Abstract
We investigated the use of infrared vibrational frequency of ligands as a potential novel molecular descriptor in three different molecular target and chemical series. The vibrational energy of a ligand was approximated from the sum of infrared (IR) absorptions of each functional group within a molecule and normalized by its molecular weight (MDIR). Calculations were performed on a set of 4-aminoquinazolines with similar docking scores for the VEGFR2/KDR receptor. 4-Aminoquinazolines with MDIR values ranging 192–196 provided compounds with KDR inhibitory activity. The correlation of KDR inhibitory activity was similarly observed in a separate chemical series, the pyrazolo[1,5-a]pyrimidines. Initial exploration of this molecular descriptor supports a tool for rapid lead optimization in the 4-aminoquinazoline chemical series and a potential method for scaffold hopping in pursuit of new inhibitors.
The lead optimization of a compound for improved binding and/or inhibitory potency remains a time consuming and challenging stage in drug discovery research. Computational chemistry provides a potential solution for rapid quantitative structure to activity relationship (QSAR) analysis to allow the efficient design of next generation analogs with improved biological activity. Molecular descriptors play a pivotal role in computational chemistry for the computational lead optimization of a chemical series. Infrared (IR) vibrations of molecules have received little attention as a molecular descriptor for QSAR analysis. Previous report utilized quantum mechanical IR values for QSAR providing predictive capability comparable to CoMFA.1 We investigated the vibrational energy of a ligand as a potential intermolecular force contributing to the binding interaction with biomolecules.
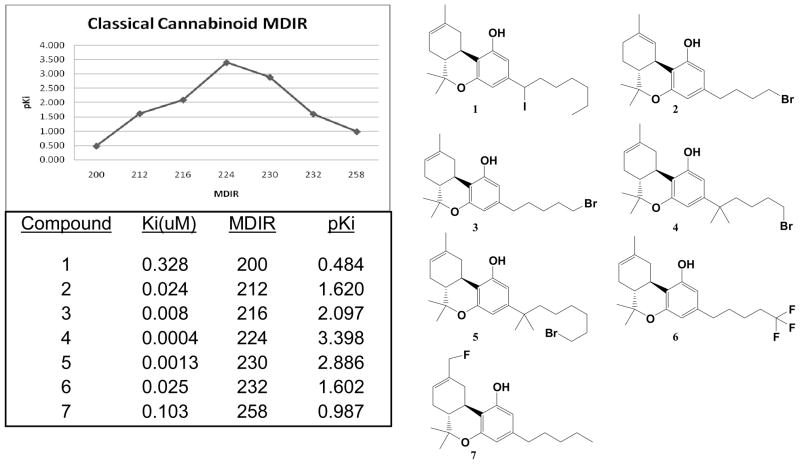
The initial QSAR study employed known classical cannabinoids with highly potent and reproducible binding affinities at the cannabinoid receptor 1 (CB1).2a A small subset of the compounds within the set was chosen based on uniform distribution of binding affinity. The average IR bond frequencies for each functional group within a molecule were summed and normalized by dividing with a known molecular descriptor (i.e. rotatable bonds, H-bond donors, molecular weight, and heavy atoms). A quadratic type of correlation was observed between the negative log of binding affinities (pKi) and the sum of all average IR bond frequencies divided by the molecular weight of the compound (MDIR). The plot of this molecular descriptor, MDIR, against pKi is shown in figure 1. The binding affinity maximizes with MDIR value of 224 for compound 4. None of the other IR normalized set of values showed an observable correlation other than molecular weight.
Figure 1.
MDIR as a molecular descriptor for QSAR of classical cannabinoids.
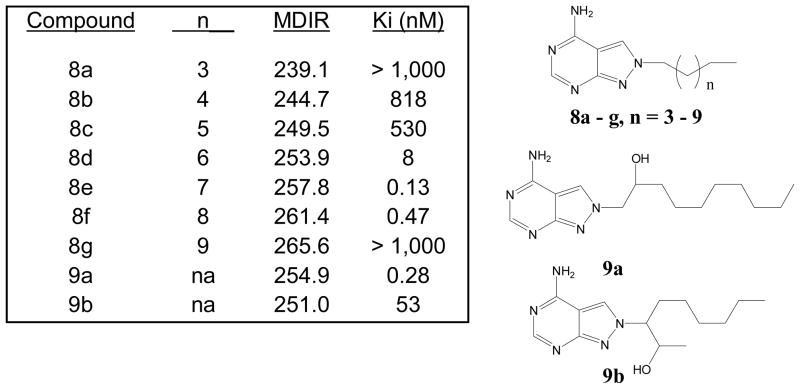
The correlation of MDIR to binding affinities employing alkyl homologation was investigated in a reported SAR of pyrazolo[3,4-d]pyrimidines as adenosine deaminase (ADA) inhibitors.2b The expected trend of increasing activity upon homologation and maximizing as seen for compounds 8e and 8f when n = 7 and 8, respectively (Figure 2), is observed. Based on the homologation SAR, ideal binding affinities are obtained for MDIR values above 250 and lower than 266. Two other compounds (9a and 9b) with additional structural modifications and containing MDIR values in the ideal range were identified and correlated to be active. The correlation of all MDIR values for compounds in figure 2 to ADA binding affinities resemble a parabolic type relationship. However, the abrupt loss of binding affinity in going from MDIR = 261.4 to 265.6 (compounds 8f and 8g, respectively) is surprising. The reported study correlated binding modes via docking studies which showed 8g lacking the ideal binding mode in their docking model. Thus, the use of docking methods coupled with MDIR may prove to be a useful modality for QSAR studies.
Figure 2.
MDIR and Binding affinities of adenosine deaminase inhibitors
We next applied this molecular descriptor within the 4-anilinoquinazoline chemical series, a series known for its inhibitory potency at the vascular endothelial growth factor receptor 2 (VEGFR2/KDR) tyrosine kinase receptor.3 In order to isolate the contribution of MDIR in QSAR, several 4-anilinoquinazoline compounds were virtually designed and docked at the human KDR receptor. A subset of these compounds was further selected based on similar Flexible Grid Docking score relative to the original ligand present in the Apo crystal structure, 3-(2-aminoquinazolin-6-yl)-4-methyl-1-[3-(trifluoromethyl) phenyl]pyridine-2(1H)-one. MDIR values were then calculated and a final subset of compounds chosen for synthesis and biological evaluation.
The docking was performed with the open source DOCK 6 suite of programs4 by University of California, San Francisco. The docking score utilized the flexible docking method allowing flexibility to both the ligand and receptor. The docking score presented is an approximation of the binding energies based on van der Waal’s interactions and coulombic electrostatic energies. The Apo structure of human KDR receptor was obtained from the Protein Data Bank (PDB No. 3CPC)5 with an X-ray diffraction resolution of 2.40 AǺ. The receptor and ligand was separately prepared for docking. Branch A of the dimeric receptor was isolated and prepared for docking by the addition of hydrogens and partial charges using Chimera. Structure of designed ligands were drawn with ChemDraw 3D and minimized using Chimera and the dot molecular surface (DMS) write tools. The ligand was saved as a Mol2 format after adding hydrogens and formal charges. The molecular surface and Spheres were generated using DMS and SPHGEN, respectively. A distance of 10 was specified from the location of the original co-crystallized ligand as the binding site. The Grid Box was then generated adding an extra 5 around the sphere. Docking was performed within the Grid Box with the bump filter on and set to 0.75. A flexible docking method was used for both the ligands and receptor and Grid Docking score6 calculated in kcal/mol.
Compounds 10-19 were synthesized via the chloride displacement of 4-chloro-6,7-dimethoxyquinazolines with the appropriate amine or alcohol. Compound 21 was synthesized from the corresponding (4-Methoxyphenyl)-acetonitrile in a three step fashion. Briefly, the (4-Methoxyphenyl)-acetonitrile is condensed with dimethylforamidedimethylacetal (DMFDMA) to afford the Aldol adduct followed by Michael addition/Cyclization with hydrazine hydrochloride. The 3-amino-4-arylpyrazole formed is then condensed/dehydrated with the corresponding 1,3-dialdehyde to afford Compound 21.
The KDR ELISA assay was performed using the HTScan®VEGF Receptor 2 Kinase Assay Kit from Cell Signaling Technology but the human N-terminal His6-tagged recombinant enzyme (aa790-end) was purchased from US Biological for a better dynamic range in the assay. DELPHIA 200 uL Streptavidin clear coated 96-well plates were purchased from Fisher Scientific Inc. The assay was found to tolerate up to 1% dimethyl sulfoxide (DMSO) to aid solubility of ligands in the kinase buffer. Final concentrations used in the assay was 20 μM ATP, 100 ng human VEGFR2 kinase and 1.5 μM substrate peptide. The absorbance of the wells was read at 450 nm on the xMark™ microplate spectrophotometer by BIO-RAD. Each assay contained a blank, control, DMSO control, and standard (Ki 8751). The average percent inhibitions are calculated from n of 3 data (unless specified otherwise) and assayed on separate days.
The KDR assay from Cell Signaling (HTSscan Kit) provided a highly reproducible assay. It also provided an enzymatic in vitro assay with potency similarity to typical cellular potency, where increase ATP concentration in cells often provide much lower potency or high IC50 values. The assay was calibrated to an internal standard, a known KDR inhibitor (Ki8751)7 with a reported IC50 value of 0.9 nM (final ATP concentration was 2 μM) and staurosporine. The reported KDR IC50 value for Staurosporine using the HTSscan kit from Cell Signalling is 250 nM. The IC50 values obtained in our assay for Ki8751 and staurosporine were measured to be 0.8 μM and 250 nM, respectively.
The final subset of compounds was chosen based on a set of MDIR values that uniformly ranged from 170 to 235 and similar grid docking scores (Table 1). These ten compounds were synthesized and evaluated in the KDR ELISA assay as described above.
Table 1.
Grid Docking score and MDIR correlation to KDR inhibitory activity of 4-Aminoquinazolines

| ||||
|---|---|---|---|---|
| Cmpd | R | Dock Scorea | MDIRb | KDR Inhibitionc |
| 10 | 4′-Chloro-anilino | −27.8 | 172 | 4% |
| 11 | 3′,4′-Difluoro-anilino | −24.9 | 178 | 5% |
| 12 | 3′-Chloro-4′-methyl-anilino | −25.0 | 180 | 9% |
| 13 | 4′-Fluoro-anilino | −24.8 | 184 | 6% |
| 14 | 3′-fluoro-4′-methyl-anilino | −27.3 | 192 | 66% |
| 15 | Anilino | −25.1 | 194 | 19% |
| 16 | Ethoxyl | −25.7 | 200 | 0% |
| 17 | Isopropoxyl | −25.5 | 207 | 2% |
| 18 | Isopropyl amine | −26.3 | 215 | 13% |
| 19 | Cyclohexyl amine | −26.0 | 234 | 17% |
Grid Scoring from Flexible Docking (kcal/mol).
amu/cm.
Percent inhibition (average taken from n of 3 performed on separate days) KDR ELISA assay (20 μM ATP) at 10 μM final compound concentration.
The most potent of these compounds contain an MDIR value of 192 (compound 14) while other compounds were either inactive (KDR percent inhibition less than 15%) or marginally active. The IC50 evaluation of compound 14 was difficult due to solubility problems at higher concentrations. A five point inhibition curve provided an IC50 = 2.5 μM for compound 14.
Additional compounds with reported KDR inhibitory activity, similar Flexible grid dock scores, and MDIR values in close proximity to 192 were identified and evaluated (Table 2). Of these four compounds, all are known inhibitors of KDR except for Compound 14. In spite of good docking scores, Compound 10 with a non-ideal MDIR value (172) is a known KDR inhibitor but with weak activity8 and relatively inactive in our assays. Compound 208 represent a fairly large structural modification towards enhanced water solubility relative to the 4-aminoquinazolines studied herein. In spite of these major structural modifications, the ideal dock score (−27 to −34 kcal/mol) and MDIR value (192) correlated with a potent KDR inhibition. Within the pyrazolo[1,5-a]pyrimidine series, we identified one compound with a dock score and MDIR value within our desired range. Compound 219 was active in our KDR assay and also a reported potent KDR inhibitor. The latter example illustrates MDIR as a potential molecular descriptor tool for scaffold hopping (design of a different chemotype). Ki 8751 was not included in our exploration of MDIR as related to KDR inhibitory activity due to its failure to dock within our designated binding pocket.
Table 2.
Grid Docking score and MDIR correlation to KDR inhibitory activity.
| Dock Scorea | MDIRb | Reported KDR Inhibition10 | Measuredc KDR Inhibition | |
|---|---|---|---|---|
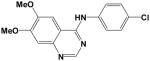 10 |
−27.8 | 172 | IC50 = 800 nM | 4% @ 10 μM (n=2) |
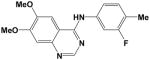 14 |
−27.3 | 192 | NR | 66% @ 10 μM (n=2) |
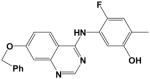 20 |
−33.9 | 192 | IC50 = 2 nM | 37% @ 20 μM (n=2) |
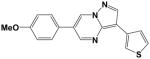 21 |
−29.6 | 199 | IC50 = 19 nM | 46% @ 25 μM (n=2) |
Grid Scoring from Flexible Docking (kcal/mol).
amu/cm.
Percent inhibition KDR ELISA assay (20 μM ATP) with HTSscan.
In summary, the preliminary study of MDIR as a molecular descriptor in QSAR is supportive. Thus, further studies are warranted for the validation of MDIR as a molecular descriptor using the method employed by Matter.11 The utility of MDIR coupled with docking methods seems to be amenable for scaffold hopping. Further examples of scaffold hopping to afford novel and active chemical series as KDR inhibitors will be required for validation studies.
Acknowledgments
This work was supported by a grant from the Hawai’i Community Foundation, Tai Up Yang Fund (Grant ID No. 20080493) and indebted to Hawai’i Pacific University for the use of their nuclear magnetic spectrometer.
Reference and notes
- 1.Turner DB, Willett P. Eur J Med Chem. 2000;35:367–375. doi: 10.1016/s0223-5234(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.a) Nikas SP, Grzybowska J, Papahatjis DP, Charalambous A, Banijamali AR, Chari R, Fan P, Kourouli T, Lin S, Nitowski AJ, Marciniak G, Guo Y, Li X, Wang CJ, Makriyannis A. AAPS J. 2004;6(4):article 30. doi: 10.1208/aapsj060430. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Settimo FD, Primofiore G, Motta CL, Taliani S, Simorini F, Marini AM, Mugnaini L, Lavecchia A, Novellino E, Tuscano D, Martini C. J Med Chem. 2005;48(16):5162–5174. doi: 10.1021/jm050136d. [DOI] [PubMed] [Google Scholar]
- 3.Plé PA, Green TP, Hennequin LF, Curwen J, Fennell M, Allen J, Lambert-van der Brempt C, Costello G. J Med Chem. 2004;47(4):871–887. doi: 10.1021/jm030317k. [DOI] [PubMed] [Google Scholar]
- 4.Park M-S, Dessal AL, Smrcka AV, Stern HA. J Chem Inf Model. 2009;49(2):437–443. doi: 10.1021/ci800384q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu E, Tasker A, White RD, Kunz RK, Human J, Chen N, Burli R, Hungate R, Novak P, Itano A, Zhang X, Yu V, Nguyen Y, Tudor Y, Plant M, Flynn S, Xu Y, Meagher KL, Whittington DA, Ng GY. J Med Chem. 2008;51:3065–3068. doi: 10.1021/jm800188g. [DOI] [PubMed] [Google Scholar]
- 6.(a) Shoichet BK, Bodian DL, Kuntz ID. J Comp Chem. 1992;13(3):380–397. [Google Scholar]; (b) Meng EC, Shoichet BK, Kuntz ID. J Comp Chem. 1992;13:505–524. [Google Scholar]
- 7.Kubo K, Shimizu T, Ohyama S-i, Murooka H, Iwai A, Nakamura K, Hasegawa K, Kobayashi Y, Takahashi N, Takahashi K, Kato S, Izawa T, Isoe T. J Med Chem. 2005;48(5):1359–1366. doi: 10.1021/jm030427r. [DOI] [PubMed] [Google Scholar]
- 8.Whittles CE, Pocock TM, Wedge SR, Kendrew J, Hennequin LF, Harper SJ, Bates DO. Microcirculation. 2002;9(6):513–522. doi: 10.1038/sj.mn.7800164. [DOI] [PubMed] [Google Scholar]
- 9.Fraley ME, Hoffman WF, Rubino RS, Hungate RW, Tebben AJ, Rutledge RZ, McFall RC, Huckle WR, Kendall RL, Coll KE, Thomas KA. Bioorg Med Chem Lett. 2002;12:2767–2770. doi: 10.1016/s0960-894x(02)00525-5. [DOI] [PubMed] [Google Scholar]
- 10.Hennequin LF, Thomas AP, Johnstone C, Stokes ESE, Plé PA, Lohmann J-JM, Ogilvie DJ, Dukes M, Wedge SR, Curwen JO, Kendrew J, Lambert-van der Brempt CL. J Med Chem. 1999;42(26):5369–5389. doi: 10.1021/jm990345w. [DOI] [PubMed] [Google Scholar]
- 11.Matter H. J Peptide Res. 1998;52:305–314. doi: 10.1111/j.1399-3011.1998.tb01245.x. [DOI] [PubMed] [Google Scholar]
-
12.Calculation of MDIR for Compound 2 as a representative example:
Compound 2 FunctionalGroup Qty IR Sub Total -C-H 23 1480 34040 =C-C- 8 1680 13440 -C=C- 4 1680 6720 -C=N- 0 1810 0 =N-H 0 1600 0 -C=N- 0 1810 0 =C-H 3 1000 3000 -C-C- 9 1680 15120 C-O-Alcohol 1 1150 1150 -O-H 1 3600 3600 -C=O 0 1820 0 -N-H 0 1600 0 -C-O-Ether 2 1300 2600 -C-N-Alkyl 0 1360 0 c-Cl 0 800 0 C-F 0 1400 0 -CN 0 2260 0 =C-N- 0 1360 0 -C-Br 1 600 600 Sum of IR Absorptions 80270 Molecular Weight 379.33 MDIR 211.6099 - 13.(4-Chloro-phenyl)-(6,7-dimethoxy-quinazolin-4-yl)-amine (10): 4-Aminoquinazo- lines were prepared according to literature methods10 with a slight procedural modification. A typical procedure utilized is demonstrated for compound 10 as a representative example. In a 25 mL seal-tube reaction vessel equipped with a magnetic stirrer, 100.0 mg (0.445 mmol) of 4-chloro-6,7-dimethoxy-quinazoline was added followed by 2.0 mL of acetonitrile and 62.5 mg (0.490 mmol) of 4-chloroaniline. The vessel was sealed and heated to 100°C. After stirring at said temperature for a period of one day, the reaction was cooled, solvent evaporated via speed-vac, and tritiated three times with cold acetonitrile. Any remaining solvent was evaporated in vacuo to afford 140 mg (quantitative yield) of 10 as a white crystalline solid. LCMS: m/z = 316.0 (M+1, 100% intensity) and 318.0 (M+1, 33% intensity). 1H-NMR (300 MHz, d6-DMSO): δ 11.4 (1H, br s), 8.83 (1H, s), 8.33 (1H, s), 7.77 (2H, br d, J = 9.3 Hz), 7.55 (2H, br d, J = 9.0 Hz), 7.35 (1H, s), 4.02 (3H, s), 4.00 (3H, s).
- 14.(3,4-Difluoro-phenyl)-(6,7-dimethoxy-quinazolin-4-yl)-amine (11): Following a similar reaction procedure to 10, 81 mg (57% yield) of 11 was isolated as a white crystalline solid. LCMS: m/z = 318.0 (M+1, 100% intensity). 1H-NMR (300 MHz, d6-DMSO): δ 11.3 (1H, br s), 8.85 (1H, s), 8.25 (1H, s), 7.96–7.89 (1H, m), 7.60–7.55 (2H, m), 7.32 (1H, s), 4.01 (3H, s), 4.00 (3H, s).
- 15.(3-Chloro-4-methyl-phenyl)-(6,7-dimethoxy-quinazolin-4-yl)-amine(12): Following a similar reaction procedure to 10, 125 mg (85% yield) of 12 was isolated as a white crystalline solid. LCMS: m/z = 330.1 (M+1, 100% intensity) and 332.1 (M+1, 37% intensity). 1H-NMR (300 MHz, d6-DMSO): δ 11.3 (1H, br s), 8.85 (1H, s), 8.28 (1H, s), 7.86 (1H, d, J = 1.8 Hz), 7.61 (1H, dd, J = 8.1, 2.4 Hz), 7.46 (1H, d, J = 8.7 Hz), 7.33 (1H, s), 4.02 (3H, s), 4.00 (3H, s), 2.37 (3H, s).
- 16.(6,7-Dimethoxy-quinazolin-4-yl)-(4-fluoro-phenyl)-amine (13): Following a similar reaction procedure to 10, 141 mg (quantitative yield) of 13 was isolated as a white crystalline solid. LCMS: m/z = 300.0 (M+1, 100% intensity). 1H-NMR (300 MHz, d6-DMSO): δ 11.3 (1H, br s), 8.80 (1H, s), 8.27 (1H, s), 7.71 (2H, br dd, J = 9.3, 5.4 Hz), 7.34 (2H, br t, J = 8.7 Hz), 7.33 (1H, s), 4.01 (3H, s), 4.00 (3H, s).
- 17.(6,7-Dimethoxy-quinazolin-4-yl)-(3-fluoro-4-methyl-phenyl)-amine (14): Following a similar reaction procedure to 10, 131 mg (94% yield) of 14 was isolated as a white crystalline solid. LCMS: m/z = 314.1 (M+1, 100% intensity). 1H-NMR (300 MHz, d6-DMSO): δ 11.4 (1H, s), 8.85 (1H, s), 8.32 (1H, s), 7.64 (1H, dd, J = 11.4, 2.1 Hz), 7.47 (1H, dd, J = 8.4, 1.8 Hz), 7.38 (1H, t, J = 8.7 Hz), 7.34 (1H, s), 4.02 (3H, s), 3.99 (3H, s), 2.27 (3H, d, J = 1.8 Hz).
- 18.(6,7-Dimethoxy-quinazolin-4-yl)-phenyl-amine (15): Following a similar reaction procedure to 10, 131 mg (quantitative yield) of 15 was isolated as a white crystalline solid. LCMS: m/z = 282.0 (M+1, 100% intensity). 1H-NMR (300 MHz, d6-DMSO): δ 11.4 (1H, br s), 8.80 (1H, s), 8.34 (1H, br d, J = 3.9 Hz), 7.69 (2H, br d, J = 7.2 Hz), 7.50 (1H, br d, J = 7.8 Hz), 7.48 (1H, br d, J = 8.4 Hz), 7.36 (1H, br d, J = 4.8 Hz), 7.32 (1H, br t, J = 7.8 Hz), 4.02 (3H, s), 3.99 (3H, s.).
- 19.4-Ethoxy-6,7-dimethoxy-quinazoline (16): Following a similar reaction procedure to 10, 129 mg (58% yield) of 16 was isolated as a white crystalline solid. LCMS: m/z = 235.0 (M+1, 100% intensity). 1H-NMR (300 MHz, CDCl3) δ 8.85 (1H, s), 7.97 (1H, s), 7.41 (1H, s), 4.83 (2H, q, J = 6.9 Hz), 4.14 (3H, s), 4.07 (3H, s), 1.59 (3H, t, J = 6.9 Hz).
- 20.4-Isopropoxy-6,7-dimethoxy-quinazoline (17): Following a similar reaction procedure to 10, 221 mg (61% yield) of 17 was isolated as a white crystalline solid. LCMS: m/z = 249.1 (M+1, 100% intensity). 1H-NMR (300 MHz, CDCl3) δ 7.98 (1H, s), 7.62 (1H, s), 7.16 (1H, s), 4.07 (1H, heptet, J = 6.9 Hz), 4.02 (3H, s), 4.02 (3H, s), 1.22 (7H, d, J = 6.9 Hz).
- 21.(6,7-Dimethoxy-quinazolin-4-yl)-isopropyl-amine (18): Following a similar reaction procedure to 10, 73 mg (66% yield) of 18 was isolated as a white crystalline solid. LCMS: m/z = 248.0 (M+1, 100% intensity). 1H-NMR (300 MHz, CDCl3) δ 8.88 (1H, s), 8.16 (1H, s), 7.44 (1H, s), 4.76 (1H, heptet, J = 6.9 Hz), 4.17 (3H, s), 4.08 (3H, s), 1.47 (6H, d, J = 6.9 Hz).
- 22.Cyclohexyl-(6,7-dimethoxy-quinazolin-4-yl)-amine (19): Following a similar reaction procedure to 10, 45 mg (35% yield) of 19 was isolated as a white crystalline solid. LCMS: m/z = 288.0 (M+1, 100% intensity). 1H-NMR (300 MHz, CDCl3) δ 8.39 (1H, s), 7.44 (1H, s), 7.28 (1H, s), 4.32 – 4.28 (1H, m), 4.11 (3H, s), 3.98 (3H, s), 3.14 (1H, tt, J = 11.0, 3.9 Hz), 2.14 – 2.10 (2H, m), 1.85 – 1.81 (2H, m), 1.63 – 1.25 (5H, m).
- 23.Compound 20 (ZM 323881) and Ki8751 were both purchased from Tocris Bioscience and used as received.
- 24.Compound 21 was prepared following a three step reaction from the corresponding (4-Methoxyphenyl)-acetonitrile based on a previously reported method.9 6-(4-Methoxy-phenyl)-3-thiophen-3-yl-pyrazolo[1,5-a]pyrimidine (21): LCMS: m/z = 308.0 (M+1, 100% intensity). 1H-NMR (300 MHz, CDCl3): δ 8.79 (1H, d, J = 2.4 Hz), 8.77 (1H, d, J = 2.4 Hz), 8.37 (1H, s), 7.90 (1H, dd, J = 3.0, 1.2 Hz), 7.70 (1H, dd, J = 5.1, 1.2 Hz), 7.54 (2H, d, J = 9.0 Hz), 7.43 (1H, dd, J = 5.1, 3.0 Hz), 7.06 (2H, d, J = 8.7 Hz), 3.89 (s, 3H).