Abstract
Objective
To evaluate whether continuing rheumatology follow-up visits and immunosuppressive therapy after starting renal replacement were associated with increased survival in lupus patients with end stage renal failure.
Methods
We identified all lupus patients over 21 years old who started renal replacement therapy between 2005 and 2011 at an urban tertiary care center. Mortality data was obtained using in-hospital records and the Social Security Death Index database.
Results
We identified 80 lupus patients on renal replacement therapy. Twenty two patients (28%) were followed in rheumatology clinics frequently (2 or more visits per year) after starting renal replacement therapy, and 58 patients (72%) were followed infrequently (fewer than 2 visits per year).
Survival rates were significantly higher in transplant patients compared with dialysis patients. However, SLE patients followed frequently after starting dialysis had significantly higher 4-year survival rates compared with patients followed infrequently after starting dialysis, log rank p = 0.03. Furthermore, in the Cox proportional hazards model, treatment with prednisone alone or with no medication was associated with a hazard ratio (HR) of death of 6.1, 95% CI (1.1, 34), p = 0.04, and HR 13, 95% CI (1.5, 106), p = 0.02, respectively, compared with patients treated with a combination of immunosuppressive therapy with or without prednisone, adjusted for age at SLE diagnosis, gender, transplant status and the frequency of rheumatology visits after the development of end stage renal failure.
Conclusion
Active disease in lupus patients on renal replacement therapy may be under-recognized and under-treated, leading to increased mortality.
Key Indexing Terms: Lupus Erythematosus, Systemic, Lupus nephritis, Kidney Failure, Chronic, Mortality
Introduction
Renal disease associated with systemic lupus erythematosus (SLE) is one of the leading causes of morbidity and mortality in these patients (1–3), with 20% to 30% of patients with lupus nephritis progressing to end stage renal failure (ESRF) over time (4). Although the availability of renal replacement therapy (RRT), including dialysis and kidney transplantation, has improved the overall survival of SLE patients with ERSF, mortality rates remain high and essentially unchanged over time (5–7). Therefore, it is important to identify modifiable risk factors for unfavorable outcomes in SLE patients on RRT (3). While several studies compared mortality rates in SLE-related ESRF and non-SLE related ESRF, factors associated with mortality in SLE patients with ESRF on RRT have not been well studied (8–12). Furthermore, it is not known whether improved survival in renal transplant patients with SLE compared with dialysis patients with SLE is due in part to better control of lupus disease activity by immunosuppressive therapy.
Several studies have shown that SLE becomes inactive once ESRF develops and patients are started on RRT (5, 13, 14). As their disease is believed to be clinically quiescent, these patients are maintained on fewer immunosuppressive medications (13) and are less likely to visit their rheumatologists. Thus, it is possible that these patients may be under-represented in observational cohort studies reported in the rheumatologic literature. More recent studies, however, have suggested that SLE can indeed remain active after starting dialysis, especially in the first few years, and even after kidney transplantation (15–20). Thus, under-recognition and under-treatment of active SLE in these patients may be contributing to higher rates of complications and mortality.
The objectives of this study were to evaluate whether continued monitoring by rheumatologists, and/or the use of immunosuppressive treatments after starting renal replacement therapy, are associated with improved survival among lupus patients with ESRF.
Materials and Methods
Patients
We identified all patients over 21 years old with ICD9 diagnoses of SLE (710.0, 695.4) and at least one of the following conditions: chronic kidney disease stage V (585.5); end stage renal disease (585.6); complications of kidney transplant (996.81); kidney transplant status (V42); encounter for dialysis (V56); or admit for renal dialysis (V56.0). Patients were followed between January 2006 and February 2011 at Montefiore Medical Center (MMC) – the University Hospital for the Albert Einstein College of Medicine, a large urban tertiary care center in the Bronx, NY. Patients were identified in the Montefiore electronic record system using Clinical Looking Glass (CLG), a software application developed at MMC, which allows clinicians and researchers to identify populations of interest, laboratory data, medications, and demographics from the MMC database (21).
Because of the retrospective nature of this study, we did not obtain informed consent from the patients, as no identifying information was stored or used in the data analysis. This project was approved by the Institutional Review Board at Albert Einstein College of Medicine/Montefiore Medical Center.
All electronic charts were reviewed by at least 2 physicians, neither of whom was aware of the outcomes prior to reviewing the charts. Although all the charts were reviewed for ACR criteria, due to the retrospective nature of this study the diagnosis of SLE was established based on the physician’s assessment documented in the medical charts. We were unable to establish the exact day/month of SLE diagnosis and ESRF onset, and we therefore used the year of SLE diagnosis and ESRF onset in our time dependent analysis.
The number of rheumatology visits post-ESRF was categorized as two or more visits per year (“frequent”) or fewer than two visits per year (“infrequent”). Time to event was defined as the number of years from the onset of ESRF to the last follow-up date or to the date of death.
Immunosuppressive therapy included at least one of the following: azathioprine; mycophenolate mofetil; tacrolimus; rituximab; or intravenous immunoglobulin (IVIG). Prednisone (Pred) and hydroxychloroquine (HCQ) use were entered and analyzed as separate variables. All patients were on a daily dose of 10 mg or less of prednisone, with the most common daily dose being 5 mg. Medication categories were as follows: no medications; Pred alone; Pred and HCQ in combination; and immunosuppressive medications with or without Pred or HCQ.
Data analysis
Statistical analysis was performed using the STATA 10.0 software package (StataCorp LP, College Station, Texas). We used the student t-test (or its non-parametric alternative, the Wilcoxon rank-sum test) and the chi-square test to evaluate bivariate relationships between continuous and categorical variables, respectively, in the “frequent” follow-up and “infrequent” follow-up groups.
We used Kaplan-Meier survival analysis and Cox proportional hazards models to evaluate all-cause mortality from the time of ESRF onset to the time of death or last follow-up at MMC.
Results
Of the 134 patients identified, 54 were excluded from the final analysis for the following reasons: never started RRT (n = 2), intermittent dialysis only (n = 1), ANCA positive crescentic glomerulonephritis (n = 2), mixed connective tissue disease (n = 2), sarcoidosis (n = 1), rheumatoid arthritis (n = 3), and “rule out SLE” (n = 43).
Based on our chart review, of the 80 patients included in the analysis, we found unequivocal evidence that 46 met at least four SLE criteria (22), 8 met fewer than four SLE criteria but had a documented biopsy consistent with lupus nephritis, 4 met three criteria for SLE, 10 met two criteria, 5 met 1 criterion, and 7 had no information available. Therefore, 58 patients had probable or definite SLE. Twenty two did not have complete information in the available records to confirm SLE by ACR criteria. However, the diagnosis of SLE was clearly documented in all patients.
The baseline characteristics of the 80 patients included in the analysis are summarized in Table 1. Of these patients, 70 (88%) were women, and 45 (63%) were African-American. The median (IQR) age at SLE onset was 27 (19, 37) years old, the median (IQR) time between diagnosis of SLE and ERSF was 5 (1, 10) years, and the median (IQR) time between starting RRT and the last follow-up was 5 (3, 9) years.
Table 1.
Baseline Characteristics of the Study Patients*
| Overall (n=80) | Infrequent follow-ups after ESRF (n = 58) | Frequent follow-ups after ESRF (n = 22) | p-value** | |
|---|---|---|---|---|
| Age at SLE onset, years | 27 (19, 37) | 28 (20, 38) | 26 (7, 33) | 0.30 |
| n (%) female | 70 (88) | 49 (84) | 21 (95) | 0.19 |
| Race, n (%) Black† | 45 (63) | 32 (65) | 13 (59) | 0.62 |
| Ethnicity, n (%) Hispanic† | 36 (51) | 27 (52) | 9 (47) | 0.73 |
| One or more visits pre-ESRF per year, n (%) | 23 (29) | 12 (21%) | 11 (50) | 0.01 |
| Yrs from SLE onset to ESRF | 5 (1, 10) | 6 (2, 10) | 2 (1, 7) | 0.21 |
| Yrs from ESRF to last follow-up | 5 (3, 9) | 6 (3, 11) | 4 (2, 9) | 0.38 |
| Yrs between ESRF and death | 3 (2, 7) | 3 (1, 6) | 5 (2, 12) | 0.42 |
| Renal Transplants, n (%) | 34 (43) | 26 (45) | 8 (36) | 0.49 |
| No medications, n (%) | 14 (18) | 13 (22) | 1 (5) | 0.02 |
| Prednisone alone, n (%) | 15 (19) | 11 (19) | 4 (18) | 0.43 |
| Immunosuppresives, n (%) | 36 (45) | 27 (47) | 9 (41) | 0.65 |
| Hydroxychloroquine, n (%) | 25 (31) | 13 (22) | 12 (55) | 0.006 |
| Pred/HCQ combination, n (%) | 15 (19 ) | 7 (12) | 8 (36) | <0.001 |
Continuous variables are reported as median (IQR)
P values in bold typeface indicate statistical significance.
9 missing race/ethnicity data
Fifteen patients (19%) died during follow-up of various causes including sepsis/probable sepsis (n = 5), seizures/status epilepticus (n = 2), subarachnoid hemorrhage (n = 1), pulmonary embolism (n = 1), colon cancer (n = 1), hypertension (n = 1), and peritonitis (n = 1), unknown (n=3). Lupus and/or ESRF (most likely related to lupus nephritis) were listed as a secondary cause of death for 8/15 (53%) deaths. Thirty four (43%) received at least one renal transplant, 7/34 (21%) failed at least one renal transplant, and 2/34 (6%) had a documented recurrence of lupus nephritis.
Among 80 patients with ESRF/SLE on RRT, 58 (63%) visited rheumatologists at MMC less than twice a year post-ESRF (“infrequent” group), and 22 (28%) visited the rheumatology practice at MMC two or more times per year post-ESRF (“frequent” group). The mean number of visits in the “infrequent” group was 0.18 per year, with over 75% of patients not followed by rheumatologists. The mean number of visits in the “frequent group” was 4.3 visits per year (median 3.7, IQR (2.3, 5.5)). Twenty seven patients (47%) in the “infrequent” group and 9 (41%) in the “frequent” group (p = 0.65) were on immunosuppressive medications. There were 26 (45%) renal transplants in the “infrequent” group and 8 (36%) in the “frequent” group (p = 0.49). The two groups were similar in terms of race, ethnicity, gender, age at SLE onset, and duration from ESRF to last follow-up.
There were several notable differences between patients followed frequently compared to patients followed infrequently by rheumatologists after starting RRT. In the “frequent” follow-up group post ESRF, 11/22 (50%) visited the rheumatology clinic more than once a year prior to developing ESRF compared with only 12/58 (21%) in the “infrequent” follow-up group post-ESR (p = 0.01). Furthermore, the median (IQR) duration between onset of SLE and onset of ESRF was 6 (2,10) years in the “infrequent” group, and 2 (1,7) years in the “frequent” group (p = 0.21). Although we did not have sufficient information about pre-ESRF disease activity for patients who were not followed by rheumatologists at MMC, the above observations suggest that patients in the “frequent” group may actually have had more rapidly progressive disease than patients in the “infrequent” group prior to developing ESRF.
The “infrequent” and “frequent” follow-up groups post-ESRF were also significantly different with respect to medication use while on RRT: 13 (22%) in the “infrequent” group were not on any medications compared with only one (5%) in the “frequent” group (p = 0.02). Only 13 (22%) patients in the “infrequent” group were on HCQ compared with 12 (55%) in the “frequent” group (p = 0.006).
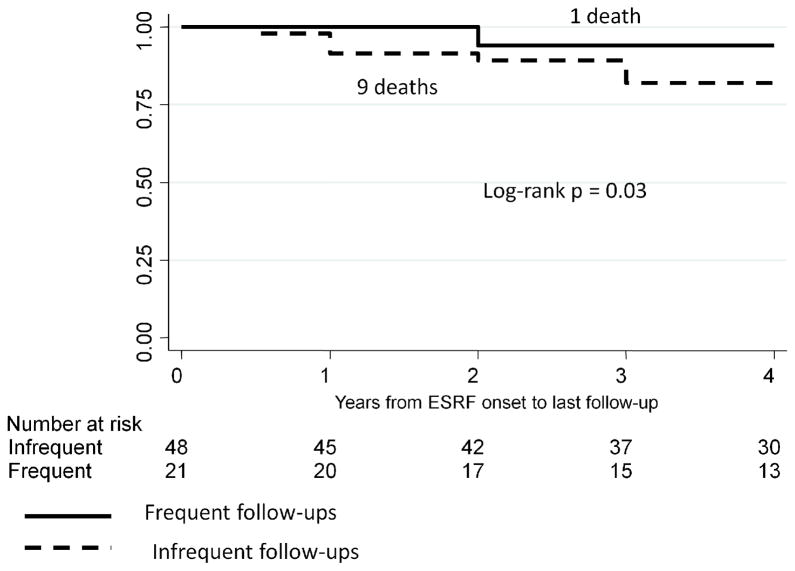
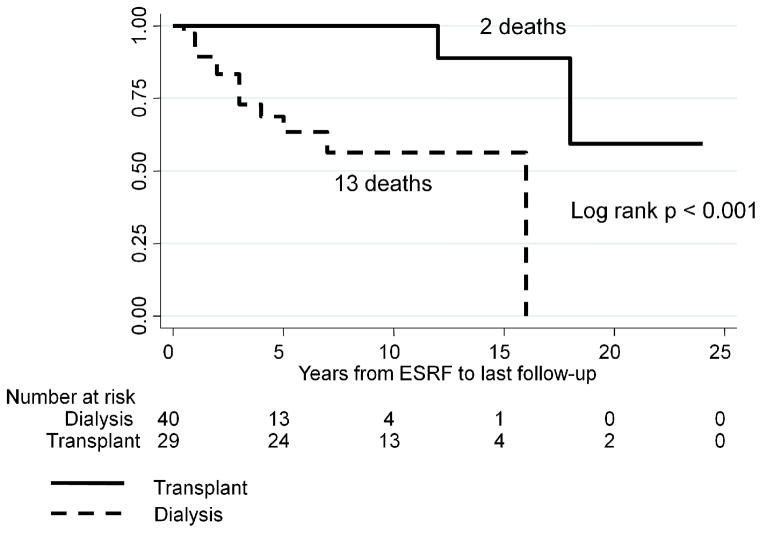
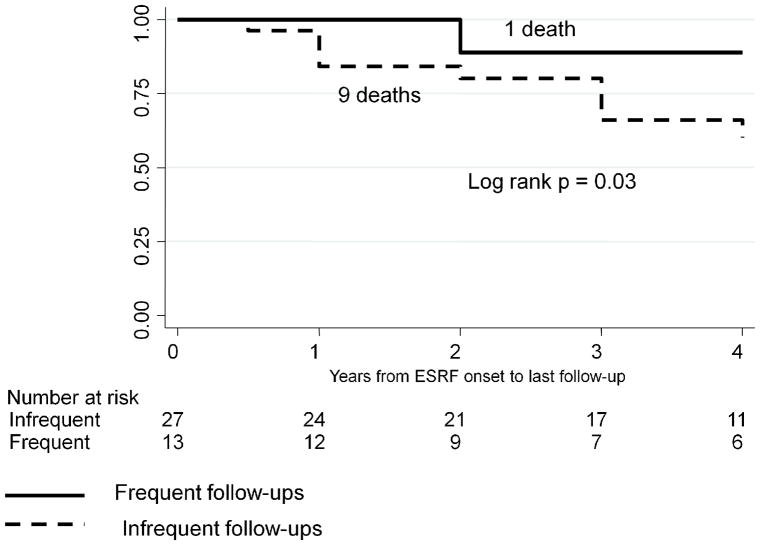
The results of Kaplan-Meier survival analysis are shown in Figures 1 – 6. We were not able to determine conclusively the time of ESRF onset for 11/80 patients. Therefore, 69 patients were included in survival analysis. SLE patients followed frequently after starting dialysis had significantly higher 4-year survival rates compared with patients followed infrequently after starting dialysis, log rank p = 0.03 (Figure 1). There was a similar trend for the 5-year survival, log rank p = 0.12, and 10-year survival, log rank p = 0.14, but these p-values did not reach statistical significance due to a relatively small group size and a relatively small number of outcomes. Since transplanted patients with SLE had much higher survival from ESRF onset, compared with SLE patients on dialysis (Figure 2), we performed survival analysis for the subgroup of 40 non-transplanted patients (Figure 3). The results were similar to the results for the entire cohort, with significantly higher 4-year survival rates among non-transplanted SLE patients followed frequently after starting dialysis, log rank p = 0.03. Again, there was a similar trend for the 5-year and 10-year survival among non-transplanted patients, log rank p = 0.09 and log rank p = 0.18, respectively.
Figure 1.
4-year Kaplan-Meier survival among SLE patients stratified by the frequency of rheumatology follow-ups post-ESRF
Figure 6.
Kaplan-Meier survival analysis among people who met at least 3 ACR criteria and/or had biopsy proven lupus nephritis stratified by medication use post-ESRF
Figure 2.
Kaplan-Meier survival in dialysis and transplant SLE patients
Figure 3.
4-year Kaplan-Meier survival among dialysis patients stratified by the frequency of rheumatology follow-ups post-ESRF
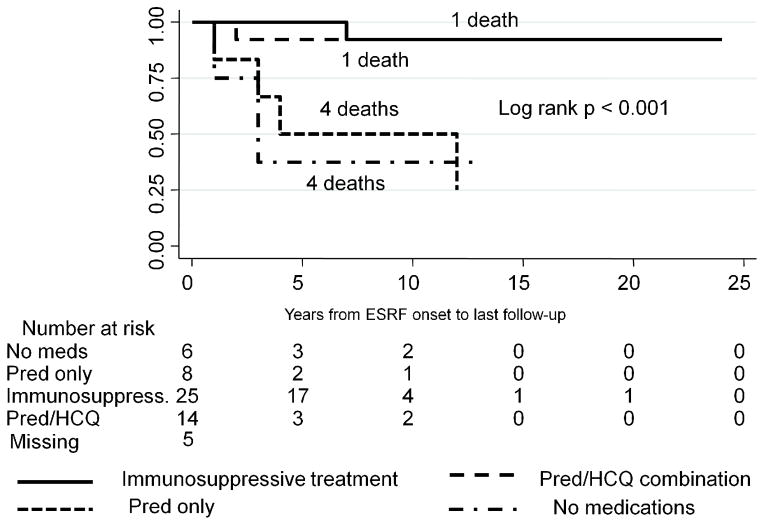
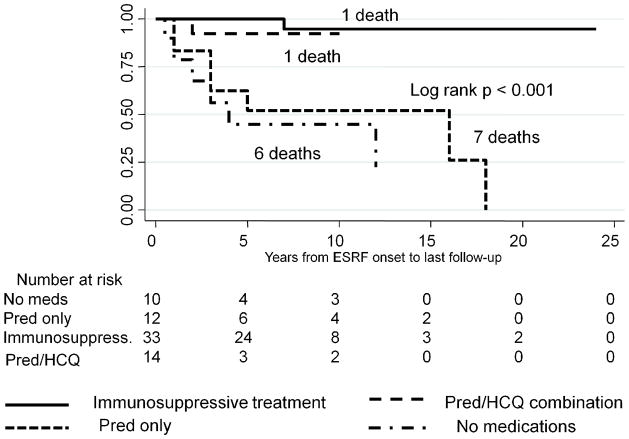
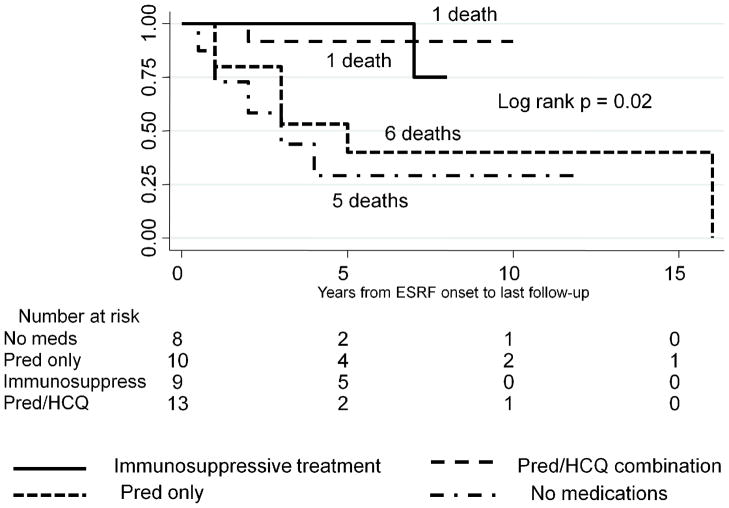
Finally, we investigated whether survival was associated with different medication regiments. ESRF/SLE patients treated with Pred alone had survival rates similar to those of untreated patients, and significantly lower survival rates, compared with patients who were treated either with Pred/HCQ alone or with a combination of immunosuppressive medications with or without Pred/HCQ, log-rank p < 0.001 (Figure 4). Similar results were observed in a subgroup of dialysis patients stratified by medication use, log-rank p = 0.02 (Figure 5).
Figure 4.
Kaplan-Meier survival stratified by medication use post-ESRF
Figure 5.
Kaplan-Meier survival among dialysis patients stratified by medication use post-ESRF
Since we did not have enough information to confirm SLE diagnosis by ACR criteria in some of the patients, we performed survival analysis in 58 patients with definite or probably SLE who met at least 3 SLE criteria and/or had biopsy proven lupus nephritis. Similar to the overall cohort, untreated patients and patients on Pred alone had much lower survival rates compared with patients treated with Pred/HCQ or other immunosuppressive medications, log rank p < 0.001 (Figure 6).
In the adjusted Cox proportional hazards model (Table 2), untreated patients had a hazard ratio (HR) of death of 13 (95% CI 1.5, 106; p = 0.02) compared with those who were on a combination of medications. Similarly, those who were on Pred alone had a HR of death of 6.1 (95% CI 1.1, 34; p = 0.04) compared with those who were on immunosuppressive therapy with or without Pred. Receiving a renal transplant was associated with a decreased HR of death of 0.08 (95% CI 0.01, 0.79; p = 0.03). This model was also adjusted for age at SLE onset, gender, and visit frequency post-ESRF.
Table 2.
Adjusted Cox proportional hazards model with death from any cause as an outcome and immunosuppressives use as the main variable of interest*
| HR | 95% CI | p-value | |
|---|---|---|---|
| Any combination of Immunosuppressives, Pred, HCQ | 1 | - | - |
| Prednisone only | 6.1 | (1.1, 34) | 0.04 |
| No medications | 13 | (1.5, 106) | 0.02 |
| History of renal transplant | 0.08 | (0.01, 0.79) | 0.03 |
Also adjusted for age, gender, and visit frequency post ESRF
Discussion
We show here that survival rates are significantly higher in patients with SLE/ESRF who follow-up at least twice a year with a rheumatologist, compared with those who follow-up less than twice a year. This novel observation concurs with more recent evidence suggesting that SLE remains active after onset of ESRF. Thus, monitoring disease activity and adjusting immunosuppressive therapy may lead to improved survival after onset of ESRF. Furthermore, patients on Pred alone had significantly lower survival rates compared with those who were on any other combination of immunosuppressive medications. The survival rates in patients treated with Pred alone were similar to those treated with no medications, suggesting that low dose Pred may be inadequate treatment for SLE/ESRF patients. Based on our review of the literature, the association between rheumatology visits and immunosuppressant use with survival post-ESRF has not been previously reported. Thus, following up with rheumatologists and maintaining immunosuppressive therapies in SLE patients with ESRF may be modifiable risk factors for improving outcomes in these patients. This is an important observation, and, if confirmed in prospective studies, may change the way SLE/ESRF patients are managed while on RRT.
Use of immunosuppressive medications post-ESRF remained a significant predictor of improved survival even after adjusting for a history of renal transplant. Furthermore, survival rates were similar between patients on the Pred/HCQ combination and patients on other immunosuppressive therapies, suggesting that the Pred/HCQ combination may provide a survival benefit in SLE/ESRF unrelated to their transplant status.
This study has several limitations related to its retrospective design and a relatively small sample size. Confidence intervals in the Cox proportional hazard model were influenced by the relatively small sample size and relatively few deaths in the stratified analysis.
We did not have sufficient information about lupus activity scores in this cohort pre- or post-ESRF, since disease activity was not monitored in those who did not follow up with rheumatologists. Nevertheless, we were able to determine information related to SLE activity pre-ESRF for 35 patients, and found no difference between the 2 groups. However, these findings need to be interpreted with caution due to the missing data.
Missing information, differential selection, as well as differential and non-differential misclassification, especially with respect to the diagnosis of SLE by rheumatologists vs. other physicians, may have affected the results in this retrospective analysis. To evaluate whether excluding 11 patients with missing time of ESRF onset from survival analysis influenced our results, we performed survival analysis using the first year of follow-up for these patients and obtained similar results.
We could not assess compliance in this study. Therefore, patients who were followed more frequently post-ESRF may have been generally more compliant and concerned about their health, which would lead to lower mortality rates. Alternatively, if patients who visit rheumatologists frequently post-ESRF have more active SLE, the true differences in mortality rates between “frequent” and “infrequent” groups would be underestimated. Furthermore, the differences in survival stratified by medication use are less likely to be explained by compliance alone.
It is also possible that patients in our study who followed up infrequently with MMC rheumatologists after starting RRT were following up with rheumatologists outside of MMC. In this case there are 2 possible interpretations of our results with respect to the visit frequency: 1) receiving centralized care at a large tertiary care center after starting RRT is associated with better survival; 2) the actual survival differences between SLE patients who follow-up with rheumatologists post-ESRF and SLE patients who don’t follow up with rheumatologists post-ESRF may be underestimated in our analysis. Nevertheless, since MMC is the only tertiary care center in the Bronx, and all of the patients included in the study were receiving medical care for other conditions at MMC, it is unlikely that a significant number of these patients were followed by outside rheumatologists.
Finally, we chose to use all-cause mortality rather than SLE-related mortality as the main outcome, since cause of death was unknown for 3 patients, and cause of death listed on death certificates are not always accurate and reliable (23). However, we did investigate whether frequent follow-up visits post ESRF were associated with SLE-related mortality. While the results showed a trend similar to all-cause mortality, they did not reach statistical significance, most likely because there were only 8 deaths attributed to SLE. We plan prospective studies to address some of the above limitations and to evaluate the associations observed in this study.
Despite these limitations, overall mortality rates and data related to ESRF progression observed in this study are consistent with those previously reported in other studies (3, 4, 24), suggesting that our results may be generalized to the overall SLE/ESRF population on RRT.
We considered whether some of the survival differences observed between patients on immunosuppressive therapy plus low dose Pred and those on no medications were due to the fact that among the 36 patients on immunosuppressive therapy post-ESRF, 27 (75%) were renal transplant patients. However, medication use post-ESRF independently predicted survival in the Cox proportional hazard model, even after adjusting for transplant rates. Another important observation is that post-ESRF, the Pred/HCQ only subgroup (not including transplant patients) had survival rates similar to the subgroup on immunosuppressive therapy (including renal transplant patients). Furthermore, similar survival analysis results were observed in the subgroup of dialysis patients.
In conclusion, treatment with immunosuppressive therapies with or without Pred in ESRF/SLE patients on RRT is associated with lower mortality rates compared to either Pred alone or no treatment in the entire study cohort, and in the subgroup of dialysis patients. Indeed, some of the observed improved survival in renal transplants may be secondary to immunosuppression and better SLE control. Our results provide preliminary evidence that active lupus disease in SLE/ESRF patients on RRT may be under-recognized and under-treated, which may in turn lead to increased all-cause mortality. As such, close monitoring of disease activity and maintenance therapy for SLE with HCQ and other immunosuppressive treatments may improve survival in post-ESRF patients on RRT.
Acknowledgments
Funding: This work was supported in part by a National Institutes of Health Grant, RO1 AR048692 (to C.P.).
We thank Miriam Gordon, PhD, for valuable assistance in editing the manuscript.
References
- 1.Waldman M, Appel GB. Update on the treatment of lupus nephritis. Kidney Int. 2006 Oct;70(8):1403–12. doi: 10.1038/sj.ki.5001777. [DOI] [PubMed] [Google Scholar]
- 2.Contreras G, Pardo V, Cely C, Borja E, Hurtado A, De La Cuesta C, et al. Factors associated with poor outcomes in patients with lupus nephritis. Lupus. 2005;14(11):890–5. doi: 10.1191/0961203305lu2238oa. [DOI] [PubMed] [Google Scholar]
- 3.Costenbader KH, Desai A, Alarcon GS, Hiraki L, Shaykevich T, Brookhart MA, et al. Trends in the incidence, demographics and outcomes of end-stage renal disease due to lupus nephritis in the U.S., 1995–2006. Arthritis Rheum. 2011 Mar 28; doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croca SC, Rodrigues T, Isenberg DA. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology (Oxford) 2011 Mar 16; doi: 10.1093/rheumatology/ker101. [DOI] [PubMed] [Google Scholar]
- 5.Lee PT, Fang HC, Chen CL, Chiou YH, Chou KJ, Chung HM. Poor prognosis of end-stage renal disease in systemic lupus erythematosus: a cohort of Chinese patients. Lupus. 2003;12(11):827–32. doi: 10.1191/0961203303lu474oa. [DOI] [PubMed] [Google Scholar]
- 6.Ippolito A, Petri M. An update on mortality in systemic lupus erythematosus. Clin Exp Rheumatol. 2008 Sep-Oct;26(5 Suppl 51):S72–9. [PubMed] [Google Scholar]
- 7.Abu-Shakra M, Urowitz MB, Gladman DD, Gough J. Mortality studies in systemic lupus erythematosus. Results from a single center. II. Predictor variables for mortality. J Rheumatol. 1995 Jul;22(7):1265–70. [PubMed] [Google Scholar]
- 8.Nossent HC, Swaak TJ, Berden JH. Systemic lupus erythematosus after renal transplantation: patient and graft survival and disease activity. The Dutch Working Party on Systemic Lupus Erythematosus. Ann Intern Med. 1991 Feb 1;114(3):183–8. doi: 10.7326/0003-4819-114-3-183. [DOI] [PubMed] [Google Scholar]
- 9.Chelamcharla M, Javaid B, Baird BC, Goldfarb-Rumyantzev AS. The outcome of renal transplantation among systemic lupus erythematosus patients. Nephrol Dial Transplant. 2007 Dec;22(12):3623–30. doi: 10.1093/ndt/gfm459. [DOI] [PubMed] [Google Scholar]
- 10.Coplon NS, Diskin CJ, Petersen J, Swenson RS. The long-term clinical course of systemic lupus erythematosus in end-stage renal disease. N Engl J Med. 1983 Jan 27;308(4):186–90. doi: 10.1056/NEJM198301273080403. [DOI] [PubMed] [Google Scholar]
- 11.Norby GE, Leivestad T, Mjoen G, Hartmann A, Midtvedt K, Gran JT, et al. Premature cardiovascular disease in patients with systemic lupus erythematosus influences survival after renal transplantation. Arthritis Rheum. 2011 Mar;63(3):733–7. doi: 10.1002/art.30184. [DOI] [PubMed] [Google Scholar]
- 12.Ward MM. Outcomes of renal transplantation among patients with end-stage renal disease caused by lupus nephritis. Kidney Int. 2000 May;57(5):2136–43. doi: 10.1046/j.1523-1755.2000.00065.x. [DOI] [PubMed] [Google Scholar]
- 13.Mojcik CF, Klippel JH. End-stage renal disease and systemic lupus erythematosus. Am J Med. 1996 Jul;101(1):100–7. doi: 10.1016/s0002-9343(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 14.Stone JH. End-stage renal disease in lupus: disease activity, dialysis, and the outcome of transplantation. Lupus. 1998;7(9):654–9. doi: 10.1191/096120398678920811. [DOI] [PubMed] [Google Scholar]
- 15.Norby GE, Strom EH, Midtvedt K, Hartmann A, Gilboe IM, Leivestad T, et al. Recurrent lupus nephritis after kidney transplantation: a surveillance biopsy study. Ann Rheum Dis. 2010 Aug;69(8):1484–7. doi: 10.1136/ard.2009.122796. [DOI] [PubMed] [Google Scholar]
- 16.Goo YS, Park HC, Choi HY, Kim BS, Park YB, Lee SK, et al. The evolution of lupus activity among patients with end-stage renal disease secondary to lupus nephritis. Yonsei Med J. 2004 Apr 30;45(2):199–206. doi: 10.3349/ymj.2004.45.2.199. [DOI] [PubMed] [Google Scholar]
- 17.Szeto CC, Li PK, Wong TY, Leung CB, Lui SF. Factors associated with active systemic lupus erythematosus after endstage renal disease. J Rheumatol. 1998 Aug;25(8):1520–5. [PubMed] [Google Scholar]
- 18.Bruce IN, Hallett DC, Gladman DD, Urowitz MB. Extrarenal disease activity in systemic lupus erythematosus is not suppressed by chronic renal insufficiency or renal replacement therapy. J Rheumatol. 1999 Jul;26(7):1490–4. [PubMed] [Google Scholar]
- 19.Krane NK, Burjak K, Archie M, O’Donovan R. Persistent lupus activity in end-stage renal disease. Am J Kidney Dis. 1999 May;33(5):872–9. doi: 10.1016/s0272-6386(99)70419-1. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro FM, Leite MA, Velarde GC, Fabris CL, Santos RC, Lugon JR. Activity of systemic lupus erythematosus in end-stage renal disease patients: study in a Brazilian cohort. Am J Nephrol. 2005 Nov-Dec;25(6):596–603. doi: 10.1159/000089708. [DOI] [PubMed] [Google Scholar]
- 21.Bellin E, Fletcher DD, Geberer N, Islam S, Srivastava N. Democratizing information creation from health care data for quality improvement, research, and education-the Montefiore Medical Center Experience. Acad Med. 2010 Aug;85(8):1362–8. doi: 10.1097/ACM.0b013e3181df0f3b. [DOI] [PubMed] [Google Scholar]
- 22.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 23.Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med. 2001 Jan 22;161(2):277–84. doi: 10.1001/archinte.161.2.277. [DOI] [PubMed] [Google Scholar]
- 24.Faurschou M, Dreyer L, Kamper AL, Starklint H, Jacobsen S. Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res (Hoboken) 2010 Jun;62(6):873–80. doi: 10.1002/acr.20116. [DOI] [PubMed] [Google Scholar]