Abstract
Over 150 mutations within the coding sequence of the V2 vasopressin receptor (V2R) gene are known to cause nephrogenic diabetes insipidus (NDI). A large number of these mutant receptors fail to fold properly and therefore are not routed to the cell surface. Here we show that selective, nonpeptidic V2R antagonists dramatically increase cell-surface expression and rescue the function of 8 mutant NDI-V2Rs by promoting their proper folding and maturation. A cell-impermeant V2R antagonist could not mimic these effects and was unable to block the rescue mediated by a permeant agent, indicating that the nonpeptidic antagonists act intracellularly, presumably by binding to and stabilizing partially folded mutants. In addition to opening new therapeutic avenues for NDI patients, these data demonstrate that by binding to newly synthesized mutant receptors, small ligands can act as pharmacological chaperones, promoting the proper folding and maturation of receptors and their targeting to the cell surface.
Introduction
Arginine vasopressin (AVP), also known as the antidiuretic hormone, is a nonapeptide hormone that is secreted by the posterior pituitary in response to low urine osmolality or reduced blood pressure. In the collecting duct of the kidney, it promotes water reabsorbtion and concentration of the urine by binding to the V2 vasopressin receptor (V2R), a member of the G protein–coupled receptor (GPCR) superfamily (1). Upon hormone binding, the V2R will promote cAMP production, protein kinase A activation, and the recruitment of aquaporin-2 water channels to the apical membrane of the principal cell. It is this final step in the antidiuresis-signaling pathway that leads to increased water permeability.
Patients suffering from congenital nephrogenic diabetes insipidus (NDI) cannot concentrate their urine, even in the presence of elevated circulating levels of AVP, as a result of defects in the V2R signal transduction pathway described above (2). To date, 155 mutations within the V2R gene have been associated with the X-linked form of this disease in 239 families. In several instances the outcome of the mutation is easily predicted. For example, the presence of premature stop codons or frame shifts leading to severely truncated or altered receptor proteins would certainly not encode a functional protein (3). However, on the basis of the structure-function relationship described for other GPCRs, the disabling effects of a number of more subtle mutations cannot be deduced. In an attempt to characterize the functional defects resulting from these mutations, heterologous expression systems have been used. Among the mutations tested, over 90% were found to lead to intracellular retention of the synthesized protein (4–9).
Inherited mutations leading to incompletely folded proteins that are retained intracellularly by the endoplasmic reticulum (ER) quality control system is a recurring observation (10–14). In some cases, it was found that the retention of the protein, and not an alteration of its functional properties, is responsible for the disease. For cystic fibrosis, it was shown that the ΔF508 mutant of the cystic fibrosis transmembrane conductance regulator (CFTR) could form functional cAMP-activated chloride channels when it could escape the ER and be expressed at the cell surface (15, 16). Given the number of subtle missense mutations identified in the V2R, it could be envisaged that promoting the release of some mutant receptors from the ER is sufficient to recover function.
Because small molecules have been shown previously to influence the folding and organelle targeting of proteins (17–20), we examined if treatment with small, cell-permeant V2R antagonists (21, 22) could stabilize mutant receptors so as to allow their maturation and cell surface expression.
Methods
Cell culture.
COS-1 cells were maintained in DMEM supplemented with 10% FBS (Immunocorp Sciences Inc., Montreal, Quebec, Canada), 100 U/mL–1 penicillin/streptomycin, 125 μg amphotericin B, 125 μg sodium desoxycholate, 2 mM L-glutamine (all from Life Technologies, Burlington, Ontario, Canada). Transient transfection of COS cells was performed using the DEAE-dextran method (23), and the cells were allowed to express the foreign DNA 48 hours before performing experiments.
HEK-293 cells were rendered stable for the expression of wild-type or del 62-64 V2R by transfection with 15 μg of receptor plasmid and 1 μg of pSV2neo (Clonetech Laboratories Inc., Palo Alto, California, USA). Single isolate clones were grown and maintained in supplemented DMEM containing 300 μg/mL–1 Geneticin (Life Technologies).
Pretreatment of cells with the nonpeptidic V2R antagonists SR121463A and VPA-985 (generous gifts from C. Serradeil-LeGal [of Sanofi Research, 31036 Toulouse Cedex, France] and W. Spinelli [of Wyeth-Ayerst Research, Princeton, New Jersey USA], respectively) was performed for 16 hours or as indicated in the figure legends. The V2R antagonist (d(CH2)51,D-Tyr(Et)2,Val4,Arg8,des-Gly9)-vasopressin was purchased from BACHEM Bioscience Inc. (King of Prussia, Pennsylvania, USA).
Genomic DNA amplification and construction of wild-type and mutant V2R expression vectors.
Genomic V2R DNA was amplified from NDI patients and unaffected related individuals using the PCR with a mutant 27-mer oligonucleotide primer bearing a KpnI site (5′-CCCAGCCCCAGGTACCTCATGTCC-3′) and a mutant 22-mer oligonucleotide primer bearing a SalI site (5′-GGATCCAAGTCGACCCCTTGCC-3′), which hybridize in the 5′ and 3′ untranslated regions (UTR) of this gene, respectively. The PCR products were digested and subcloned 3′ to the c-Myc epitope tag sequence (MEQKLISEEDLNA) into the pBC12BI mammalian expression vector (23).
Saturation binding and cAMP accumulation.
Saturation binding isotherms were performed on attached cells with increasing concentrations of [3H]arginine vasopressin (NEN Life Sciences Products Inc., Boston, Massachusetts, USA) in the presence (to define nonspecific binding) and absence of an excess (10–5 M) of unlabeled AVP (Peninsula Laboratories Inc., Belmont, California, USA) as described previously (24). Total cAMP accumulation was measured by assessing the transformation of [3H]ATP into [3H]cAMP as described previously (25).
Metabolic labeling and immunoprecipitation.
Stable HEK-293 cells expressing the wild-type or del 62-64 V2R were starved for 30 minutes in methionine-free DMEM, then labeled for 30 minutes with 150 μCi/mL–1 [35S]-Translabel (ICN Radiochemicals Inc., Irvine, California, USA) in the same medium. Cells were lysed by sonication and membranes were sedimented by centrifugation at 25,000 g for 30 minutes. The crude membrane preparation was solubilized in 0.5% n-dodecyl-β-D-maltoside (Alexis Corp., San Diego, California, USA). The solubilized receptor was immunoprecipitated with an anti-myc agarose conjugate (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), and, where indicated, the immunoprecipitated V2R was digested for 16 hours at 37°C with 4 mU endoglycosidase H (Endo H) or 40 mU peptide N-glycosidase F (PNGase F; Roche Molecular Biochemicals, Laval, Quebec, Canada). Immunopurified receptors were subsequently resolved on 12% SDS-PAGE, treated with En3Hance autoradiography enhancer (NEN Life Science Products), and exposed to Biomax film (Eastman Kodak Co. Scientific Imaging Systems, Rochester New York, USA) at –80°C.
Immunofluorescence microscopy and flow cytometry.
To determine cell-surface receptor expression, cells were incubated with the 9E10 mAb (anti-myc; Santa Cruz Biotechnology Inc.) in DMEM/HEPES on ice for 1 hour. The cells were rinsed with DMEM/HEPES, followed by ice-cold PBS, and fixed with 3% paraformaldehyde (PFA). Nonspecific sites were quenched with a blocking buffer (PBS containing 0.2% BSA). After extensive rinsing, the cells were incubated with Oregon green–conjugated donkey anti-mouse antibody (Molecular Probes, Eugene, Oregon, USA) in blocking buffer for 30 minutes in the dark. Labeling of actin filaments was performed with rhodamine-conjugated phalloidin (Molecular Probes). After extensive washing, the coverslips were mounted upside-down on glass microscope slides in Airvol (Air Products and Chemicals Inc., Allentown, Pennsylvania, USA) and viewed with a Zeiss Axioskop fluorescent microscope (Carl Zeiss Inc., Thornwood, New York, USA) equipped with ×40 and ×100 planapochromat objective lenses. For intracellular labeling, cells were first fixed with 3% PFA in PBS and incubated with 0.2% Triton X-100 for 10 minutes. The cells were treated with the 9E10 or anti-calreticulin antibody (StressGen Biotechnologies Corp., Victoria, British Columbia, Canada) in blocking buffer at room temperature for 30 minutes. Labeling with Oregon green–conjugated donkey anti-mouse antibody is as described above. Double labeling was viewed on a Biorad MRC 600 laser confocal microscope (BioRad Laboratories Ltd., Mississauga, Ontario, Canada).
For flow cytometry, the cells were washed with PBS and incubated with 9E10 antibody in PBS for 1 hour at room temperature. After this incubation, the cells were washed with PBS and incubated with phycoerythrin-conjugated goat anti-mouse antibody (Immunotech, Westbrook, Maine, USA) in PBS for 1 hour at room temperature. The cells were washed with PBS, fixed with 3% paraformaldehyde, and analyzed on a FACS® calibur Becton-Dickson flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, California, USA) set up to detect phycoerythrin fluorescence (585 ± 21 nm); 10,000 cells were analyzed for each sample.
Results
Intracellular retention of the del 62-64 V2R.
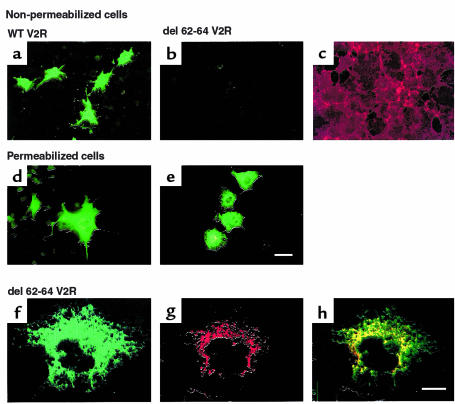
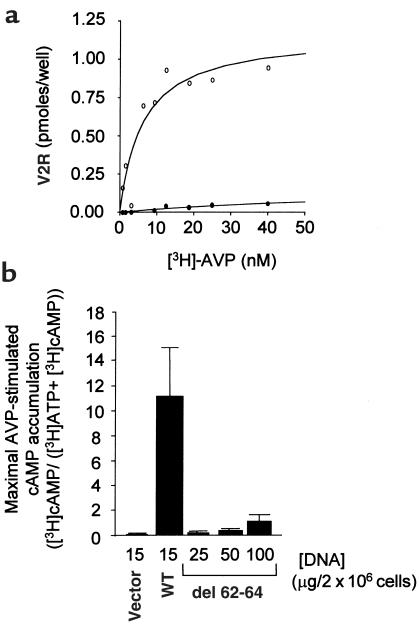
Given that the first cytoplasmic loop of GPCR has never been implicated in ligand binding or G protein coupling, we reasoned that a mutation in this domain could lead to a nonfunctional receptor as a consequence of ER retention. Therefore, the expression and functional characteristics of an NDI mutant V2R bearing a 3–amino acid deletion in the first cytoplasmic loop (del 62-64 V2R) was examined. Immunofluorescence analysis of nonpermeabilized cells shows that in contrast to the wild-type receptor, the del 62-64 V2R was not expressed at the cell surface (Figure 1, a–c). However, upon cell permeabilization the del 62-64 V2R could be detected intracellularly (Figure 1, d and e), indicating that it was properly synthesized. Synthesis of the full-length receptor was further confirmed by Western blot analysis (data not shown). Higher magnification of permeabilized cells expressing del 62-64 V2R reveals a perinuclear punctate labeling that is characteristic of ER staining (Figure 1f). Colocalization with the ER-resident protein calreticulin confirms that the del 62-64 V2R is indeed retained intracellularly at the level of the ER (Figure 1, g and h). Consistent with this lack of cell-surface receptor immunoreactivity is the absence of [3H]vasopressin binding (Figure 2a) and vasopressin-stimulated cAMP production (Figure 2b) in whole cells expressing the del 62-64 V2R.
Figure 1.
The del 62-64 V2R mutant is not expressed at the cell surface and is retained in the ER. (a and b) Nonpermeabilized cell immunofluorescence microscopy of COS-1 cells expressing the myc-tagged wild-type (WT) or myc-tagged del 62-64 V2R, respectively. (c) The same field of cells as in b stained with rhodamine-conjugated phalloidin to label actin filaments. (d and e) Permeabilized cell immunofluorescence microscopy of COS-1 cells expressing the myc-tagged WT or myc-tagged del 62-64 V2R, respectively. Bar = 50 μm. (f–h) Confocal immunofluorescence microscopy on permeabilized COS-1 cells expressing the myc-tagged del 62-64 V2R using the anti-myc antibody (f), an antibody directed against the ER-resident protein calreticulin (g). (h) Illustration of the superimposed localization of both proteins. Bar = 20 μm. Representative of 5 separate experiments.
Figure 2.
Cells expressing the del 62-64 V2R mutant do not display AVP-binding or signaling on whole cells. (a) Saturation-binding isotherm of [3H]AVP to whole cells expressing the WT (open circles) and the del 62-64 V2R (filled circles). COS-1 cells were transfected with 15 μg of plasmid encoding either WT or del 62-64 V2R. Specific binding is expressed as picomoles per well of [3H]AVP bound per well. Representative of 3 separate experiments. (b) Accumulation of cAMP in cells expressing WT or del 62-64 V2R. COS-1 cells were transfected with the indicated amounts of plasmid DNA. The data are expressed as an increase in cAMP levels above basal levels obtained at a 10–5 M AVP concentration and expressed as the mean ± SEM (n = 4).
Cell-surface targeting and functional rescue of the del 62-64 V2R upon antagonist treatment.
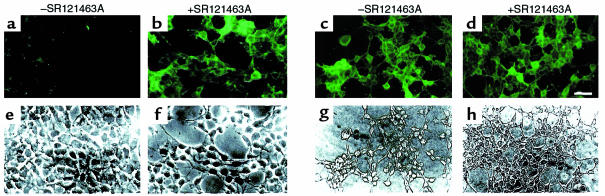
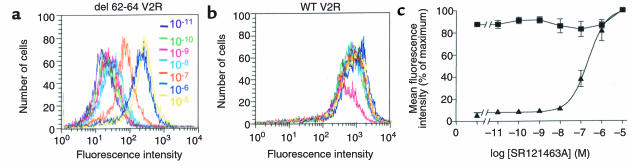
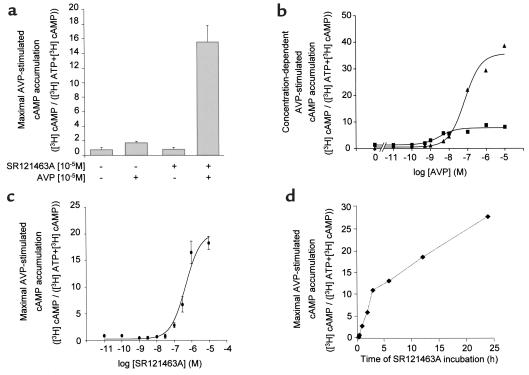
As shown in Figure 3, treating cells with the selective, cell-permeant V2R antagonist SR121463A for 16 hours dramatically increased the cell-surface expression of the del 62-64 V2R (Figure 3, a and b), whereas it did not affect that of the wild-type receptor (Figure 3, c and d). In an effort to quantitate the effect of the treatment on cell-surface expression, flow cytometry analysis in the presence and absence of SR121463A was carried out. Increasing the concentration of SR121463A results in an increase of the cell-surface fluorescence intensity (Figure 4a) with a half-maximal effective concentration (EC50) of 2.2 × 10–7 M (Figure 4c). It should be noted that the absolute mean fluorescence intensity observed after treatment of del 62-64 V2R-expressing cells with 10–5 M SR121463A was as high as 30% of that observed for the cells expressing the wild-type V2R. Interestingly, under the same conditions, vasopressin stimulation of del 62-64 V2R cells produced 30% of the cAMP accumulation obtained in cells expressing the wild-type V2R. This indicates that the mutant receptor is as efficiently coupled to the cyclase stimulation pathway as the wild-type V2R once it is expressed at the cell surface. The antagonist treatment did not affect the already maximal fluorescence intensity of cells expressing the wild-type V2R (Figure 4b).
Figure 3.
SR121463A treatment increases the presence of the del 62-64 V2R at the cell surface. Nonpermeabilized cell immunofluorescence microscopy of HEK-293 cells stably expressing the del 62-64 V2R that were incubated for 16 hours in the absence (a) or presence (b) of 10–5 M SR121463A. (c and d) Identical treatment as a and b performed on HEK-293 cells expressing the WT V2R. Bar = 50 μm. (e–h) Same field of cells as a–d, viewed by phase-contrast microscopy. Representative of 3 separate experiments.
Figure 4.
Concentration-dependent increase of cell-surface del 62-64 V2R after SR121463A treatment. Cell-surface receptor expression was measured by FACS of HEK-293 cells stably expressing either del 62-64 (a) or WT (b) V2R. Cells were treated with the indicated concentrations of SR121463A. Representative of 3 separate experiments. (c) Dose-dependent effect of SR121463A treatment on cell-surface del 62-64 (filled triangles) and WT (filled squares) V2R expression as quantified by the mean cell-surface fluorescence intensity obtained by FACS. The 100% point was taken from cells exposed to a 10–5 M concentration of SR121463A, and the 0% point was taken from cells incubated without anti-myc antibody. There was no difference in cell-surface fluorescence intensity between untransfected HEK-293 cells incubated with both antibodies and HEK-293 cells expressing the del 62-64 V2R that were incubated without anti-myc antibody. There was no difference in cell-surface fluorescence intensity between untransfected HEK-293 cells incubated with both antibodies and HEK-293 cells expressing the del 62-64 V2R that were incubated without anti-myc antibody. The data are expressed as means ± SEM (n = 3).
The increase in cell-surface expression of the del 62-64 V2R promoted by SR121463A pretreatment led to the functional rescue of this mutant protein. Indeed, after removal of the antagonist, stimulation of the del 62-64 V2R with vasopressin promoted cAMP accumulation (Figure 5a) that was found to be concentration dependant (Figure 5b). This is in sharp contrast to what is observed in the absence of SR121463A pretreatment. As was the case for cell-surface expression, vasopressin responsiveness was restored in an SR121463A concentration-dependent manner (Figure 5c) with an EC50 value similar for the 2 phenomena (4.6 × 10–7 vs 2.2 × 10–7 M). This functional rescue of the del 62-64 V2R is time dependent and could be observed as early as 1 hour after the addition of SR121463A to the cells (Figure 5c). In addition, the functional rescue observed in cells expressing the del 62-64 V2R lasted for at least 24 hours after the removal of antagonist (data not shown). Taken together, these data indicate that treatment with the cell-permeant antagonist permits the trafficking of receptor to the cell surface and that the major defect of the del 62-64 V2R is its inability to escape the ER in an appreciable quantity. Once at the plasma membrane, this mutant receptor is functional and can induce cAMP production in response to vasopressin stimulation.
Figure 5.
Rescued signaling activity of cells expressing the del 62-64 V2R after pretreatment with SR121463A. (a) AVP-stimulated cAMP accumulation was measured in whole HEK-293 cells stably expressing the del 62-64 V2R. Cells were treated (or not) with 10–5 M SR121463A for 16 hours, washed extensively, and incubated (or not) with 10–5 M AVP for 20 minutes. The data are expressed as means ± SEM (n = 3). (b) Concentration-dependent AVP-stimulated cAMP accumulation was measured in whole COS-1 cells transiently expressing the del 62-64 V2R. Cells were treated with (filled triangles) or without (filled squares) 10–5 M SR121463A for 16 hours, washed extensively, and incubated with the indicated AVP concentrations. Representative of 3 separate experiments. (c) Dose-dependent effect of SR121463A on AVP-stimulated cAMP accumulation. HEK-293 cells stably expressing the del 62-64 V2R were treated for 16 hours with the indicated concentrations of SR121463A before the determination of cAMP accumulation. The data are expressed as means ± SEM (n = 4). (d) Onset of the effect of SR121463A pretreatment on AVP-dependent cAMP accumulation. HEK-293 cells stably expressing the del 62-64 V2R were treated with 10–6 M SR121463A for the indicated times before assessing AVP-stimulated cAMP accumulation. Representative of 3 separate experiments.
Antagonist treatment promotes the proper maturation of the del 62-64 V2R.
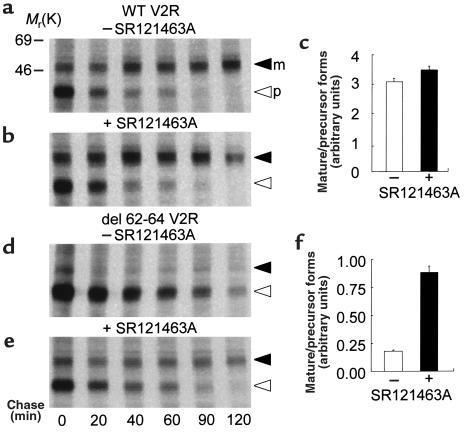
In an effort to further characterize the mechanism by which the antagonist promotes proper cell-surface targeting and rescues function of the del 62-64 V2R, pulse-chase metabolic labeling experiments were carried out. As shown in Figure 6, the V2R is synthesized as a 38-kDa precursor form that is processed to a mature 48-kDa species. In cells expressing the wild-type receptor, this maturation process is unaffected by the SR121463A treatment (Figure 6, a–c). In the absence of SR121463A, the del 62-64 V2R precursor is very poorly processed to the mature form and accumulates for longer periods of time before being degraded (half-life of over 3 hours compared with 30 minutes for the wild-type V2R precursor; Figure 6d). However, SR121463A treatment favored the maturation of the mutant receptor as evidenced by the appearance of the 48-kDa mature receptor form (Figure 6, e and f).
Figure 6.
SR121463A treatment promotes del 62-64 V2R protein maturation. Metabolic labeling of the WT (a, b) and del 62-64 (d, e) V2R in HEK-293 cells incubated in the absence or presence of 10–5 M SR121463A for 16 hours. Labeling was carried out with 150 μCi/mL–1 [35S]methionine/cysteine for 30 minutes followed by the indicated times of chase in complete medium. The migration of the precursor (open arrowhead) and mature (filled arrowhead) species is indicated. Representative of 3 separate experiments. (c and f) Mature/precursor receptor ratio used as an index of the maturation efficiency was calculated at the 60-minute chase time by densitometric analysis of the autoradiograms.
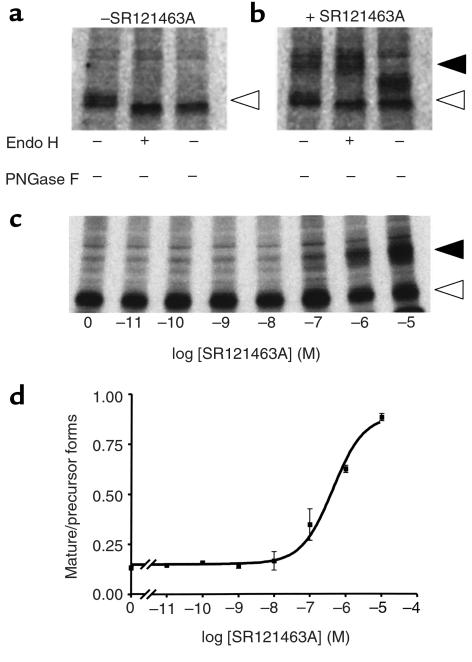
The nature of the precursor and mature forms of the receptor was confirmed by their sensitivity to Endo H and the peptide PNGase F enzymes. Indeed, once proteins mature past the medial saccule of Golgi complex, they become resistant to oligosaccharide digestion by Endo H but remain sensitive to the actions of PNGase F (26). In the absence of SR121463A treatment, the 38-kDa del 62-64 V2R species was found to be sensitive to both enzymes, as indicated by the increase in its electrophoretic mobility, confirming it as a core glycosylated precursor receptor (Figure 7a). In contrast, the 48-kDa form that accumulated upon SR121463A treatment acquired Endo H resistance, indicating the presence of complex carbohydrates characteristic of the mature receptor (Figure 7b). The absence of complete digestion by PNGase F reflects the presence of O-linked carbohydrates in the mature V2R (27).
Figure 7.
Sensitivity of the mature and precursor V2R species to Endo H and PNGase F. The nature of the del 62-64 V2R species was assessed in pulse-chase metabolic-labeling experiments carried out in cells treated (or not) with SR121463A. The glycosylation state was determined at the 60-minute chase time by treating the purified receptor with the indicated enzymes. In the absence of SR121463A treatment (a), only the precursor (open arrowhead) form that is sensitive to both enzymes is observed, whereas following a 10–5-M SR121463A treatment for 16 hours (b), the Endo H–resistant mature species is observed (closed arrowhead). (c) Concentration dependence of the SR121463A-promoted maturation of the V2R. The effect of the indicated concentrations of SR121463A (for 16 hours) on the maturation of the del 62-64 V2R was assessed at the 60–minute chase time. (d) The mature/precursor receptor ratio of the autoradiogram shown in c was calculated by densitometric analysis of the autoradiogram. Representative of 3 separate experiments.
The mature/precursor receptor protein ratio at the 60-minute chase time was then used as an index of the maturation process to determine the EC50 of SR121463A in this assay. As seen in Figures 7, c and d, the EC50 for the effect of SR121463A on the maturation of del 62-64 V2R was found to be 4.3 × 10–7 M, a value comparable to those found for its effects on function and cell surface expression (4.6 and 2.2 × 10–7 M, respectively).
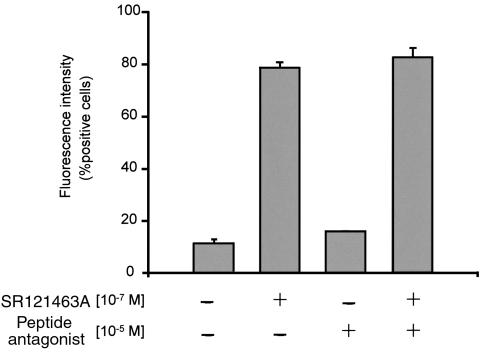
The results described above, and in particular the observation that the SR121463A treatment decreases the half-life of the del 62-64 V2R, suggest that the antagonist acts by binding to the intracellular precursor form to favor its proper folding. This concept is further supported by the observation that the cell-impermeant V2R antagonist (d(CH2)51,D-Tyr(Et)2, Val4,Arg8,des-Gly9-vasopressin) (28) does not mimic the effect of SR121463A and, when used at concentrations that saturate cell-surface vasopressin receptors (10–5 M), did not interfere with the ability of SR121463A to promote cell-surface targeting of the del 62-64 V2R as assessed by flow cytometry analysis (Figure 8).
Figure 8.
Intracellular mode of action of SR121463A on the rescue of cell-surface del 62-64 V2R. HEK-293 cells stably expressing the del 62-64 V2R were treated with or without 10–5 M of the peptidic antagonist d(CH2)51,D-Tyr(Et)2,Val4,Arg8,des-Gly9-vasopressin for 1 hour before the addition of SR121463A. A concentration of 10–7 M of SR121463A was then added (or not) to the cells for 6 hours. To ensure that the peptidic antagonist did not become degraded during the incubation period it was added every hour. Cell-surface receptor expression was measured by FACS® as described above. Cells were scored as positive based on a 0% point taken without anti-myc antibody and without any drug treatments. The data are expressed as means ± SEM (n = 3).
Several NDI mutant receptors can be functionally rescued.
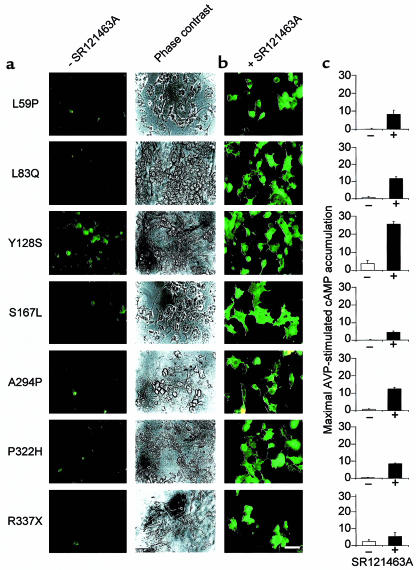
Of 15 mutants tested that showed little or no cell-surface receptor expression under control conditions, SR121463A treatment restored both cell-surface targeting and agonist-stimulated signaling for 8 of these (Figure 9). The variation in the extent of functional rescue as assessed by cAMP production (e.g., the poor functional recovery of R337X), may reflect the fact that, in addition to being retained intracellularly, some mutants may also be somewhat impaired in their ability to productively interact with Gs and stimulate adenylyl cyclase activity (4, 5). Interestingly, the same subset of mutant receptors that was rescued by SR121463A was also rescued by the another cell-permeant nonpeptidic V2R antagonist, VPA-985 (data not shown), suggesting that it is not a property that is unique to SR121463A.
Figure 9.
SR121463A increases the cell-surface expression and signaling activity of several distinct NDI-V2R mutants. (a) cos-1 cells transiently transfected with plasmids encoding the indicated myc-tagged mutant V2 receptors detected by whole-cell immunofluorescence and phase-contrast microscopy. All mutants were found to be poorly expressed at the cell surface. (b) Treatment with 10–5 M SR121463A for 16 hours leads to cell-surface appearance of these receptor mutants as assessed by fluorescence microscopy. Bar = 50 μm. (c) Effects of a pretreatment with 10–5 M SR121463A for 16 hours on vasopressin-stimulated cAMP accumulation in cos-1 cells expressing the various mutants as indicated in a. The position and nature of the amino acid replacement in each NDI-causing mutation is indicated by the single letter amino acid code. X indicates a stop codon. Representative of 3 separate experiments.
Discussion
Antagonists have been shown previously to stabilize GPCR into conformations that are more resistant to downregulation. For instance, treatment of cells expressing the histamine-H2 receptor with the antagonists cimetidine and ranitidine, lead to an increase of the receptor number (29). Such stabilization effects have been more apparent for constitutively active mutant receptors (30–32), which are known to be less stable than their wild-type counterparts. In these cases, it was assumed that the antagonists bind to the cell-surface receptors and stabilize a conformation that is less amenable to internalization and degradation. Following this reasoning, one could suggest that the effect of SR121463A in promoting the accumulation of functional receptors at the cell surface could also be due to the stabilization of a small contingent of mutant receptors already expressed there. The data presented here do not support such a hypothesis. First, a peptide antagonist that does not penetrate the cell did not mimic nor block (even when used at 100-fold excess concentration) the functional rescue promoted by the SR121463A treatment. Second, the treatment with SR121463A caused a more rapid and efficient processing of the del 62-64 V2R as reflected by the reduction of the half-life of the precursor and the increase in the amount of mature receptor detected.
Taken together, these data allow us to propose a model in which small, nonpeptidic V2R antagonists permeate the cell and bind to incompletely folded mutant receptors. This would then stabilize a conformation of the receptor that allows its release from the ER quality control apparatus. The stabilized receptor would then be targeted to the cell surface where it could bind vasopressin and promote signal transduction. As such, these compounds would act as pharmacological chaperones that promote receptor processing through their specific binding activity. However, one cannot exclude the possibility that SR121463A could act as a chemical chaperone by an undefined mechanism.
The model proposed above is consistent with the finding that 2 NDI mutants that were found to be normally expressed at the cell surface in the absence of SR121463A but that are deficient in vasopressin-mediated cAMP production (D85N and V88M; ref. 33 and ref. 8, respectively) could not be functionally rescued by the antagonist treatment (data not shown).
The observation that antagonist treatment rescues the function of NDI vasopressin receptor mutants by promoting their maturation and targeting to the plasma membrane may have important clinical implications because it could offer a new therapeutic avenue for the treatment of this disease. The potential for the use of nonpeptidic vasopressin antagonists in clinical settings is further supported by the fact that the functional rescue observed in cells expressing the del 62-64 V2R lasted for at least 24 hours after the removal of the antagonists (data not shown). Therefore, antagonist treatment could offer a simple alternative to the gene therapy approach that was proposed recently (34). Whether or not a nonpeptidic agonist could also promote rescue of the receptor is an interesting possibility that remains to be investigated.
It has been argued that, in contrast to cystic fibrosis where the majority of patients share a unique mutation (ΔF508) that leads to ER retention, the diversity of mutations (over 150 mutations) in NDI complicates the search for rescue strategies of intracellularly retained mutant proteins (35). The results presented in this study challenge this notion because 8 distinct mutations could be rescued by the same treatment.
Stabilization of protein conformation(s) that are compatible with their export from the ER using small, cell-permeable ligands may represent a generally applicable rescue strategy. The generality of this approach is further supported by the observation that at least 2 different nonpeptidic V2R antagonists, SR121463A and VPA-985, rescued the same V2R mutants. Given that many pathologies stem from intracellular retention of otherwise functional proteins (for a review see ref. 36), this mechanism may offer a new therapeutic approach to the treatment of several different diseases resulting from errors in protein kinesis.
Acknowledgments
We thank C. Serradeil-LeGal and W. Spinelli for the generous gifts of SR121463A and VPA-985, respectively; I. Robert Nabi for antisera and his help in immunofluorescence experiments; L. Cournoyer and S. Sénéchal for their technical assistance; M. Moreau for assistance with the figures; H. Ansanay, R. Qanbar, T.E. Hebert, and S. Michnick for helpful discussions and critical reading of the manuscript. This work was supported by grants from the Kidney Foundation of Canada and the Medical Research Council of Canada (MRC) to M. Bouvier and D.G. Bichet. D.G. Bichet thanks la Fondation J. Rodolphe-La Haye for generous support. J.P. Morello held a studentship from the Heart and Stroke Foundation of Canada. A. Salahpour and S. Angers hold studentships from the MRC. M. Bouvier holds an MRC Scientist Award.
References
- 1.Knoers NV, Monnens LL. Nephrogenic diabetes insipidus. Semin Nephrol. 1999;19:344–352. [PubMed] [Google Scholar]
- 2.Bichet, D.G., and Fujiwara, T.M. 2000. Nephrogenic diabetes insipidus. In The metabolic and molecular basis of inherited disease. 8th edition. C.R. Scriver et al., editors. McGraw-Hill. New York, NY. In press.
- 3.Schülein R, Rutz C, Rosenthal W. Membrane targeting and determination of transmembrane topology of the human vasopressin V2 receptor. J Biol Chem. 1996;271:28844–28852. doi: 10.1074/jbc.271.46.28844. [DOI] [PubMed] [Google Scholar]
- 4.Wenkert D, et al. Functional characterization of five V2 vasopressin receptor gene mutations. Mol Cell Endocrinol. 1996;124:43–50. doi: 10.1016/s0303-7207(96)03926-3. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghi HM, Innamorati G, Birnbaumer M. An X-linked NDI mutation reveals a requirement for cell surface V2R expression. Mol Endocrinol. 1997;11:706–713. doi: 10.1210/mend.11.6.9919. [DOI] [PubMed] [Google Scholar]
- 6.Tsukaguchi H, et al. Binding-, intracellular transport-, and biosynthesis-defective mutants of vasopressin type 2 receptor in patients with X-linked nephrogenic diabetes insipidus. J Clin Invest. 1995;96:2043–2050. doi: 10.1172/JCI118252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schöneberg T, Yun J, Wenkert D, Wess J. Functional rescue of mutant V2 vasopressin receptors causing nephrogenic diabetes insipidus by a co-expressed receptor polypeptide. EMBO J. 1996;15:1283–1291. [PMC free article] [PubMed] [Google Scholar]
- 8.Ala Y, et al. Functional studies of twelve mutant V2 vasopressin receptors related to nephrogenic diabetes insipidus: molecular basis of a mild clinical phenotype. J Am Soc Nephrol. 1998;9:1861–1872. doi: 10.1681/ASN.V9101861. [DOI] [PubMed] [Google Scholar]
- 9.Oksche A, et al. Vasopressin V2 receptor mutants that cause X-linked nephrogenic diabetes insipidus: analysis of expression, processing, and function. Mol Pharmacol. 1996;50:820–828. [PubMed] [Google Scholar]
- 10.Cheng SH, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 11.Lehrman MA, et al. The Lebanese allele at the low density lipoprotein receptor locus. Nonsense mutation produces truncated receptor that is retained in endoplasmic reticulum. J Biol Chem. 1986;262:401–410. [PubMed] [Google Scholar]
- 12.Gow A, Friedrich VL, Lazzarini RA. Many naturally occurring mutations of myelin proteolipid protein impair its intracellular transport. J Neurosci Res. 1994;37:574–583. doi: 10.1002/jnr.490370504. [DOI] [PubMed] [Google Scholar]
- 13.Singh N, et al. Prion protein aggregation reverted by low temperature in transfected cells carrying a prion protein gene mutation. J Biol Chem. 1997;272:28461–28470. doi: 10.1074/jbc.272.45.28461. [DOI] [PubMed] [Google Scholar]
- 14.Hou W-S, et al. Characterization of novel cathepsin K mutations in the pro and mature polypeptide regions causing pycnodysostosis. J Clin Invest. 1999;103:731–738. doi: 10.1172/JCI653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalemans W, et al. Altered chloride ion channel kinetics associated with the ΔF508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 16.Drumm ML, et al. Chloride conductance expressed by ΔF508 and other mutant CFTRs in Xenopus oocytes. Science. 1991;254:1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- 17.Eilers M, Schatz G. Methotrexate strongly inhibits DHFR import into yeast mitochondria. Nature. 1986;322:228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- 18.Loo TP, Clarke DM. Correction of defective protein kinesis of human P-glycoprotein mutants by substrates and modulators. J Biol Chem. 1997;272:709–712. doi: 10.1074/jbc.272.2.709. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, White AL. 6-aminohexanoic acid as a chemical chaperone for apolipoprotein (a) J Biol Chem. 1999;274:12883–12889. doi: 10.1074/jbc.274.18.12883. [DOI] [PubMed] [Google Scholar]
- 20.Tamarappoo BK, Verkman AS. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest. 1998;101:2257–2267. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serradeil-LeGal C, et al. Characterization of SR121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist. J Clin Invest. 1996;98:2729–2738. doi: 10.1172/JCI119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albright JD, et al. 5-Fluoro-2-methyl-N-[4-(5H-pyrrolo[2,1-c]-[1,4]benzodiazepin-10(11H)-carbonyl)-3-cholorophenyl]benzamide (VPA-985): an orally active arginine vasopressin antagonist with selectivity for V2 receptors. J Med Chem. 1998;41:2442–2444. doi: 10.1021/jm980179c. [DOI] [PubMed] [Google Scholar]
- 23.Cullen BR. Use of eucaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- 24.Morin D, et al. The D136A mutation of the V2 vasopressin receptor induces a constitutive activity which permits discrimination between antagonists with partial agonist and inverse agonist activities. FEBS Lett. 1998;441:470–475. doi: 10.1016/s0014-5793(98)01585-3. [DOI] [PubMed] [Google Scholar]
- 25.Wong YH, et al. Mutant α subunits of Gi2 inhibit cAMP accumulation. Nature. 1990;351:63–65. doi: 10.1038/351063a0. [DOI] [PubMed] [Google Scholar]
- 26.Kornfeld R, Kornfeld S. Assembly of asparigine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 27.Sadeghi H, Birnbaumer M. O-glycosylation of the V2 vasopressin receptor. Glycobiology. 1999;9:731–737. doi: 10.1093/glycob/9.7.731. [DOI] [PubMed] [Google Scholar]
- 28.Jard S, et al. Vasopressin antagonists allow demonstration of a novel type of vasopressin receptor in the rat adenohypophysis. Mol Pharmacol. 1986;30:171–177. [PubMed] [Google Scholar]
- 29.Smit M, et al. Inverse agonism of histamine H2 antagonists accounts for upregulation of spontaneously active histamine H2 receptors. Proc Natl Acad Sci USA. 1996;93:6802–6807. doi: 10.1073/pnas.93.13.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gether U, et al. Structural instability of a constitutively active G protein-coupled receptor. J Biol Chem. 1997;272:2587–2590. doi: 10.1074/jbc.272.5.2587. [DOI] [PubMed] [Google Scholar]
- 31.Heinflink M, et al. A constitutively active mutant thyrotropin-releasing hormone receptor is chronically down-regulated in pituitary cells: evidence using chlordiazepoxide as a negative antagonist. Mol Endocrinol. 1995;9:1455–1460. doi: 10.1210/mend.9.11.8584022. [DOI] [PubMed] [Google Scholar]
- 32.Lee TW, Cotecchia S, Milligan G. Up-regulation of the levels of expression and function of a constitutively active mutant of the hamster α1B-adrenoceptor by ligands that act as inverse agonists. Biochem J. 1997;325:733–739. doi: 10.1042/bj3250733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadeghi H, et al. Biochemical basis of partial nephrogenic diabetes insipidus phenotypes. Mol Endocrinol. 1997;11:1806–1813. doi: 10.1210/mend.11.12.0017. [DOI] [PubMed] [Google Scholar]
- 34.Schöneberg T, et al. Reconstitution of mutant V2 vasopressin receptors by adenovirus-mediated gene transfer. J Clin Invest. 1997;100:1547–1556. doi: 10.1172/JCI119678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oksche A, Rosenthal W. The molecular basis of nephrogenic diabetes insipidus. J Mol Med. 1998;76:326–337. doi: 10.1007/s001090050224. [DOI] [PubMed] [Google Scholar]
- 36.Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]