Abstract
Introduction
Although implantable cardioverter defibrillator (ICD) therapy reduces mortality in moderately symptomatic heart failure patients with an ejection fraction ≤35%, many such patients do not require ICD shocks over long-term follow-up.
Methods
Using a modification of a previously validated risk prediction model based on routine clinical variables, we examined the relationship between baseline predicted mortality risk and the relative and absolute survival benefits of ICD treatment in the primary prevention Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT).
Results
In the placebo arm, predicted 4-year mortality grouped into 5 equal-sized risk groups varied from 12% to 50% (c statistic=0.71), while the proportion of SCD in those same risk groups decreased from 52% to 24% of all deaths. ICD treatment decreased relative risk of SCD by 88% in the lowest risk group vs. 24% in the highest risk group (p =0.009 for the interaction), and relative risk of total mortality by 54% in the lowest risk group vs. no benefit (2%) in the highest risk group (p =0.014 for the interaction). Absolute 4-year mortality reductions were 6.6%, 8.8%, 10.6%, 14.0% and −4.9% across risk quintiles. In highest risk patients (predicted annual mortality >20%), no benefit of ICD treatment was seen. Projected over each patient’s predicted life span, ICD treatment added 6.3, 4.1, 3.0, 1.9, and 0.2 additional years of life in the lowest to highest risk groups, respectively.
Conclusions
A clinical risk-prediction model identified subsets of moderately symptomatic heart failure patients in SCD-HeFT in whom single lead ICD therapy was of no benefit and other subsets in which benefit was substantial.
Keywords: Arrhythmias, clinical electrophysiology, drugs, Congestive Heart Failure, Ablation/ICD/surgery, Health policy and outcome research
INTRODUCTION
Both the 2005 Clinical Practice Guidelines on the Management of Chronic Heart Failure1 and the 2008 Guidelines on Pacemakers and Implanted Devices from the American College of Cardiology and the American Heart Association rate as “class I” use of prophylactic ICD therapy in heart failure (HF) patients with NYHA class 2–3 symptoms and ejection fraction (EF) ≤35%, suggesting ICDs should be routinely placed in such patients as a part of evidence-based medicine.2 However, actual use of ICD treatment in this large population appears to have lagged behind these recommendations.3 Several reasons for this slow adoption can be offered, but two may be particularly relevant. First, patients with chronic heart failure and a depressed ejection fraction are prognostically heterogeneous both for overall mortality and for sudden death mortality.4 Second, because only approximately 20–25% of primary prevention ICD patients receive appropriate shocks within 5 years of implantation, many nominally eligible patients appear to not actually need this therapy.5,6 Although much interest exists in developing various novel testing strategies to identify subsets of patients most likely to benefit, to-date none of these prediction strategies have proven sufficiently discriminative nor received independent validation for use in general clinical practice.7
The Seattle Heart Failure Model (SHFM) is a multivariable risk model that predicts both all-cause and cause-specific mortality in HF patients. The model was developed in the PRAISE I Trial cohort and prospectively validated in 5 additional cohorts derived from both large clinical trials and outpatient community practice settings in the US and Europe.8,9 The SHFM uses routinely collected clinical variables to make predictions, not requiring specialized or costly testing. We postulated that ICDs may be more beneficial in relatively lower risk patients, in whom the predominant mode of death would be sudden cardiac death (SCD), and in whom SCD may more often be due to ventricular tachycardia/ventricular fibrillation (more amenable to ICD therapy) than electromechanical dissociation, pulmonary embolus, or ventricular tachycardia storm (less amenable to ICD therapy). We used patient-level data from the Sudden Cardiac Death in Heart Failure5 (SCD-HeFT- ClinicalTrials.gov number, NCT00000609) randomized trial to test the hypothesis that, among patients with moderate systolic HF, the SHFM-predicted risks could identify subsets of patients in whom clinically relevant differences in single lead ICD treatment benefit would be present.
METHODS
Study Patients
Patients were eligible for the SCD-HeFT Trial if they had NYHA class 2 or 3 heart failure with an ejection fraction of ≤35%.5 Compared to medical therapy alone, randomization to single-lead, ICD therapy (829 patients) reduced total mortality by 23% (p=0.007) in the overall trial, while amiodarone had no benefit. Of the 2,521 enrolled patients, 38 were excluded from the present analysis due to missing baseline variables for SHFM calculation.
Calculation of the SHFM
The SHFM is a validiated risk-prediction model based on routinely collected clinical variables.8 In SCD-HeFT, most SHFM variables were available, including age, gender, ischemic etiology, systolic blood pressure, ejection fraction, and medication use (ACEI, ARB, beta blocker, statin, and daily diuretic dose), serum sodium, but data were not available on allopurinol use, total cholesterol, hemoglobin, % lymphoctes, or uric acid. To account for the impact of these missing variables, we used a separate derivation cohort of 10,038 HF patients from 5 other studies including 23,037 patient-years of observation 10–14 to develop a modified version of the SHFM that included the SHFM predictor variables available in the SCD-HeFT population and additional prognostic variables using the Cox proportional hazards model and previously described methods.8 This new model, SHFM-D (differential ICD benefit), for simplicity will be abbreviated as SHFM in the manuscript. The final model, SHFM-D, derived in the separate derivation dataset, included the original SHFM variables age, gender, systolic blood pressure, ischemic etiology, NYHA class, ejection fraction, ACEI/ARB use, beta blocker use, statin use, furosemide equivalent daily dose in mg/kg, serum sodium, and new variables of digoxin use, carvedilol use, and creatinine, with each individual’s SHFM score derived as previously described.8 Baseline survival (survival for Score=0) was derived from measured survival in the derivation cohort between 0 and 5 years, fit using a third degree polynomial curve. The SHFM was then applied prospectively to patients in SCD-HeFT to provide individual estimates of annual survival through year 5. Number needed to treat for 4 years to save one life was calculated as 1/absolute risk reduction for ICD versus placebo at 4 years15. Total life expectancy was estimated by the Gompertz method using the SHFM estimated 1-year survival.16,17 Years needed to treat (YNT) were calculated as the number of years needed to treat a patient with an ICD to add 1 year of life as previously described 17, using the Gompertz method to estimate total life expectancy:
For the YNT analysis, the estimated lifespan for all patients within each quintile were averaged and the placebo and ICD groups were compared.
Ascertainment of Mortality
A centralized adjudication committee classified modes of death in SCD-HeFT.5 For this analysis, the primary outcomes were all-cause mortality, sudden cardiac death, and all other deaths, which include pump failure deaths (non-SCD). Patients who underwent transplant (n=61) or crossed over to an ICD (n=188) were analyzed using intention-to-treat principles. Median follow-up was 3.8 years (range 2.1–6.0 years). Vital status was known on all patients.
Statistical analysis
The SHFM regression coefficients derived on the external data set were used to calculate a risk score and predicted survival for each SCD-HeFT patient using each individual’s specific values of the variables that were included in the SHFM. Quintiles of SHFM-predicted survival were plotted against observed (Kaplan-Meier) survival for the placebo group at 1 and 4 years. The ability of the risk score to provide different predictions for patients who lived versus those who died (i.e., discrimination) was evaluated using the c-statistic for time-to-event data. Confidence intervals for c-statistics were generated by drawing 200 bootstrap samples from the placebo group, fitting a Cox model using the SHFM risk score, and calculating the c-statistic for each sample. The confidence interval for the c-statistic was then calculated as ±1.96 * the standard deviation of the 200 c-statistics. The SHFM score and randomization group (ICD, amiodarone, or placebo) were entered into a Cox model to determine the risk-adjusted effects (hazard ratio, hereafter referred to as relative risk) of ICD and amiodarone therapy on all-cause and cause-specific mortality. We used the same SHFM risk score (derived using all cause mortality in the external data set) for examining all three outcomes (all-cause mortality, sudden cardiac death, and all other deaths) rather than building a separate cause-specific model for each outcome. Potential interaction (effect modification) between SHFM-predicted mortality and randomization group was evaluated by respectively adding multiplicative interaction terms (SHFM score*amiodarone, SHFM score*ICD) to the Cox model as continuous variables. Potential interaction between SHFM-predicted risk and ICD therapy was further evaluated in stratified analyses by quintiles of SHFM-predicted mortality. We used Statview 5 (SAS Institute, NC) for the external derivation of the SHFM and SAS v. 8.2 (SAS Institute, NC) for analyses in SCD-HeFT. Statistical significance was defined as alpha<0.05 (two-tailed). The authors had full access to the data and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
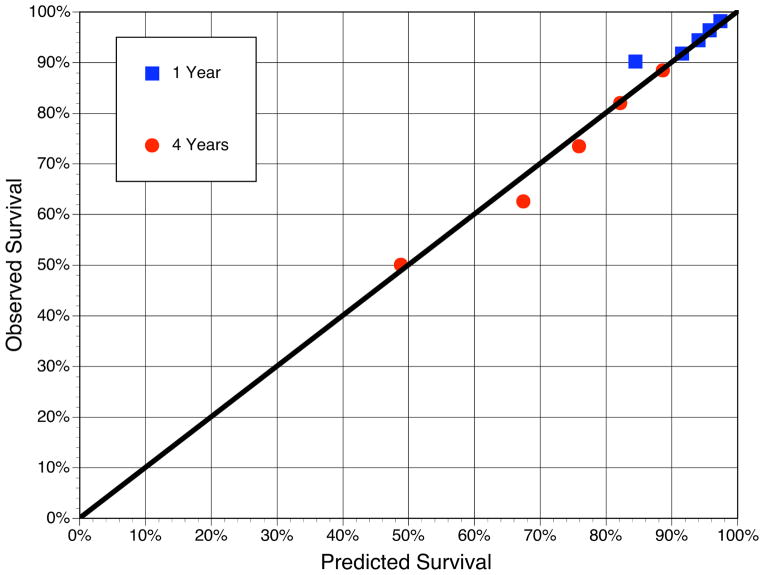
The baseline variables in ascending quintiles of the Seattle HF Score (lower risk to higher risk) are shown in Table 1. QRS width and 6-minute walk distance were not part of the model, but showed higher risk values with higher risk quintiles. The SHFM had excellent model calibration, with overall 4-year predicted and actual survival of 71% (Figure 1). The c-statistic was 0.71 in the external derivation dataset and 0.71 (95% CI 0.69–0.73) in the SCD-HeFT cohort. Although the SHFM was designed to estimate all cause mortality, when applied to the SCD-HeFT data, it was more accurate in predicting pump failure death (c statistic 0.79, 95% CI 0.76–0.82) and non-SCD (which includes pump failure death, c statistic 0.74, 95% CI 0.72–0.77), but still discriminative for predicting SCD (c statistic 0.66, 95% CI 0.63–0.70).
Table 1.
Baseline characteristics by SHFM subgroups.
| SHFM Annual Mortality | Quintile 1 (1.11%–3.58%) (N = 497) | Quintile 2 (3.59%–5.26%) (N = 497) | Quintile 3 (5.26%–7.30%) (N = 496) | Quintile 4 (7.30%–11.14%) (N = 496) | Quintile 5 (11.15%–63.48%) (N = 497) |
|---|---|---|---|---|---|
| Randomized treatment | |||||
| ICD | 32% (159) | 33% (164) | 33% (164) | 30% (150) | 36% (181) |
| Amiodarone | 35% (173) | 33% (165) | 31% (152) | 38% (187) | 31% (152) |
| Placebo | 33% (165) | 34% (168) | 36% (180) | 32% (159) | 33% (164) |
| Age (yrs) | 50 (42, 57) | 57 (50, 64) | 61 (53, 68) | 64 (57, 72) | 68 (62, 74) |
| Male | 69% (341) | 72% (357) | 77% (380) | 81% (403) | 86% (425) |
| NYHA Class III | 5% (27) | 18% (88) | 27% (133) | 41% (201) | 61% (302) |
| Ischemic HF | 30% (147) | 42% (208) | 52% (258) | 61% (304) | 75% (375) |
| Ejection fraction (%) | 25 (20, 30) | 25 (20, 30) | 24 (19, 30) | 25 (19, 30) | 21 (17, 28) |
| Serum sodium (mEq/L) | 139 (138, 141) | 139 (138, 141) | 139 (137, 141) | 139 (137, 141) | 138 (136, 141) |
| Serum creatinine (mg/dL) | 1.0 (0.8, 1.1) | 1.0 (0.9, 1.2) | 1.1 (1.0, 1.3) | 1.2 (1.0, 1.5) | 1.4 (1.2, 1.8) |
| Systolic BP (mm Hg) | 126 (112, 140) | 120 (110, 134) | 120 (107, 130) | 116 (104, 130) | 110 (100, 120) |
| Furosemide equivalent (mg/kg) | 20 (0, 40) | 40 (20, 80) | 40 (20, 80) | 60 (40, 80) | 80 (40, 160) |
| Digoxin | 51% (253) | 65% (321) | 74% (367) | 77% (382) | 81% (401) |
| ACE-I or ARB | 99% (495) | 98% (487) | 99% (490) | 97% (479) | 89% (444) |
| Beta blocker | 88% (439) | 80% (396) | 69% (342) | 63% (310) | 46% (229) |
| Statin | 45% (223) | 37% (183) | 40% (199) | 39% (194) | 31% (154) |
| Carvedilol | 52% (257) | 50% (249) | 36% (181) | 32% (157) | 24% (119) |
| QRS≥120 ms | 33% (166) | 36% (180) | 42% (206) | 43% (215) | 50% (250) |
| QRS width (ms) | 104 (92, 134) | 107 (96, 134) | 112 (98, 144) | 114 (100, 140) | 120 (100, 142) |
| 6-min walk distance (ft) | 1247 (1050,1495) | 1200 (928,1445) | 1180 (928,1382) | 1019 (755,1274) | 920 (640,1177) |
Continuous variables are shown as median (25th, 75th percentiles).
N=2483 pts included (same as model); others excluded for missing data.
Figure 1.
SHFM-Predicted vs. Observed (Kaplan-Meier) Survival among Patients in the SCD-HeFT Placebo Arm
The predicted survival by the Seattle Heart Failure Model and the observed (Kaplan-Meier) survival is shown for quintiles of the placebo group at 1- and 4-years. The predicted and observed mortality at 4 years was 71%. The diagonal line is the line of identity.
As a percent of all deaths, the proportion of SCDs in the placebo group decreased with increasing annual SHFM-predicted mortality, from 52% in low risk patients (quintiles 1 and 2) to 40% in moderate risk patients (quintiles 3 and 4) to 24% in higher risk patients (quintile 5).
In the overall population, the SHFM-adjusted relative risk of mortality was 0.73 (p=0.0016; 95% CI 0.60–0.89) for ICD therapy vs. placebo and 1.04 (p=0.68; 95% CI 0.87–1.12) for amiodarone vs. placebo, similar to the values in the original report of 0.77 and 1.06 (as would be expected given randomization). The benefit of ICD therapy on all-cause mortality varied significantly according to SHFM-predicted risk (interaction term p=0.014). In the lowest SHFM-predicted risk quintile, the relative risk reduction due to ICD therapy was 54%, decreasing to 31% in the 4th quintile and no benefit in the highest risk quintile (Table 2 and Figure 2).
Table 2.
| I. All-cause Mortality According to ICD Therapy by Quintiles of SHFM-Predicted Risk | |||||
|---|---|---|---|---|---|
| Quintile | Total deaths | Placebo Mortality Rate (events per 1,000 person-years) | Relative risk comparing ICD therapy to placebo | Relative risk comparing ICD therapy to placebo using a linear interaction term in the model (SHFM x ICD)* | |
| RR (95% CI) | p | RR (95% CI) | |||
| I (n=497) | 42 | 33 | 0.46 (0.20, 1.04) | 0.063 | 0.46 (0.30, 0.70) |
|
| |||||
| II (n=497) | 73 | 51 | 0.48 (0.26, 0.89) | 0.019 | 0.57 (0.43, 0.76) |
|
| |||||
| III (n=496) | 106 | 73 | 0.63 (0.39, 1.01) | 0.054 | 0.63 (0.50, 0.80) |
|
| |||||
| IV (n=496) | 178 | 113 | 0.69 (0.46, 1.03) | 0.067 | 0.70 (0.57, 0.86) |
|
| |||||
| V (n=497) | 256 | 176 | 0.98 (0.73, 1.32) | 0.89 | 1.00 (0.73, 1.36) |
| II. Sudden Cardiac Death | |||||
|---|---|---|---|---|---|
| Quintile | Total deaths | Placebo Mortality Rate (events per 1,000 person-years) | Relative risk comparing ICD therapy to placebo | Relative risk comparing ICD therapy to placebo using a linear interaction term in the model (SHFM x ICD)* | |
| RR (95% CI) | p | RR (95% CI) | |||
| I (n=497) | 17 | 15 | 0.12 (0.016, 0.97) | 0.047 | 0.14 (0.059, 0.35) |
|
| |||||
| II (n=497) | 29 | 29 | 0.12 (0.028, 0.53) | 0.005 | 0.23 (0.13, 0.42) |
|
| |||||
| III (n=496) | 42 | 29 | 0.47 (0.21, 1.08) | 0.076 | 0.29 (0.18, 0.47) |
|
| |||||
| IV (n=496) | 52 | 46 | 0.30 (0.13, 0.69) | 0.005 | 0.37 (0.25, 0.56) |
|
| |||||
| V (n=497) | 52 | 41 | 0.76 (0.39, 1.47) | 0.42 | 0.83 (0.43, 1.62) |
| III. Non-sudden cardiac death | |||||
|---|---|---|---|---|---|
| Quintile | Total deaths | Placebo Mortality Rate (events per 1,000 person-years) | Relative risk comparing ICD therapy to placebo | Relative risk comparing ICD therapy to placebo using a linear interaction term in the model (SHFM x ICD)* | |
| RR (95% CI) | p | RR (95% CI) | |||
| I (n=497) | 25 | 18 | 0.76 (0.29, 1.96) | 0.57 | 0.79 (0.48, 1.30) |
|
| |||||
| II (n=497) | 44 | 22 | 0.90 (0.42, 1.95) | 0.79 | 0.85 (0.61, 1.19) |
|
| |||||
| III (n=496) | 64 | 44 | 0.73 (0.41, 1.30) | 0.28 | 0.88 (0.67, 1.16) |
|
| |||||
| IV (n=496) | 126 | 67 | 0.96 (0.60, 1.54) | 0.86 | 0.91 (0.72, 1.16) |
|
| |||||
| V (n=497) | 204 | 134 | 1.05 (0.75, 1.47) | 0.79 | 1.03 (0.73, 1.46) |
This is the quintile point estimate, 95% CI, and the p value derived from the SHFM, ICD, and SHFM
ICD interaction terms in the Cox model using continuous variables.
Figure 2.
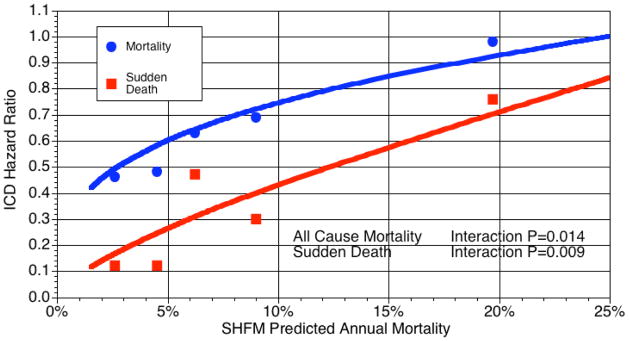
The ICD hazard ratio varied with the Seattle Heart Failure Model predicted mortality.
The relative risk of all-cause mortality and sudden cardiac death due to ICD therapy varied according to SHFM-predicted risk. The estimated hazard ratios of ICD treatment across the SHFM predicted annual mortality generated from a Cox proportional hazards model including a SHFM*ICD multiplicative interaction term.are plotted for total mortality (blue) and sudden cardiac death (red) for quintiles of predicted risk. The points shown in the plot are the hazard ratios generated separately for each quintile of SHFM predicted risk.
As might be expected, this interaction was driven by the effects of the ICD on SCD. In the overall trial population, the ICD decreased the relative risk of SCD by 62% (RR=0.38, 95% CI 0.26–0.57, p<0.0001). This ICD benefit for SCD also varied significantly according to SHFM-predicted mortality (interaction term p=0.009-Table 2 and Figure 2). There was an 88% relative risk reduction in SCD in the lowest risk quintiles (annual mortality ~2.5–4.5%) but only a 24% reduction in the highest risk quintile (annual mortality ~19%, Figure 2).
To further delineate how the SHFM-predicted risk modified benefits of ICD treatment, the 5th risk quintile was split into 2 groups (9th and 10th deciles, with ~13% and ~24% predicted annual mortality, respectively). In the 9th decile the ICD trended towards benefit (hazard ratio 0.72, p=0.15, 95% CI 0.44–1.13), whereas no benefit was seen in the 10th decile (hazard ratio 1.23, p=0.31, 95% CI 0.82–1.83). The plot of the interaction term SHFM score*ICD on total mortality suggested the benefit of the ICD approached null at ~20–25% annual mortality (Figure 2).
The ICD patients who had an appropriate shock for ventricular tachycardia (VT) or ventricular fibrillation (VF) were 16%, 20%, 19%, 22%, and 33% respectively for quintiles 1 to 5. The proportion of the first appropriate shock for VT/VF that was for VF was ~50% for quintiles 1–4 and 35% for quintile 5. Thus, the first appropriate shock that was for ventricular fibrillation was very similar in all quintiles (~10% over 4 yours).
To explore whether the SHFM adds to ICD decision-making based on NYHA class, we evaluated the effect of ICD in strata of the NYHA with and without including the patients in whom the SHFM model appeared to predict no benefit (i.e., patients with SHFM-predicted annual mortality >20%). In the overall population, exclusion of these patients improved the hazard ratio for mortality benefits of ICD therapy from 0.77 to 0.63 (95% CI 0.51–0.78). Among NYHA 2 patients alone, the hazard ratio change was trivial (from HR 0.54 to 0.57), as only 1% of these patients were excluded. Among NYHA 3 patients, however, 15% of patients had SHFM-predicted annual mortality >20%; exclusion of these patients altered the hazard ratio from possible harm (HR=1.16, 95% CI = 0.87–1.54) to potential benefit (HR=0.75, 95% CI 0.53–1.06).
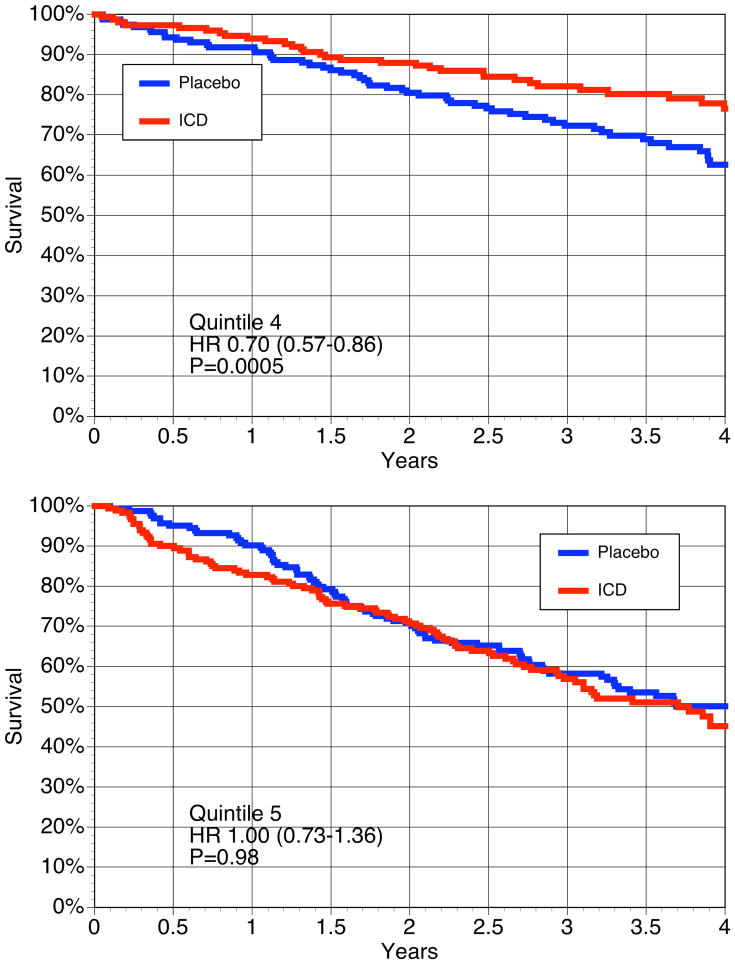
The Kaplan-Meier survival curves according to both ICD treatment and the 5 quintiles of SHFM-estimated risk are shown in Figure 3. The ICD had a survival advantage in quintiles 1–4, whereas in quintile 5, the survival curves were not different at 4 years.
Figure 3.
Kaplan-Meier Survival curves for Quintiles of SHFM predicted survival.
The Kaplan-Meier survival curves for SHFM predicted quintiles are shown for the placebo and the ICD groups. The hazard ratio and p values using a linear interaction model for SHFM*ICD is shown for each quintile.
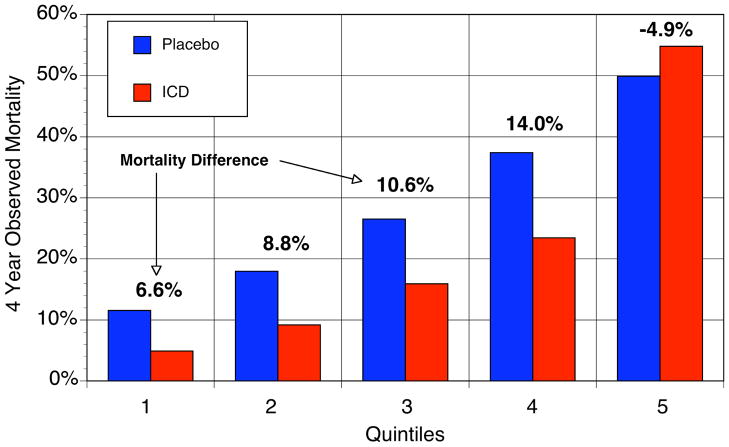
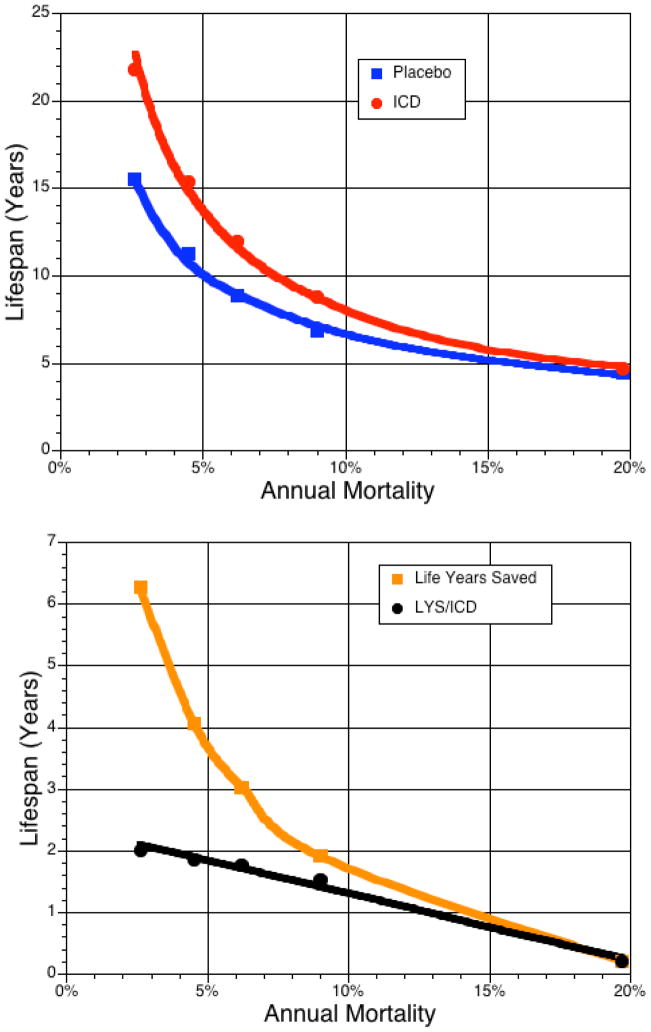
Absolute 4-year reductions in mortality with ICD treatment were 6.6%, 8.8%, 10.6%, 14.0% and −4.9% across SHFM quintiles 1 to 5, respectively. The number needed to treat to add 1 year of life over 4 years of follow-up was 15.2, 11.4, 9.4, 7.1, and −20.4 (no benefit in quintile 5 – Figure 4). Treatment with an ICD added 6.3, 4.1, 3.0, 1.9 and 0.2 additional years of life in the low- to high-risk quintiles, when projected over the patients’ predicted life span (Figure 5). Assuming a 7-year ICD battery life, for each ICD one would add 2.0, 1.9, 1.8, 1.5, and 0.2 years of life across the 5 quintiles. The years needed to treat to add 1 year of life with an ICD (YNT) were 4.0 for the overall trial and 3.5, 3.8, 3.9, 4.6, and 21.5 in the low to high risk quintiles.
Figure 4.
Kaplan-Meier 4 year mortality for placebo and ICD groups.
The observed (Kaplan-Meier) mortality at 4 years of the placebo and ICD groups are shown above for each SHFM estimated quintile of risk. The absolute reduction in mortality (shown above each quintile) ranged from ~7 to 14% in quintiles 1 to 4 with no benefit in quintile 5.
Figure 5.
The projected lifespan for each quintile of the placebo and ICD group
Figure 5a. The projected total lifespan estimate (Gompertz method) for each patient within each quintile was averaged for all placebo and ICD patients within the quintile according to SHFM-predicted risk. Figure 5b shows the difference in total lifespan between the placebo and ICD group averaged over a lifetime. In quintile 1, the average patient will live ~6 years longer but will require ~3 ICDs over the 22 year projected lifespan. Assuming a 7-year ICD battery life, 2.0 life-years were saved per ICD for patients with an average SHFM-predicted 2.5% annual mortality but decreased to 0.2 life-years for quintile 5.
Amiodarone had no significant effect on all cause mortality, SCD or non-SCD. There was no significant interaction of amiodarone with SHFM score for any mode of death (data not shown).
DISCUSSION
The primary finding of our study is that an externally derived risk stratification model containing only routine clinical variables can accurately partition and quantify the treatment benefit from primary prevention ICD therapy in systolic heart failure patients. In particular, the model identified subsets with large differences in both relative and absolute risk reduction. For example, numbers needed to treat for 4 years to save 1 life varied from 15 in the lowest risk quintile to 7 in the 4th risk quintile to no benefit in the highest risk quintile. Using an alternative metric to measure the ICD benefit, the years needed to treat to add one year of life was 3.5 to 4.6 in the first four quintiles17. In terms of absolute survival benefits provided, primary prevention ICD therapy is one of the most effective treatments for moderately symptomatic heart failure patients tested in several decades. Effectiveness has been demonstrated in large contemporary clinical trials and the resulting evidence has been used as the basis for Class I clinical practice guideline recommendations.1,2 Nonetheless, many eligible patients are not currently receiving ICDs, and many expert clinicians remain less enthusiastic about this therapy than would appear to be warranted from the evidence.3 While much of the focus on refining the use of primary prevention ICD has been to try to identify some novel test-based measure of the risk of sudden cardiac death (e.g., microvolt T wave alternans), to-date no study has yet provided evidence that any single test can serve that purpose.4,7 Our results suggest that a regression-based risk model that uses only standard clinical variables, without specialized or expensive testing, can identify clinically useful and statistically valid risk subsets that have different levels of benefit from ICD therapy. Providing clinicians with a simple quantitative tool that can identify the patients in whom ICD therapy offers little potential of benefit, and can also quantify the anticipated additional life expectancy of patients who would be expected to benefit, provides a cost effective method for matching patient preferences and toleration of risk with an invasive but highly effective therapy that is currently significantly underused according to the present guidelines.
Clear variation of primary prevention ICD efficacy based on estimated annual mortality has not previously been demonstrated in a large clinical trial population, although, the patients at lower risk of total mortality die mainly from SCD.9 In the present analysis, these relatively lower risk groups (estimated annual mortality of ~2.5–4.5%) comprised ~40% of all patients, and a single-lead ICD therapy was 88% effective in reducing SCD and decreased all-cause mortality by ~50%. These patients were projected to gain, on average, approximately 5 years of life with an ICD. Patients with higher annual mortalities (up to ~11%) had less relative risk reduction but greater absolute risk reduction with ICD therapy. Conversely, patients with in the highest quintile of predicted annual mortality (~19%) did not benefit from ICD therapy; exploratory analyses suggested that a threshold of benefit may be present at an annual mortality of greater than 20–25% for primary prevention ICD therapy. In these patients, we found no significant benefit of the ICD in preventing SCD and no overall benefit on all-cause mortality.
Current guidelines suggest that ICDs are indicated in class II and III patients but not in class IV patients.1,2 Our results suggest that a multivariable risk model can provide a more nuanced and likely more reproducible method of assessing candidacy for ICD therapy. The standard SHFM model includes hemoglobin, % lymphocytes, uric acid, and total cholesterol (commonly available clinical variables), with a 1-year receiver operating characteristic of 0.68 for SCD and 0.85 for pump failure death (Http://SeattleHeartFailureModel.org).9 Due to absent data on these laboratory variables in the present cohort, the SHFM was modified (SHFM-D) for this analysis, but with similar overall results, although the c statistic was modestly lower than for the original model for all-cause mortality (0.71 vs 0.73), pump failure death (0.79 vs. 0.85), and SCD (0.66 vs. 0.68). Having complete covariate information on these patients would likely have strengthened the discriminative properties even further.
Our findings are consistent with an analysis of ADHERE LM registry, in which an ICD in Stage D heart failure patients was not associated with improved survival; only 17% of deaths in this high risk population (annual mortality 28%) were due to an arrhythmia18. Our results are also consistent with a MADIT II analysis, in which patients who were at highest risk for 2-year all-cause mortality had no benefit from the ICD.6 Similar results to ours were found in a recent ICD propensity analysis where increasing number of comorbidities was associated with increased mortality (4.5% to 13.8%) along with a trend for diminishing ICD benefit (53% to 11%, p=0.18)19.
In the SCD-HeFT population, we did not find a subgroup of patients who were at such low risk of SCD that they did not derive benefit from the ICD. This differs from MADIT II, in which a U-shaped relationship of ICD benefit was seen, with no benefit in either high or low risk patients.6 The low risk group in MADIT II, in whom no ICD benefit was seen, had a 4% annual mortality. In comparison, the lowest risk quintile in SCD-HeFT, in whom substantial ICD benefit was seen, had a 3% annual mortality. The reasons for the different results of MADIT II versus SCD-HeFT for these low risk patients is not clear; the MADIT II model results should likely be validated in an independent cohort before low risk patients otherwise meeting criteria are denied ICD therapy.
Risk stratification using the SHFM should be most beneficial in NYHA class III patients, as 98% of the NYHA class II patients in the derivation cohorts and 99% in SCD-HeFT had a less than 20% annual mortality compared to ~85% of NYHA class III patients. The present analysis cannot determine whether patients with severe symptoms (NYHA class IV) but at lower risk (less than or equal to 15% SHFM estimated annual mortality) would benefit from an ICD; this is not a small subgroup in clinical practice, comprising for example ~20% of the NYHA class IV patients in the derivation cohorts. These patients in the derivation cohorts (less than or equal to 15% SHFM annual mortality) had a similar ratio of sudden death to pump failure death at 2 years whether they were NYHA class II-III (2.4) or NYHA class IV (2.6).
The 1-year mortality in Medicare patients who received an ICD is 13.5%, ~2.5 fold higher risk than the patients in SCD-HeFT. It is quite likely that a significant proportion of Medicare patients have an estimated 1year mortality of greater than 20–25% where the benefit of a primary prevention ICD may be minimal20.
Strengths of this analysis include external derivation of the modified model in a large separate cohort of HF patients that preceded prophylactic ICD use. This differs from MADIT II6, AVID21, and MUSTT4 in which the risk models were derived within the same database and not externally validated. Several caveats should also be considered. Although the SHFM-D performed well in this analysis, addition of other variables, such as BNP, might improve the predictive accuracy of the model even further. Additionally, all trials and cohort studies are subject to the possibility of varying amounts of unrecognized misclassification of SCD. However, the benefit of ICD therapy for total mortality also varied with SHFM-predicted risk. This study also does not address the effect of 2-lead and 3-lead systems on outcome; only single lead systems, conservatively programmed devices were included in this analysis. Some co-morbidities may increase risk of all-cause mortality without a corresponding increase in risk of preventable SCD20,22. These may include, for example, cancer, stroke, lung disease, peripheral vascular disease, dementia, and cirrhosis23. HF populations with an increased prevalence of one or more of these conditions may experience diminished benefits from an ICD by increasing the non-SCD rate. Caution will need to be exercised if this approach is used in the general population who often have more comorbidities than patients in clinical trials.
In conclusion, a clinical risk-prediction model that was externally derived using HF patient cohorts from the pre-primary prevention ICD era and validated in the present cohort was able to identify subgroups of moderately symptomatic HF patients in whom clinically relevant differences were seen in the therapeutic benefit of primary prevention ICD therapy.
Acknowledgments
The authors would like to acknowledge all the subjects, clinical investigators, and clinical coordinators who participated in SCD-HeFT.
Funding Source
This was an investigator initiated research proposal initiated by Wayne Levy, David Linker, and Jeanne Poole, and funded by Medtronic via University of Washington Technology Transfer. The SCD-HeFT trial was supported by grants (UO1 HL55766, UO1 HL55297, and UO1 HL55496) from the National Heart, Lung and Blood Institute, NIH, and by Medtronic, and Wyeth–Ayerst.
Footnotes
SCD-HeFT- ClinicalTrials.gov number, NCT00000609
Disclosures
Drs. Levy and Linker received research support for the SHFM from, Medtronic, HeartWare, CardiacDimensions, and Scios. Dr. Levy received lecture fees from GlaxoSmithKline and Medtronic. Dr. Lee received grant support and consulting fees from Medtronic. Dr. Poole received lecture fees from Medtronic, Boston Scientific, St. Jude Medical and grant support from NIH, Biotronik, and consulting fees from Phillips. Dr. Mozaffarian received research funding from Sigma Tau, Pronova, and GlaxoSmithKline. Dr. Fishbein received lecture fees from Medtronic. Dr. Mark received research grants from Medtronic. Dr. Bardy received grant support from, and having intellectual property rights with, Medtronic, received consulting fees and grant support from Philips, board position and equity and intellectual property rights with Cameron Health, and received grant support from Laerdal. No other potential conflict of interest relevant to this article was reported.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. Circulation. 2008;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 3.Feder B. New York Times. New York: 2008. Defibrillators are lifesaver, but risk gives pause (September 13, 2008) p. 1. [Google Scholar]
- 4.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–7. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 5.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–96. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 7.Gold M, IJ, Costantini O, Bloomfield D, Poole J, McNulty S, Mark D, Lee K, Bardy G S-H Investigators. The Role of Microvolt T-Wave Alternans to Assess Arrhythmia Vulnerability Among Patients with Heart Failure and Systolic Dysfunction: Primary Results from the TWA SCD-HeFT Substudy. Circulation. 2008;118:2022–2028. doi: 10.1161/CIRCULATIONAHA.107.748962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JG, Carson PE, Maggioni AP, Mann DL, Pitt B, Poole-Wilson PA, Levy WC. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation. 2007;116:392–8. doi: 10.1161/CIRCULATIONAHA.106.687103. [DOI] [PubMed] [Google Scholar]
- 10.Packer M, O’Connor CM, Ghali JK, Pressler ML, Carson PE, Belkin RN, Miller AB, Neuberg GW, Frid D, Wertheimer JH, Cropp AB, DeMets DL. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med. 1996;335:1107–14. doi: 10.1056/NEJM199610103351504. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan MD, Newton K, Hecht J, Russo JE, Spertus JA. Depression and health status in elderly patients with heart failure: a 6-month prospective study in primary care. Am J Geriatr Cardiol. 2004;13:252–60. doi: 10.1111/j.1076-7460.2004.03072.x. [DOI] [PubMed] [Google Scholar]
- 13.Maggioni AP, Opasich C, Anand I, Barlera S, Carbonieri E, Gonzini L, Tavazzi L, Latini R, Cohn J. Anemia in patients with heart failure: prevalence and prognostic role in a controlled trial and in clinical practice. J Card Fail. 2005;11:91–8. doi: 10.1016/j.cardfail.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 15.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318:1728–33. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- 16.Haybittle JL. The use of the Gompertz function to relate changes in life expectancy to the standardized mortality ratio. Int J Epidemiol. 1998;27:885–9. doi: 10.1093/ije/27.5.885. [DOI] [PubMed] [Google Scholar]
- 17.Levy WC, Mozaffarian D, Linker DT, Kenyon KW, Cleland JG, Komajda M, Remme WJ, Torp-Pedersen C, Metra M, Poole-Wilson PA. Years-needed-to-treat to add 1 year of life: a new metric to estimate treatment effects in randomized trials. Eur J Heart Fail. 2009;11:256–263. doi: 10.1093/eurjhf/hfn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costanzo MR, Mills RM, Wynne J. Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM) Am Heart J. 2008;155:339–47. doi: 10.1016/j.ahj.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Chan PS, Nallamothu BK, Spertus JA, Masoudi FA, Barone C, Kereiakes DJ, Chow T. Impact of age and medical comorbidity on effectiveness of implantable cardioverter-defibrillators for primary prevention. Circ Cardiovas Qual Outcomes. 2009;2:16–24. doi: 10.1161/CIRCOUTCOMES.108.807123. [DOI] [PubMed] [Google Scholar]
- 20.Al-Khatib SN, Greiner MA, Peterson ED, Hernandez AF, Schulman KA, Curtis LH. Patient and Implanting Physician Factors Associated With Mortality and Complications After Implantable Cardioverter-Defibrillator Implantation, 2002–2005. Circ Arrhythmia Electrophysiol. 2008;1:240–249. doi: 10.1161/CIRCEP.108.777888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brodsky MA, McAnulty J, Zipes DP, Baessler C, Hallstrom AP. A history of heart failure predicts arrhythmia treatment efficacy: data from the Antiarrythmics versus Implantable Defibrillators (AVID) study. Am Heart J. 2006;152:724–30. doi: 10.1016/j.ahj.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Lee DS, Tu JV, Austin PC, Dorian P, Yee R, Chong A, Alter DA, Laupacis A. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–15. doi: 10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 23.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. Jama. 2003;290:2581–7. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]