search model for VcCheY4 (low pH) and the refined structure of VcCheY4 (low pH) was used as search model to determine the structure high pH CheY4 (VcCheY4free). Low pH VcCheY4 (VcCheY4sulf) was refined to Rcryst 21.8% and Rfree 24.6% and VcCheY4free was refined to Rcryst 22.5% and Rfree 26.0%. VcCheY3-BeF3 − structure was solved by using the coordinates of E. coli activated CheY i.e. EcCheY-BeF3 − (PDB code: 1F4V) as the search model. Strong electron density of beryllofluoride was found close to the active-site residue D60. The structure was refined upto Rcryst of 23.1% and Rfree of 24.3% by several rounds of refinements and manual rebuilding by using the programs CNS [27] and COOT [28], respectively. The structure of VcCheY3-Q97A was solved using VcCheY3 as template and refined by the similar protocol to Rcryst of 22.5% and Rfree of 25.2%. The structure of Mg2+ bound VcCheY3 was also solved using VcCheY3 (Ca2+ bound) as template after removing the coordinates of Ca2+ and waters and refined by the similar protocol to Rcryst of 20.0% and Rfree of 22.5%. Details of the refinement parameters for all the structures along with the geometric parameters determined by PROCHECK [29] are given in Table 2.
Table 2. Refinement statistics.
| VcCheY3 Ca2+bound | VcCheY3 Mg2+bound | VcCheY3-Q97A | VcCheY3-BeF3 − | VcCheY4sulf | VcCheY4free | |
| Rcryst (%)a | 20.2 | 20.0 | 22.5 | 23.1 | 21.8 | 22.5 |
| Rfree (%)b | 22.9 | 22.5 | 25.2 | 24.3 | 24.6 | 26.0 |
| r.m.s.d bond (Å) | 0.005 | 0.016 | 0.019 | 0.012 | 0.006 | 0.009 |
| r.m.s.d angle (°) | 1.3 | 1.6 | 2.17 | 1.6 | 1.3 | 1.59 |
| No. of waters | 176 | 94 | 93 | 107 | 114 | 144 |
| B-factors (Å2) | 19.05 | 25.69 | 48.194 | 27.350 | 19.215 | 46.52 |
| Ramachandran plot (%) c | ||||||
| Most favored(%) | 97.5 | 95.1 | 97.5 | 92.7 | 98.3 | 94.9 |
| Allowed(%) | 2.5 | 4.8 | 2.5 | 5.7 | 1.7 | 34.2 |
| Disallowed(%) | 0.0 | 0.0 | 0.0 | 1.6 | 0.0 | 0.9 |
| PDB code | 3TO5 | 4LX8 | 4HNQ | 4HNS | 4H60 | 4HNR |
Rmerge = ∑hkl∑i|Ihkl-〈Ihkl〉|/∑hkl∑i(Ihkl), where Ihkl is the intensity of an individual reflection and 〈I hkl〉 is the average intensity over symmetry equivalents.
Rcryst = ∑hkl|Fobs Fcalcd|/∑hklFobs, where Fobs and Fcalcd are the observed and calculated structure factor amplitudes, respectively.
R free is the equivalent of R-factor, calculated for a randomly chosen set of the reflections (5%) that were omitted throughout the refinement process. VM is the partial specific volume.
As defined by PROCHECK.
Calculation of Normalized B Factor
Since VcCheY4free and VcCheY4sulf crystals grew in different space groups and their diffraction resolutions are different, to compare their B factors we have plotted their normalized B-factor or B′-factor. Crystallographic B-factors of proteins determined even at high resolutions show large variations from one structure to another but the B-factors expressed in units of standard deviation about their mean value (normalized B-factor or B′-factor) shows consistent behaviour [30]–[32]. The equation used by us to calculate the normalized B-factor is B′ = B-<B>/σ<B>; where <B> is the average B value for the whole molecule based on Cα atoms and σ<B> is the standard deviation of the B values.
Results
Overall Structures of VcCheY3 and VcCheY4
As expected, both VcCheY3 and VcCheY4 possess (β/α)5 fold (Figure 1 b, c) typical of the response regulators. Structure of VcCheY3 in free state superposes on S. typhimurium CheY (StCheY; PDB code: 2 CHE) with a root mean square deviation (rmsd) of 0.4 Å (for 108 Ca atoms) (Figure 1b). VcCheY4 was crystallized in two different states; one is in free state with no ligand attached (VcCheY4free) and another with a sulfate and a Ca2+ ion bound at the active site (VcCheY4sulf). Interestingly, when VcCheY4free is superposed on VcCheY4sulf significant differences are observed at the active site, together with helix α4, β4α4 loop and β3α3 loop (Figure 1c). Since VcCheY4free and VcCheY4sulf were crystallized in different space groups, we have checked the probable influence of the crystal packing on the observed structural differences. Our packing analysis suggests that, in either case, these regions are rather loosely packed and their conformations are not influenced by crystal packing. VcCheY4, in either state, is significantly different from that of VcCheY3 (Figure 1b) and superposition of VcCheY4free and VcCheY4sulf on VcCheY3 produces rmsd values of 1.4 Å and 1.2 Å respectively. VcCheY4, in either state, differs from VcCheY3 mainly in the α1, α5, α4 regions and in the β3α3 loop (Figure 1b). It is to be noted that α1 and α5 were implicated previously in CheA and FliM binding respectively [33].
We have solved the structures of VcCheY3 in Ca2+ and Mg2+ bound states to the resolutions of 1.67 Å (Figure S1a) and 2.2 Å (Figure S1b) respectively. The location of the Ca2+ (or Mg2+) ion in VcCheY3 is similar to that of Mg2+ ion in StCheY. The Ca2+ (or Mg2+) of VcCheY3 is heptacoordinated where four coordinations occur with protein atoms and three with water molecules (Figure 2a). In contrast to that, the Mg2+ of StCheY is hexacoordinated. Although D12 of StCheY is not coordinated to Mg2+, D15 of VcCheY3 that corresponds to D12 of StCheY, coordinates with the metal ion (Figure 2a). Except this residue the disposition of the side chains of the other residues that coordinate with the metal ion are more or less similar in these structures (Figure 2a). The average coordination distance between Ca2+ and the protein atoms is about 2.4 Å while this is of about 2.1 Å in case of Mg2+ which is due to the size difference of the ions.
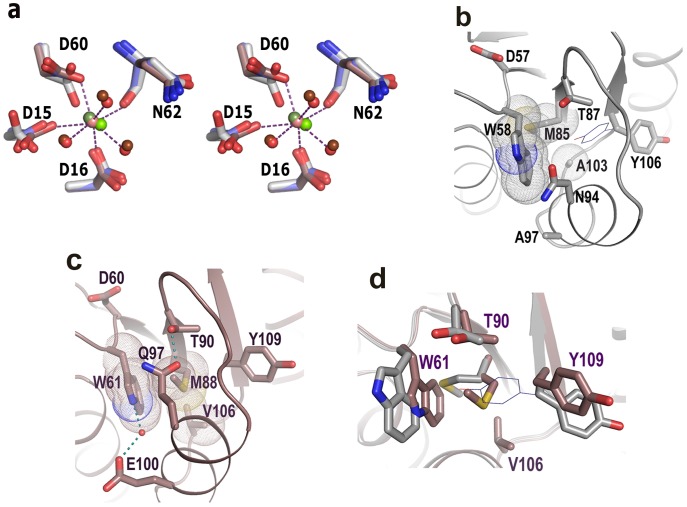
Figure 2. Metal binding and conformational barrier in VcCheY3.
(a) Stereo representation to compare the Ca2+ and Mg2+ binding at the active site of VcCheY3 (violet) with the Mg+2 binding of StCheY3 (grey). Ca2+ bound to VcCheY3 is shown as pink sphere, Mg2+ bound to VcCheY3 is shown as dark green sphere and Mg2+ bound to StCheY3 is shown as light green sphere. Waters bound to of Ca2+ and Mg2+ are shown as light and dark red spheres respectively. Only the hydrogen bonds, observed in Ca2+ bound VcCheY3 are shown for clarity; (b) preformed pocket for the ‘in’ position for Y106 in EcCheY (thin line), coordinates for the ‘in’ position of Y106 is taken from the activated EcCheY structure (PDB code:1F4V), (c) The hydrophobic packing of W61, M88, V106, hydrogen bond between T90 and Q97, and water mediated hydrogen bond between E100 and W61 that make a conformational barrier in VcCheY3, (d) superposition of ‘b’ and ‘c’ showing the buried conformation of W61 and its packing with M88 in VcCheY3 (violet) compared to StCheY (grey), ‘in’ position of Y109 (thin line) makes clashes with VcCheY3 residues,
Identification of a Conformational Barrier Towards Activation of VcCheY3
In StCheY or EcCheY, upon phosphorylation at D57, a series of structural changes occur near the active site. T87 along with β4α4 loop moves toward the active site and stabilizes the bound phosphate through hydrogen bonding. Y106 of β5 executes an ‘inward’ movement (shown in line in Figure 2b) with minimal conformational adjustments of W58 and M85 and that inward movement of Y106 is essential for the binding of FliM at α4-β5-α5 face of CheY. K109 and the Mg2+ contribute to stabilize phosphorylated D57 [34]. In the free state StCheY, W58 stays more on the surface (with χ1 of 174°, χ2 of 101°) and M85 side chain adopts such a χ1 value (−155°) that together these residues leave a preformed cavity for the ‘inward’ positioning of Y106 upon activation (Figure 2b).
D60 is the site of phosphorylation in VcCheY3 as it corresponds to D57 of StCheY (Figure 1a). Both in the Mg2+ and Ca2+ bound free state structures of VcCheY3, the side chain of W61 (that corresponds to W58 of StCheY) is observed in a conformation, substantially different from that of StCheY (Figure 2c, 2d). In the free state structure of VcCheY3, the side chain of W61 buries unusually deeply with a χ1 of −135° and χ2 of −133° (Figure 2c). Y109 stays in its ‘out’ position and the side chain of M88 (with χ1 of 64°, χ2 of 175°) stays between W61 and Y109, packing snugly with W61, Y109 and V106 through hydrophobic interactions (Figure 2c). This packing essentially fills up the pocket, required for the ‘inward’ positioning of Y109 upon activation (Figure 2c, 2d).
Moreover, in this inactivated structure of VcCheY3, the crucial T90 of β4α4 loop (that corresponds to T87 of StCheY), which stabilizes the bound phosphate on D60 upon activation, is hydrogen bonded with Q97 (Figure 2c). To the best of our knowledge, this kind of interaction involving the Thr of β4α4 loop was not observed so far in any other response regulator. In VcCheY3, T90 and Q97 are oriented in such a fashion that together they form a capping on the aforesaid hydrophobic packing and at the same time block the ‘out to in’ trajectory of Y109 (Figure 2d). Additionally, the side chain carboxylate group of E100 (which is Ala in EcCheY or StCheY) forms a water mediated hydrogen bond with NE1 of W61 (Figure 2c; Figure S3a, S3b). Therefore, the hydrophobic packing of W61 with M88 and V106, together with the hydrogen bond between T90 and Q97 and the water mediated interaction between W61 and E100 seem to make a conformational barrier that may affect the process of activation in VcCheY3.
Comparison of Phosphorylation Events Through Fluorescence Spectroscopy
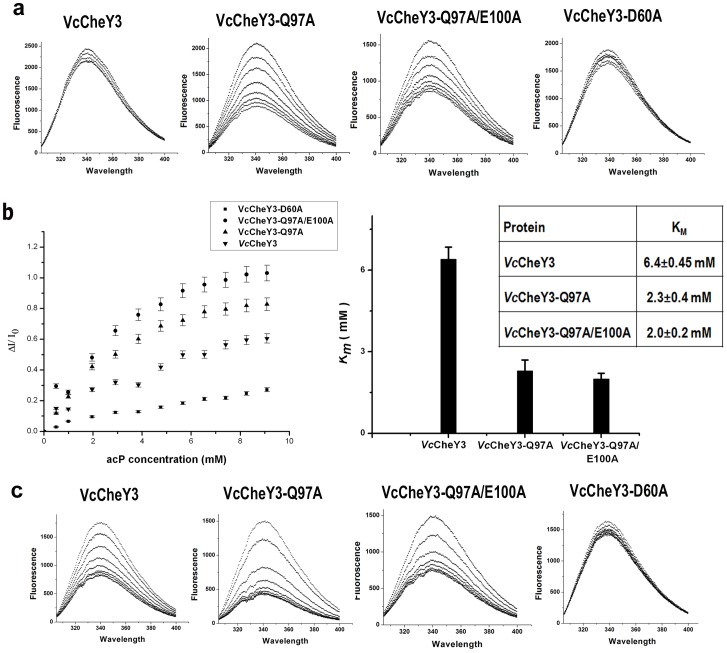
To investigate the contribution of the proposed ‘conformational barrier’ of VcCheY3 towards its activation, we prepared three mutants VcCheY3-Q97A, VcCheY3-Q97A/E100A and VcCheY3-D60A. Since W61 is within the Forster distance of D60, tryptophan quenching study was performed with VcCheY3 and its mutants to monitor the phosphorylation event using acetyl phosphate (acP) as substrate. Interestingly, VcCheY3 showed very low quenching (Figure 3a) indicating that phosphorylation at D60 does not induce any conformational change in W61 and W61 remains buried even after the treatment with acP. VcCheY3-Q97A and VcCheY3-Q97A/E100A, on the other hand, showed considerable quenching in the presence of acP (Figure 3a), suggesting that in the absence of the hydrogen bond between T90 and Q97 (and also in absence of E100), conformational alteration of W61 may take place more easily and it can move toward the surface of the molecule. As expected, quenching is almost negligible for the nonphosphorylatable analog VcCheY3-D60A (Figure 3a). Based on these experiments we have calculated the Km (Km = Ks. k3/k2) values where a higher Km value implies a decrease in the binding affinity between CheY and the phosphodonor (i.e. larger Ks), a slower rate of phosphorylation of bound CheY (i.e. smaller k2) or a faster rate of autodephosphorylation (i.e. larger k3) [35]. Km value, obtained by us, was the highest for VcCheY3 (6.4±0.45 mM) followed by VcCheY3-Q97A (2.3±0.4 mM) and VcCheY3-Q97A/E100A (2.0±0.2 mM) (Figure 3b) which are in accordance with our structural observations.
Figure 3. Activation of VcCheY3 and its mutants, measured through fluorescence quenching.
(a) Tryptophan quenching of VcCheY3 and its different mutants (indicated at top of the figure) using acetyl phosphate (acP) as substrate. (b) Plot of ΔI/I0 vs acP concentration (in mM) and corresponding Km values (both in graphical and numerical modes); (c) Tryptophan quenching of VcCheY3 and its different mutants (indicated at top of the figure) using BeF3 − as substrate.
Structure of VcCheY3-Q97A
To investigate whether the hydrogen bond between T90 and Q97 affects the hydrophobic packing of W61, M88 and V106, we have solved the structure of VcCheY3-Q97A. As expected, the overall structure of VcCheY3-Q97A is almost identical to that of VcCheY3 and the Mg2+ ion bound at the active site occupies the equivalent position to that of Mg2+ (or Ca2+) of VcCheY3 (Figure 4a). Interestingly, even in the absence of the hydrogen bond between T90 and Q97, the conformation and packing of W61, M88 and V106 are found to be unaltered with respect to the wild type VcCheY3 (Figure 4b). However, the water mediated hydrogen bond between W61 and E100 is not seen in this mutant. E100 is slightly reoriented here and has moved toward the CD1 atom of the adjacent I69 (Figure 4b). These observations, coupled with the quenching results, point to the fact that although the hydrophobic packing of W61, M88 and V106 is independent of the hydrogen bond between T90 and Q97 in free state, in the absence of the later interaction, reorientation of W61 and M88 occurs more smoothly upon phosphorylation.
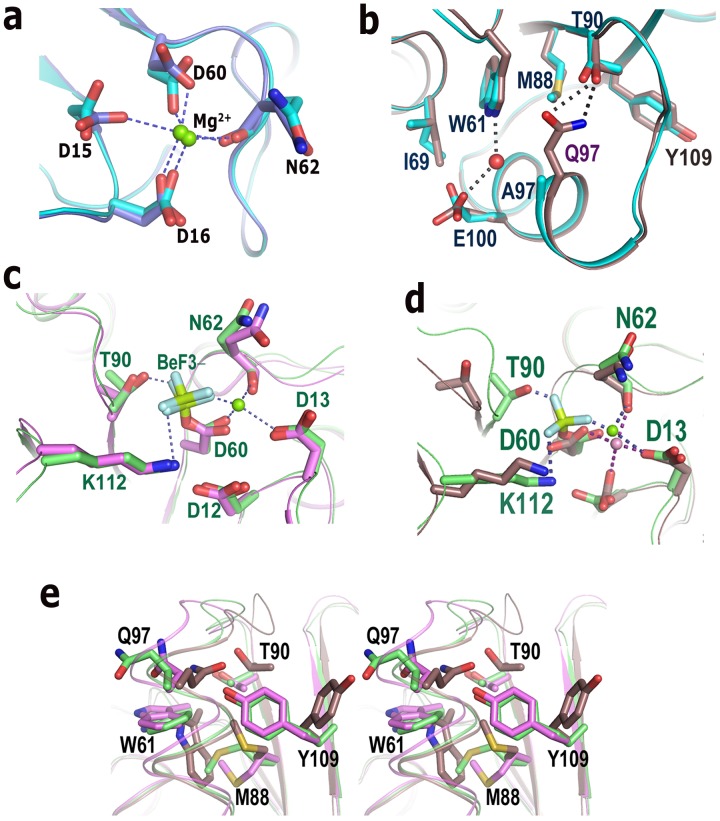
Figure 4. Mg2+ binding, activation of VcCheY3 and comparison with EcCheY.
(a) Comparison of the Mg2+ binding in VcCheY3 (blue) and VcCheY3-Q97A (cyan); (b) superposition of VcCheY3 (violet) and VcCheY3-Q97A (cyan) showing that the hydrogen bond between Q97 and T90 does not directly influence the conformation of W61 and M88; (c) comparison of the active site of VcCheY3-BeF3 − (green) with EcCheY3-BeF3 − (magenta); (d) comparison of the active site of VcCheY3-BeF3 − (green) with free state VcCheY3 (violet); (e) stereoscopic representation comparing the ‘in’ position and the conformation of the neighbouring residues in VcCheY3-BeF3 − (green), EcCheY3-BeF3 − (magenta) with respect to VcCheY3 (violet).
Structure of VcCheY3-BeF3 −
Quenching data using acP (Figure 3a) clearly indicate that obtaining of stable VcCheY3-P for crystallographic study is not possible. Since BeF3 − readily forms persistent activated complexes with many response regulators, regardless of the half-lives of their phosphorylated states, this is regularly used to structurally mimic the phosphorylated state of the response regulators [36]. Fluorescence quenching experiment for VcCheY3 and its mutants, performed in the presence of BeF3 −, showed approximately 30 fold lowering of the Km values (219.0±0.6 µM, 110.0±2.1 µM, and 96.4±1.4 µM for VcCheY3, VcCheY3-Q97A and VcCheY3-Q97A/E100A respectively) compared to that of acP (Figure 3c). Thus, to visualize the structural changes in VcCheY3 upon phosphorylation, we have activated VcCheY3 using BeF3 − and solved the structure of VcCheY3-BeF3 − to 2.1 Å.
The active site of VcCheY3-BeF3 − largely resembles to that of EcCheY-BeF3 − (PDB code: 1F4V) (Figure 4c). In VcCheY3-BeF3 −, BeF3 − is covalently linked with D60 and Mg2+ is properly poised to interact with BeF3 − (Figure 4c, 4d). To stabilize the bound BeF3 −, the side chain of K112 reorients and T90 along with the β4α4 loop moves toward the active site with a conformational change, hallmark for the activation of this type of CheYs (Figure 4d). The hydrogen bond between T90 and Q97 is abolished and Q97 side chain moves away from T90 (Figure 4e). Breaking the hydrophobic packing with M88, the side chain of W61 moves toward the surface (with χ1 of −166°, χ2 of −34°) acquiring a conformation similar to that observed in EcCheY-BeF3 − (Figure 4e). Under that situation, M88 occupies the space left by W61 and creates a pocket, sufficient to accommodate the ‘in’ position of Y109 which is essential for FliM binding (Figure 4e).
Free and Sulfated Structures of VcCheY4
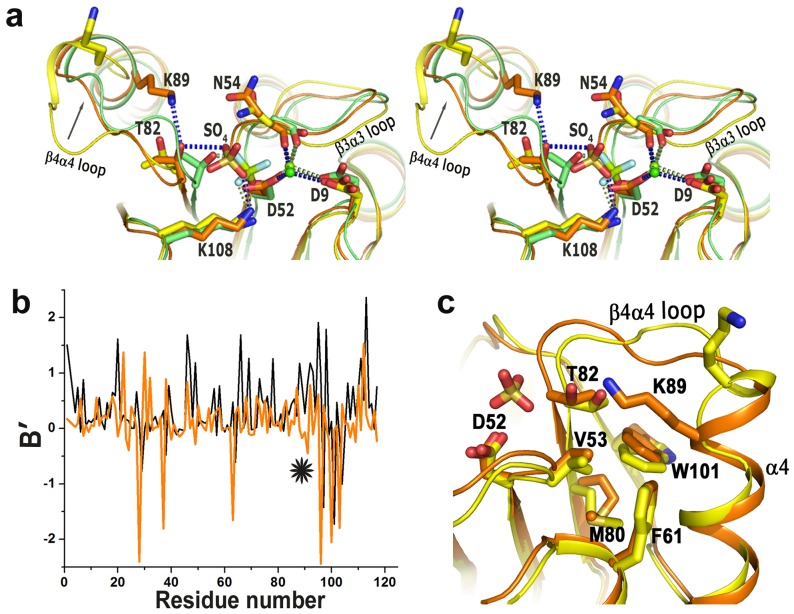
Although the overall structures of VcCheY4free and VcCheY4sulf are similar, substantial conformational differences are observed between these two, especially around the active site, in helix α4 and β4α4 loop. A Ca2+ ion is located at the active site of VcCheY4sulf which coordinates with D9, D52 and main chain carbonyl oxygen of N54 with an average coordination distance of 2.4 Å (Figure 5a; Figure S2a). A tetrahedral positive electron density was observed in the active-site pocket of VcCheY4sulf during refinement which was interpreted as a sulfate ion because VcCheY4 was crystallized using ammonium sulfate as precipitant (Figure S2a). In contrast to that, neither a metal ion nor a sulfate ion was observed at the active site of VcCheY4free although both of these components were added during crystallization (Figure 5a; Figure S2b). Absence of the divalent metal ion do not cause any change in the side chain conformation of D9 and D52 compared to VcCheY4sulf, but the carbonyl oxygen of N54 points away from the metal binding side (Figure 5a). As a result, the β3α3 loop of VcCheY4free takes a different conformation and moves about 3 Å away from the active site (Figure 5a). In VcCheY4free, helix α4 is shorter and β4α4 loop is unusually longer compared to those of VcCheY4sulf (Figure 1c). Electron density around the β4α4 loop of VcCheY4free is shown in the Figure S2c. The plot of B′-values indicated that the crystallographic B-factor of the β4α4 loop is much lower in VcCheY4sulf compared to that of VcCheY4free (Figure 5b). In VcCheY4sulf part of the β4α4 loop is stabilized and adopts a helical structure effectively extending the length of α4 (Figure 5c) and overall, the VcCheY4sulf structure seems to be more compact compared to VcCheY4free.
Figure 5. Structure of VcCheY4 in free and sulphated states.
(a) stereo view of the superposition of VcCheY4free (yellow) and VcCheY4sulf (orange) on activated VcCheY3-BeF3 − (green) showing the location and the interactions of the sulfate ion in VcCheY4sulf, relative movement of T82, hydrogen bond between K89 and T82 in VcCheY4sulf and the interactions of the metal ion with the neighbouring residues; (b) B′ plot of VcCheY4free (black) and VcCheY4sulf (orange) showing reduction of flexibility of the β4α4 loop (*) in VcCheY4sulf; (c) superposition of VcCheY4free (yellow) on VcCheY4sulf (orange) showing the conformational difference at the β4α4 loop and packing of W101 in its exclusive ‘in’ position.
The location of the sulfate ion at the active site of VcCheY4sulf is somewhat similar to BeF3 − of VcCheY3-BeF3 − (Figure 5a). T82 and K104, which are well known to stabilize the phosphoryl group in the other reported CheY structures, stabilize the sulfate ion in VcCheY4sulf through hydrogen bonding. A movement of about 2 Å towards the active site occurs for T82 along with the β4α4 loop (Figure 5a). Interestingly, in VcCheY4sulf, an additional hydrogen is generated between T82 and K89 (K89 corresponds to Q97 of VcCheY3) which might further contribute to the compactness of α4 in VcCheY4sulf (Figure 5a, 5c).
The crucial residue at β5 that acquires ‘in’ position upon activation is a Trp (W101) in case of VcCheY4 and in both the structures of VcCheY4 the side chain of W101 acquired ‘in’ position. In fact, this is the first structure of a naturally occurring CheY where Trp at this crucial position is observed to spontaneously occupy ‘in’ position, even without activation. In this case, W101 fits in a hydrophobic pocket made of V53, F61 and M80 (Figure 5c) and apart from making a hydrogen bond with T82, the hydrophobic part of K89 packs with W101 further contributing to the stability of VcCheY4sulf.
Molecular Mechanism of FliM Binding in V. Cholerae
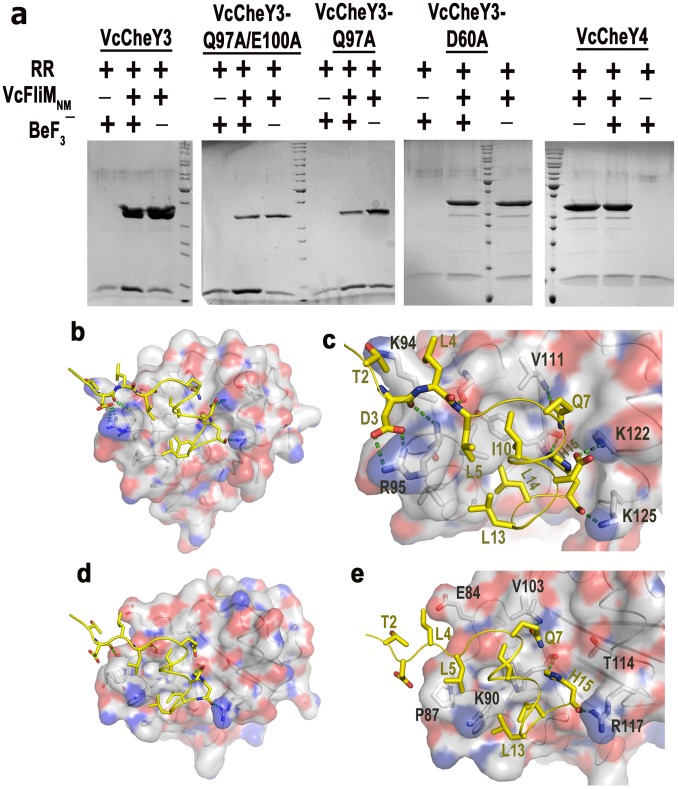
To investigate the binding ability of VcCheY3 and VcCheY4 with VcFliM, we performed an in-vitro pull down assay. VcFliMNM (a construct having the N-terminal and the middle domain of VcFliM with a C-terminal 6×His-tag) was immobilized on Ni-NTA resin, which was then incubated with VcCheY3, VcCheY3-Q97A, VcCheY3-Q97A/E100A, VcCheY3-D60A and VcCheY4, individually, in presence of Mg2+ but with or without BeF3 −. Our results showed that while the activated VcCheY3, VcCheY3-Q97A and VcCheY3-Q97A/E100A can interact with VcFliMNM, VcCheY3-D60A and VcCheY4 do not show any significant interaction with VcFliMNM even in presence of BeF3 − and Mg2+ (Figure 6a). VcCheY3-D60A was used as the negative control, and the experiment performed with BeF3 − and without VcFliMNM quantified the basal level of adherence of VcCheYs in Ni-NTA agarose during experiment.
Figure 6. Interactions of FliMNM with VcCheY3, VcCheY4 and VcCheY3 mutants.
(a) Pull-down assays of VcCheY3, VcCheY3-Q97A, VcCheY3-Q97A/E100A, VcCheY3-D60A and VcCheY4 with VcFliMNM. Purified VcFliMNM in 0.2 mg/ml was immobilized on pre-washed resin. VcCheY3, VcCheY3-D60A and VcCheY4 in a 1∶1 molar ratio to VcFliMNM was incubated with immobilized VcFliMNM with or without BeF3 − at 25°C for 10 mins; (b) Docking of VcFliMN (16 residues) at the FliM binding face of VcCheY3-BeF3 −; (c) Zoomed view of (b) showing the probable interactions in detail; (d) Docking of VcFliMN (16 residue) at the probable FliM binding face of VcCheY4sulf; (e) Zoomed view of (d) showing the probable interactions in detail.
To identify the structural features of VcCheY3 and VcCheY4, responsible for the difference in affinity towards VcFliM, it was necessary to critically analyse their FliM binding surface. To start with, we prepared a model of the N-terminal 16 peptide of VcFliMN by 3D-JIGSAW and VcFliMN, thus prepared, was docked at the FliM binding face of VcCheY3-BeF3 − and VcCheY4sulf. The FliMN part of the coordinates of EcFliMN-EcCheY-BeF3 − complex structure (PDB code: 1F4V) were used as a template to prepare the model of VcFliMN and EcFliMN-EcCheY structure as a whole was used as template for the docking. The resulting models of VcFliMN-VcCheY3-BeF3 − or VcFliMN-VcCheY4sulf were then analysed to identify the structural determinants responsible for the differential FliM binding of VcCheY3 and VcCheY4. VcFliMN is observed to fit properly at the α4-β5-α5 cleft of VcCheY3-BeF3 − with considerable number of hydrogen bonds and hydrophobic interactions (Figure 6b, 6c) which are comparable with those of EcFliMN-EcCheY-BeF3 − (Table 3). In contrast to that, the probable interactions of VcCheY4sulf with VcFliMN are inadequate (Figure 6d, 6e; Table 3). The FliM binding face of VcCheY4sulf is not compatible enough for VcFliM. In VcCheY4sulf, the space between α4 and α5 is ∼2 Å wider compared to that of VcCheY3-BeF3 − which might cause a loose fit of VcFliMN at α4-β5-α5 face of VcCheY4sulf. Residues T2 and D3 of VcFliMN are found to interact with VcCheY3-BeF3 −, but no such interaction is possible with VcCheY4sulf (Figure 6c, 6e). Furthermore, in VcCheY3-BeF3 −, K122 of α5 is poised to form a salt bridge with D12 of VcFliMN, corresponding residue of VcCheY4sulf is T114 which is spatially away from D12 of VcFliMN and naturally no interaction is expected between this pair (Figure 6e). As a result, the overall interactions between VcFliMN and VcCheY4sulf are reduced significantly (Figure 6e, Table 3) supporting the observation of the pull down assay (Figure 6a).
Table 3. Residues of VcFliMN model, involved in the probable interactions with VcCheY3 and VcCheY4 structures, are compared with that of EcFliM-EcCheY structure.
| EcCheY3-BeF3 − | EcFliMN | VcCheY3-BeF3 − | VcFliMN | VcCheY4 |
| Polar interactions | ||||
| K91 | D3 | K94 NZ | T3 OG1 | – |
| K92 N | S4 O | R95 N | D4 O | – |
| R95 NH1, NH2 | D4 OD1, OD2 | – | ||
| A90 O | L6, N | A93 O | L6 N | T85 O |
| V108 N | Q8 OE1 | V111 N | Q8 OE1 | V104 N |
| K119 | D12 | K122 NZ | D12 OD1 | – |
| Y106 N | D16 OD1 | Y109 O | H16 NE2 | – |
| Y106 O | D16 N | |||
| K122 NZ | D16 O | K125 NZ | H16 O | R117 NH1 |
| Hydrophobic interactions | ||||
| I95 | L6 | R95, I98 | L6 | P87 |
| I95, A99, Y106 | I11, L14 | I98, I99, Y109,V106, A102 | I11, L14, L15 | K90, W101 |
Discussion
Unlike E. coli two-component chemosensory pathway that relies on a single copy of response regulator CheY, V. cholerae possesses four CheY homologues. Occurrence of multiple CheYs is not unusual in bacteria as these are also found in R. sphaeroides and B. Burgdorferi [5]. Recent studies have demonstrated that multiple copies of CheY play specific roles in the chemotactic signal transduction mechanisms. As for example, among the three CheYs of B. burgdorferi only CheY3 directly regulates motor action while the other two cannot bind to the motor and act as signal terminating phosphate sink [37]. Similarly, in R. sphaeroides only CheY6 can change the direction of the flagellar motor, although the others bind FliM probably to regulate the level of the phosphodonor [38], [39]. An intriguing question, therefore, arises about the role of multiple copies of CheY in V. Cholerae, especially of VcCheY3 and VcCheY4.
Together, phosphorylation at the active site Asp, hallmark movement of the Thr and the β4α4 loop toward the active site to stabilize the bound phosphate, ‘in’ positioning of the crucial hydrophobic residue of β5 and FliM binding at the α4-β5-α5 face to reverse the flagellar motion constitute the general mode of action of the chemotactic response regulators. In EcCheY or StCheY, a preformed pocket was seen to accommodate the ‘in’ position of the crucial β5 residue Y106 upon activation (Figure 2b). In contrast to that, in VcCheY3, this pocket is preoccupied by the hydrophobic packing of W61, M88 and V106 (Figure 2c). A unique hydrogen bond between T90 and Q97 additionally restricts the outward movement of W61, which is necessary to make a pocket for the ‘in’ positioning of Y109. This hydrogen bond also obstructs the movement of T90 toward the active site essentially hindering the stabilization of the phosphoryl group by T90. VcCheY3 shows minimum quenching in the presence of acP which further support the hindered movement of W61 upon phosphorylation at D60 (Figure 3a). VcCheY3-Q97A and VcCheY3-Q97A/E100A, on the other hand, show considerable quenching in the presence of acP indicating that in the absence of the hydrogen bond between T90 and Q97, W61 can easily be reoriented toward solvent and T90 can move toward the active site to stabilize the phosphoryl group.
Higher Km value of VcCheY3 compared to its mutants VcCheY3-Q97A and VcCheY3-Q97A/E100A further establishes the hindrance caused by the hydrogen bond between T90 and Q97 in stabilizing the acyl phosphate on D60. The lower Km values of VcCheY3-Q97A and VcCheY3-Q97A/E100A are due to the loss of the coupling between T90 and Q97 which facilitates the movement of T90 toward the active site and stabilize the acyl phosphate. A comparison of the Km value of VcCheY3 with the CheYs from Helicobacter pylori or E. coli shows that the Km of VcCheY3 is also higher than that of HpCheY1 (1.07±0.31 mM) and EcCheY (3.2±0.4 mM). As mentioned by Lam et al. (2010), Km increases with the increase in the ionic strength of the buffer used in the experiment [24]. While 200 mM salt was used in the experiment of EcCheY, only 50 mM salt was used for HpCheY1and VcCheY3 (and its mutants). Since our experimental condition is same as that of HpCheY1, we can clearly infer that the Km value of VcCheY3 is about six fold higher than that of HpCheY1.
As mentioned earlier, a higher Km (Km = Ks. k3/k2) implies a decrease in the binding affinity between CheY and the phosphodonor (larger Ks), a slower rate of phosphorylation of CheY (smaller k2) or a faster rate of autodephosphorylation (larger k3) [35]. The high Km value of VcCheY3 implies that either its phosphorylation occurs slowly or it has a higher rate of autodephosphorylation. Based on the swarming assay and swimming behaviour Hyakutake et al, (2005) reported that only the VcCheY3 directly switches the flagellar rotation [14]. Our pull down assay shows that VcCheY3 and its mutants VcCheY3-Q97A and VcCheY3-Q97A/E100A bind VcFliMNM efficiently in the presence of BeF3 − and Mg2+. Docking results suggest that VcFliMN can fit properly at the α4-β5-α5 face of the activated VcCheY3 with significant number of hydrogen bonding and hydrophobic interactions (Figure 6a, 6b; Table 3). Moreover, sequence comparison of VcCheY3 with EcCheY or StCheY denotes that the crucial residues implicated in binding the kinase CheA are conserved in VcCheY3 (Figure 1a). These observations indicate that although VcCheY3 has all the requisites for the phosphorylation, stabilization of the acyl phosphate is hindered due to the obstructed movement of T90 towards the active site. Lesser stabilization of the bound phosphate might be implicated in enhanced autodephosphorylation (larger k3) for VcCheY3, effectively causing lower rate of activation which is reflected in its higher Km value. The conformational barrier of VcCheY3, therefore, acts as a molecular switch to control the level of VcCheY3-P. Elevated temperature and/or adequacy of phosphate pool might break the barrier of the free-state VcCheY3 and flip it to the phosphorylated state for FliM binding.
Two distinct conformations, differing at helix α4 and the crucial β4α4 loop, are observed for VcCheY4. Among these two structures, VcCheY4sulf possesses a bound sulfate ion near the active site which occupies a position similar to the BeF3 − of StCheY-BeF3 − and VcCheY3-BeF3 − (Figure 5a). A bound sulfate ion was also observed in HpCheY1 structure (PDB code: 3GWG) where that sulfate ion caused conformational changes similar to the activated structure [24]. However, in HpCheY1, along with the conventional conformational changes, an unusual orientation of D53 was observed [24]. In VcCheY4sulf, the sulfate ion did not alter the side chain conformation of catalytic D52 but stayed very close (∼2.5 Å) to it (Figure 5a). Since VcCheY4sulf was crystallized at pH 4.0, at this pH D52 might be protonated allowing the sulfate ion to come to its close vicinity. In VcCheY4sulf, the sulfate ion is properly coordinated with the Ca2+ ion and is stabilized through the interactions with T82 and K104 (Figure 5a). Considering the compactness of the VcCheY4sulf structure having a shorter β4α4 loop with low B-factors, long α4 helix, movement of T82 and β4α4 loop to stabilize the sulfate ion and additional hydrogen bond between T82 and K89, it can be said that VcCheY4 has a strong tendency to be phosphorylated in the presence of a divalent metal ion and the phosphorylated state is more stable compared to its free state.
Despite the fact that the crucial β5 residue W101 of VcCheY4 consistently acquires ‘in’ position, VcCheY4 fails to interact with VcFliMNM (Figure 6a). Through mutagenesis and structure-function studies Matsumura and collaborators showed that substitution of Y106 of EcCheY with tryptophan (Y106W) produces a phosphorylation-dependent, hyperactive mutant that generates mainly clockwise rotational bias upon interacting with FliM [40]. In contrast to that, despite the consistent ‘in’ position of W101, VcCheY4 does not interact with VcFliM, as the N terminal part of VcFliM does not fit at the α4-β5-α5 face of VcCheY4 because of their spatial and electrostatic incompatibility (Table 3, Figure 6e). This apparent contradiction suggest that FliM binding by CheY is not just influenced by the ‘in’ positioning of the β5 hydrophoc residue but the spatial and electrostatic compatibility of the α4-β5-α5 face of CheY with the N-terminal part of FliM plays a vital role in this process. Since, CheZ and FliM share a common face of CheY for binding with similar mode of interactions [41], VcCheY4 is expected not to interact efficiently with CheZ as well. This observation corroborates with the fact that no cheZ is found in the cluster III where cheY4 is located. Since VcCheY4 can be phosphorylated but cannot bind FliM and probably not CheZ as well, VcCheY4 might act as phosphate sink or it might induce the expression of some other genes upon phosphorylation which can indirectly modulate flagellar action and/or virulence.
VcCheY4 was seen to slightly enhance the spreading of an E. coli cheZ mutant in semisolid agar and based on that Hyakutake et al proposed that VcCheY4 can affect chemotaxis by removing a phosphoryl group from VcCheY3 [14]. Our observations intend us to hypothesise that if a phosphate pull is shared by VcCheY3 and VcCheY4 then VcCheY4 can cause a phosphate depleted situation for VcCheY3, as phosphorylated state of VcCheY4 is more stable compared to its unphosphorylated state, which is other way round for VcCheY3. Alternatively, in a phosphate depleted situation, additional energy might help phosphorylated VcCheY4 to release the phosphoryl group through conformation dependent autodephosphorylation, as proposed by Pazy et al., 2009 [42] based on their observations of the mutant EcCheY.
Supporting Information
Metal binding in Vc CheY3. (a) Electron density maps (2Fo-Fc) around the active site of VcCheY3 contoured at 1.2 σ level, Ca2+ is shown in pink sphere and water molecules as red dots. Ca2+ binding residues are labelled; (b) Electron density maps (2Fo-Fc) around the active site of VcCheY3 contoured at 1.0 σ level, Mg2+ is shown as white star and waters are shown in red stars. Mg2+ binding residues are labelled.
(DOCX)
Electron density map of Vc CheY4. Electron density map (2Fo-Fc) contoured at 1.0 σ level (a) around the active site of VcCheY4sulf in stereo, (b) around the active site of VcCheY4free, (c) around the β4α4 loop of VcCheY4free.
(DOCX)
Interaction of W61 with E100. (a) Electron density map (2Fo-Fc) contoured at 1.0 σ level around the water molecule that connects W61, M88, E100 along with the water molecule in Ca2+ bound VcCheY3; (b) Water mediated interaction of W61 with E100 in Mg2+ bound VcCheY3.
(DOCX)
(PDF)
Acknowledgments
We are extremely thankful to Prof. Anirban Siddhanta of the Dept of Biochemistry, University of Calcutta and Prof. Abhijit Chakrabarti of SINP for generously allowing us to use the spectrofluorometer. JD and MB are grateful to Mr. Abhijit Bhattacharya and Mr. Samir Das of SINP for their technical and academic supports. JD is grateful to Dr. J Felix Raj SJ, Principal, St. Xavier’s College, Kolkata for his encouragement and constant support.
Accession Codes
Protein Data Bank: Coordinates and structure factor files have been deposited with the accession codes 3TO5, 4HNQ, 4HNS, 4HNR 4H60 and 4LX8 [see Summary Reports in “Supporting Information S1”].
Funding Statement
Partial funding from: CSIR/37(1381)/09/EMR-II, Govt. of India. No additional external funding were received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Butler SM, Camilli A (2004) Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc Natl Acad Sci U S A 101: 5018–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freter R, Jones GW (1976) Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun 14: 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boin MA, Austin MJ, Hase CC (2004) Chemotaxis in Vibrio cholerae. FEMS Microbiol Lett 239: 1–8. [DOI] [PubMed] [Google Scholar]
- 4. Eisenbach M (1990) Functions of the flagellar modes of rotation in bacterial motility and chemotaxis. Mol Microbiol 4: 161–167. [DOI] [PubMed] [Google Scholar]
- 5. Wuichet K, Zhulin IB (2010) Origins and diversification of a complex signal transduction system in prokaryotes. Sci Signal 3: ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Szurmant H, Ordal GW (2004) Diversity in chemotaxis mechanisms among the bacteria and archaea. Microbiol Mol Biol Rev 68: 301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mauriello EM, Mignot T, Yang Z, Zusman DR (2010) Gliding motility revisited: how do the myxobacteria move without flagella? Microbiol Mol Biol Rev 74: 229–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhaya D, Takahashi A, Grossman AR (2001) Light regulation of type IV pilus-dependent motility by chemosensor-like elements in Synechocystis PCC6803. Proc Natl Acad Sci U S A 98: 7540–7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Black WP, Schubot FD, Li Z, Yang Z (2010) Phosphorylation and dephosphorylation among Dif chemosensory proteins essential for exopolysaccharide regulation in Myxococcus xanthus. J Bacteriol 192: 4267–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berleman JE, Bauer CE (2005) A che-like signal transduction cascade involved in controlling flagella biosynthesis in Rhodospirillum centenum. Mol Microbiol 55: 1390–1402. [DOI] [PubMed] [Google Scholar]
- 11. Porter SL, Roberts MA, Manning CS, Armitage JP (2008) A bifunctional kinase-phosphatase in bacterial chemotaxis. Proc Natl Acad Sci U S A 105: 18531–18536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, et al. (2000) DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406: 477–483 Vibrio cholerae. Innate Immun 15: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Banerjee R, Das S, Mukhopadhyay K, Nag S, Chakrabortty A, et al. (2002) Involvement of in vivo induced cheY-4 gene of Vibrio cholerae in motility, early adherence to intestinal epithelial cells and regulation of virulence factors. FEBS Lett 532: 221–226. [DOI] [PubMed] [Google Scholar]
- 14. Hyakutake A, Homma M, Austin MJ, Boin MA, Hase CC, et al. (2005) Only one of the five CheY homologs in Vibrio cholerae directly switches flagellar rotation. J Bacteriol 187: 8403–8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bandyopadhaya A, Chaudhuri K (2009) Differential modulation of NF-kappaB-mediated pro-inflammatory response in human intestinal epithelial cells by cheY homologues of Vibrio cholerae. Innate Immun 15(3): 131–42. [DOI] [PubMed] [Google Scholar]
- 16. Stock AM, Mottonen JM, Stock JB, Schutt CE (1989) Three-dimensional structure of CheY, the response regulator of bacterial chemotaxis. Nature 337: 745–749. [DOI] [PubMed] [Google Scholar]
- 17. Volz K, Matsumura P (1991) Crystal structure of Escherichia coli CheY refined at 1.7-A resolution. J Biol Chem 266: 15511–15519. [DOI] [PubMed] [Google Scholar]
- 18. Bourret RB, Hess JF, Simon MI (1990) Conserved aspartate residues and phosphorylation in signal transduction by the chemotaxis protein CheY. Proc Natl Acad Sci U S A 87: 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu X, Rebello J, Matsumura P, Volz K (1997) Crystal structures of CheY mutants Y106W and T87I/Y106W. CheY activation correlates with movement of residue 106. J Biol Chem 272: 5000–5006. [DOI] [PubMed] [Google Scholar]
- 20. Zhu X, Volz K, Matsumura P (1997) The CheZ-binding surface of CheY overlaps the CheA- and FliM-binding surfaces. J Biol Chem 272: 23758–23764. [DOI] [PubMed] [Google Scholar]
- 21. Khamrui S, Biswas M, Sen U, Dasgupta J (2010) Cloning, overexpression, purification, crystallization and preliminary X-ray analysis of CheY3, a response regulator that directly interacts with the flagellar ‘switch complex’ in Vibrio cholerae. Acta Crystallogr Sect F Struct Biol Cryst Commun 66: 944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Biswas M, Khamrui S, Sen U, Dasgupta J (2011) Overexpression, purification, crystallization and preliminary X-ray analysis of CheY4 from Vibrio cholerae O395. Acta Crystallogr Sect F Struct Biol Cryst Commun 67: 1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lukat GS, McCleary WR, Stock AM, Stock JB (1992) Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc Natl Acad Sci U S A 89: 718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lam KH, Ling TK, Au SW (2010) Crystal structure of activated CheY1 from Helicobacter pylori. J Bacteriol 192: 2324–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Collaborative Computational Project N (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50: 760–763. [DOI] [PubMed] [Google Scholar]
- 26. Jones TA, Zou JY, Cowan SW, Kjeldgaard M (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A 47 (Pt 2): 110–119. [DOI] [PubMed] [Google Scholar]
- 27. Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, et al. (1998) Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54: 905–921. [DOI] [PubMed] [Google Scholar]
- 28. Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60: 2126–2132. [DOI] [PubMed] [Google Scholar]
- 29. Laskowski RA, MacArthur MW, Thornton JM (1998) Validation of protein models derived from experiment. Curr Opin Struct Biol 8: 631–639. [DOI] [PubMed] [Google Scholar]
- 30. Parthasarathy S, Murthy MR (1997) Analysis of temperature factor distribution in high-resolution protein structures. Protein Sci 6: 2561–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carugo O, Argos P (1998) Accessibility to internal cavities and ligand binding sites monitored by protein crystallographic thermal factors. Proteins 31: 201–213. [PubMed] [Google Scholar]
- 32. Yuan Z, Zhao J, Wang ZX (2003) Flexibility analysis of enzyme active sites by crystallographic temperature factors. Protein Eng 16: 109–114. [DOI] [PubMed] [Google Scholar]
- 33. Bell CH, Porter SL, Strawson A, Stuart DI, Armitage JP (2010) Using structural information to change the phosphotransfer specificity of a two-component chemotaxis signalling complex. PLoS Biol 8: e1000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee SY, Cho HS, Pelton JG, Yan D, Berry EA, et al. (2001) Crystal structure of activated CheY. Comparison with other activated receiver domains. J Biol Chem 276: 16425–16431. [DOI] [PubMed] [Google Scholar]
- 35. Silversmith RE, Appleby JL, Bourret RB (1997) Catalytic mechanism of phosphorylation and dephosphorylation of CheY: kinetic characterization of imidazole phosphates as phosphodonors and the role of acid catalysis. Biochemistry 36: 14965–14974. [DOI] [PubMed] [Google Scholar]
- 36. Cho H, Wang W, Kim R, Yokota H, Damo S, et al. (2001) BeF(3)(-) acts as a phosphate analog in proteins phosphorylated on aspartate: structure of a BeF(3)(-) complex with phosphoserine phosphatase. Proc Natl Acad Sci U S A 98: 8525–8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sourjik V, Schmitt R (1998) Phosphotransfer between CheA, CheY1, and CheY2 in the chemotaxis signal transduction chain of Rhizobium meliloti. Biochemistry 37: 2327–2335. [DOI] [PubMed] [Google Scholar]
- 38. Porter SL, Wadhams GH, Martin AC, Byles ED, Lancaster DE, et al. (2006) The CheYs of Rhodobacter sphaeroides. J Biol Chem 281: 32694–32704. [DOI] [PubMed] [Google Scholar]
- 39. Porter SL, Armitage JP (2004) Chemotaxis in Rhodobacter sphaeroides requires an atypical histidine protein kinase. J Biol Chem 279: 54573–54580. [DOI] [PubMed] [Google Scholar]
- 40. Zhu X, Amsler CD, Volz K, Matsumura P (1996) Tyrosine 106 of CheY plays an important role in chemotaxis signal transduction in Escherichia coli. J Bacteriol 178: 4208–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guhaniyogi J, Wu T, Patel SS, Stock AM (2008) Interaction of CheY with the C-terminal peptide of CheZ. J Bacteriol 190: 1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pazy Y, Wollish AC, Thomas SA, Miller PJ, Collins EJ, et al. (2009) Matching biochemical reaction kinetics to the timescales of life: structural determinants that influence the autodephosphorylation rate of response regulator proteins. J Mol Biol 392: 1205–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Metal binding in Vc CheY3. (a) Electron density maps (2Fo-Fc) around the active site of VcCheY3 contoured at 1.2 σ level, Ca2+ is shown in pink sphere and water molecules as red dots. Ca2+ binding residues are labelled; (b) Electron density maps (2Fo-Fc) around the active site of VcCheY3 contoured at 1.0 σ level, Mg2+ is shown as white star and waters are shown in red stars. Mg2+ binding residues are labelled.
(DOCX)
Electron density map of Vc CheY4. Electron density map (2Fo-Fc) contoured at 1.0 σ level (a) around the active site of VcCheY4sulf in stereo, (b) around the active site of VcCheY4free, (c) around the β4α4 loop of VcCheY4free.
(DOCX)
Interaction of W61 with E100. (a) Electron density map (2Fo-Fc) contoured at 1.0 σ level around the water molecule that connects W61, M88, E100 along with the water molecule in Ca2+ bound VcCheY3; (b) Water mediated interaction of W61 with E100 in Mg2+ bound VcCheY3.
(DOCX)
(PDF)