Abstract
Objective Determine the effectiveness of the temporoparietal fascia flap (TPFF) with adipose tissue in preventing cerebrospinal fluid (CSF) leaks for lateral skull base tumor reconstruction.
Design A retrospective chart review from 2005 to 2010 was conducted of patients undergoing skull base tumor resection. Patients with TPFF reconstruction were compared with those with adipose packing alone based on lumbar drain placement, tumor size, extent of dissection, and incidence of CSF leak. Data was analyzed with a Fisher exact test at p < 0.05.
Setting Tertiary care institution.
Main Outcome Measures Incidence of CSF leak.
Results A total of 16 patients had a TPFF reconstruction; 20 had adipose only. Four TPFF patients had lumbar drain placement, as did six in the adipose-only group. Six patients had a CSF leak, all in the adipose-only group (p = 0.02). Patients with a lumbar drain were more likely to have larger tumors (p = 0.01) and to have a CSF leak if they had adipose-only reconstruction (p = 0.07).
Conclusions Lateral skull base reconstruction using TPFF with adipose tissue is easily performed and has a low operative morbidity. Early results show a significant decrease in the rate of CSF leak using TPFF, particularly in high-risk patients.
Keywords: cerebrospinal fluid leak, skull base surgery, skull base reconstruction, paraganglioma
Introduction
Lateral skull base tumors are amongst the most surgically challenging tumors of the head and neck. These include benign conditions such as paragangliomas, meningiomas, and schwannomas, as well as other conditions such as cholesteatomas and cholesterol granulomas. Malignant tumors include squamous cell and basal cell carcinomas with involvement of the temporal bone. Surgical management of these tumors can involve both transcervical and transtemporal dissection and may result in a significant bone and soft tissue defect with an inherent risk of postoperative cerebrospinal fluid (CSF) leak.1 Although most CSF leaks can be managed conservatively, the presence of a postoperative CSF leak can lead to increased patient morbidity as well as a longer hospital stay and a greater cost of care.
The risk of postoperative CSF leak depends on the extent of tumor involvement, the size and type of surgical resection, and the method of reconstruction.2 Traditionally, adipose tissue has been used to provide soft-tissue support for these wounds. Though effective, the extensive removal of the bony skull base that is common in these cases removes the structural architecture that is needed to achieve a tight seal with fat patching. More recently vascularized tissue has been explored as a more durable reconstructive option, particularly in the management of skull base glomus tumors.3 Both local and free tissue flaps can be used for reconstruction, though free tissue transfer has the disadvantages of significantly prolonged operative time and donor site morbidity. The ideal reconstructive option would provide durable and reliable wound closure, with minimal additional surgical morbidity and operative time.
The temporoparietal fascia flap (TPFF) has several properties that make it ideal for skull base reconstruction. It is an anatomic continuation of the galea aponeurosis from the scalp and becomes the superficial musculoaponeurotic system below the zygomatic arch. In the mastoid region the TPFF is found just deep to the subcutaneous tissue (Fig. 1). The fascia has a reliable vascular supply from the superficial and middle temporal arteries, as well as the occipital and posterior auricular vessels.4 When pedicled on the superficial temporal artery, the TPFF has a length of 4 to 6 cm from the tragus to the superior temporal line5 (Fig. 2). This area can be extended to 17 × 14 cm by incorporating posterior arterial branches.6 The use of temporoparietal fascia as a flap in otologic reconstruction was first described in the mid 1990s.6 We sought to determine the efficacy of the TPFF in preventing postoperative CSF leaks as compared with adipose tissue packing alone.
Fig. 1.
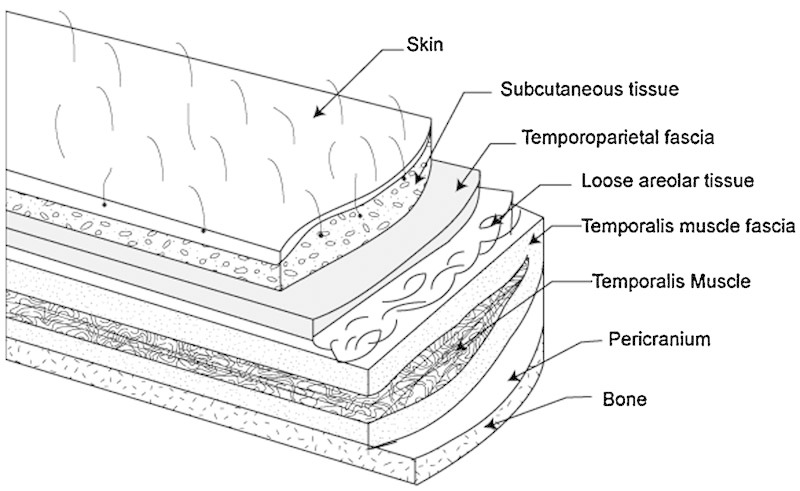
Layers of the scalp with temporoparietal fascia.
Fig. 2.
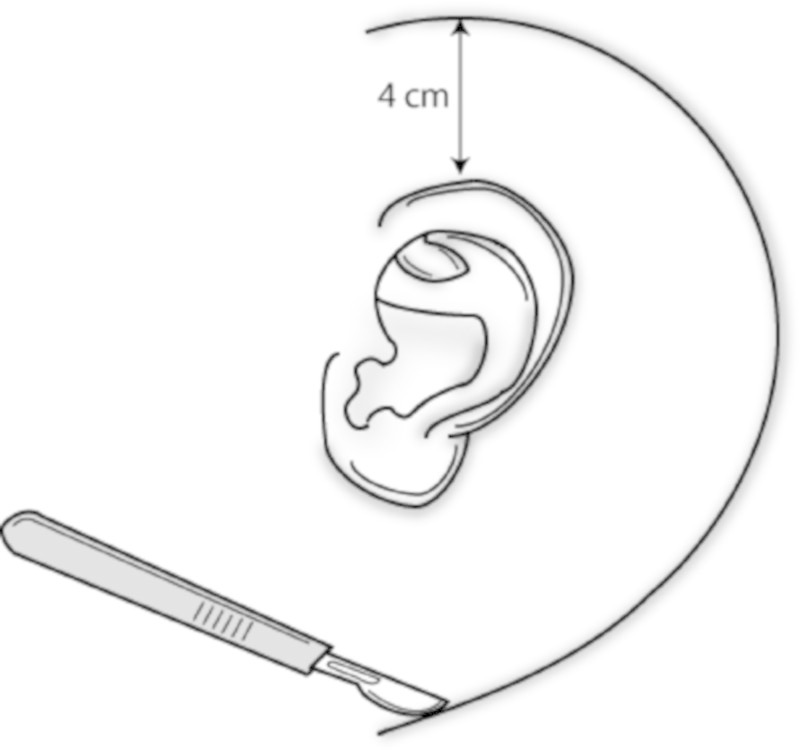
Postauricular incision.
Methods
A retrospective review was conducted of all lateral skull base cases from 2005 to 2010 at our tertiary-care hospital. Study approval was obtained from the Institutional Review Board. Operative Current Procedural Terminology (CPT) codes specific to lateral skull base surgery were used to create a patient cohort (CPT Codes: 61590-61592, 61595-61598, 69535, 61600, 61605-61608, 61615-61616). Exclusion criteria included a diagnosis of an acoustic neuroma, prior radiation therapy, and inadequate chart documentation. Note that patients with an acoustic neuroma were excluded because these defects seldom require extensive skull base dissection or reconstruction. Charts were reviewed to determine the type and size of the original tumor, extent of surgical resection, placement of intraoperative lumbar drain, and the method of defect reconstruction. The presence of a CSF leak was determined by review of patient inpatient notes and clinical notes up to 2 months out from discharge. Tumor size was measured based on surgical pathology reports and, when available, was graded as small (< 2.5 cm), medium (2.41 to 4 cm), or large (> 4.1 cm) based on the tumor's largest dimension. The extent of surgical resection was characterized as intradural or extradural as determined from operative reports. In addition, intraoperative violation of the dura during an otherwise extradural case was also recorded based on operative reports. Patients with adipose tissue packing alone were compared with those patients who had a TPFF in addition to adipose tissue packing for reconstruction. These groups were further subdivided into patients with and without intraoperative lumbar drain placement. The histologic tumor type, size of tumor, intraoperative violation of the dura, and rate of postoperative CSF leak was calculated as a percentage for each of the four patient groups.
Statistical Analysis
Two-tailed Fisher exact test was used to compare the incidence of CSF leak in TPFF patients with those who had adipose tissue packing, as well as between subgroups with and without lumbar drains. The data on tumor size and extent of surgical dissection were similarly analyzed for patients between the four patient groups. All analysis was conducted using GraphPad Prism version 5.04 (GraphPad Software, Inc., La Jolla, California, USA).7 Statistical significance was set at p < 0.05.
Surgical Methods
Patients who were candidates for TPFF were those with intradural tumor extension, an intraoperative CSF leak, or risk of dural violation due to the extent of tumor resection. Our method of harvesting the TPFF is as follows: after induction of general anesthetic and sterile preparation, a superficial c-shaped incision is made from the scalp approximately 4 cm above the ear extending postauricularly to the neck (Fig. 2). The incision is extended superior in a linear fashion. Dissection is started in the subcutaneous plane at the postauricular aspect and changed to a subplatysmal dissection in the neck. The subcutaneous tissue is gently dissected off the underlying temporoparietal fascia, taking care to preserve axial blood vessels. The anterior limit of dissection is the temporal hairline to avoid injury to the temporal branch of the facial nerve. Once the appropriate area has been exposed, the temporoparietal fascia is sharply incised and the distal superficial temporal vessels are ligated or cauterized. This fascia is then elevated off the temporalis fascia bluntly (Fig. 3). This is continued until the superficial temporal artery is reached at the level of the tragus. The flap is then lifted out of the surgical field while remaining pedicled on the superficial temporal artery vessels. The remainder of the surgery is then completed. If an inadvertent intraoperative durotomy is made, the dura is reapproximated with suture ligation when possible. The flap is then rotated over the surgical defect, and abdominal fat packing is placed to stabilize the flap and provide contour. A lumbar drain is surgically placed based on surgeon preference based on anticipated risk of dural entry or intracranial dissection. Postoperatively, patients have a mastoid dressing in place and are monitored in the neurosurgical intensive care unit. Signs of CSF leak are assessed daily, including leakage from the nose or incision site or severe positional headaches. All patients with a CSF leak diagnosed in the hospital are initially managed conservatively with elevation of the head of the bed, avoidance of Valsalva, and placement of a lumbar drain. In cases of a persistent or complicated CSF leak, operative intervention is undertaken for definitive closure.
Fig. 3.
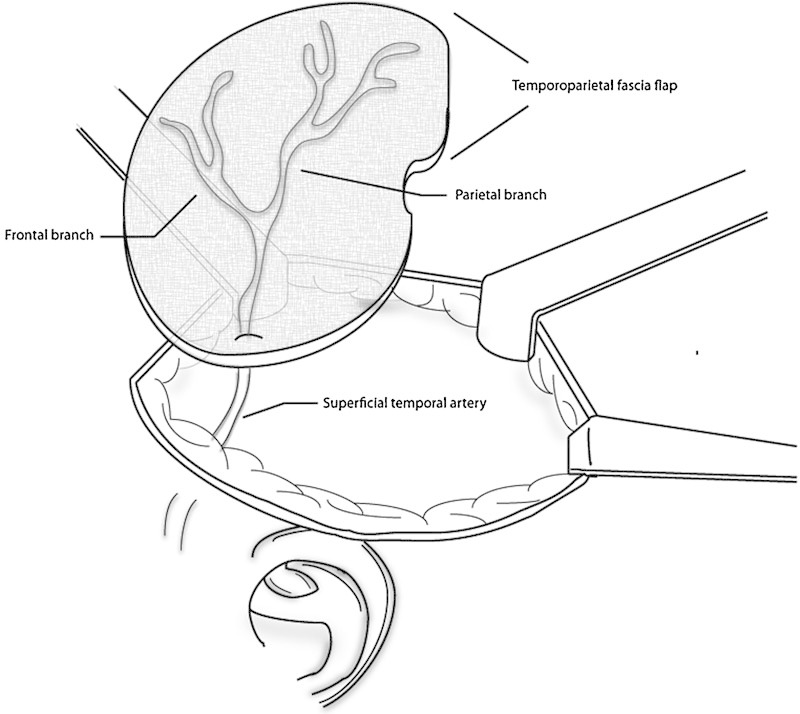
Temporoparietal flap pedicled on superficial temporal artery.
Results
Patient Characteristics
A total of 61 patients underwent lateral skull base resection and were available for review. Data on tumor subtype, size, the extent of surgical dissection, and type of reconstruction are summarized in Table 1. After exclusion criteria, 16 patients who were reconstructed with TPFF and adipose packing and 20 patients with adipose tissue packing as their sole method of reconstruction met criteria for analysis.
Table 1. Patient characteristics.
| TPFF | Adipose | TPFF-LD | Adipose-LD | |
|---|---|---|---|---|
| Total | 12 | 14 | 4 (25%) | 6 (30%) |
| Tumor type | ||||
| Paraganglioma | 10 | 4 | 3 | 4 |
| Schwannoma | - | 2 | - | 1 |
| Cholesteatoma | 2 | 1 | - | - |
| Meningioma | - | 1 | 1 | 1 |
| Other | - | 6 | - | - |
| Tumor size* | ||||
| Small | 5 (42%) | 8 (57%) | 1 (25%) | 3 (50%) |
| Medium | 7 (58%) | 6 (43%) | 1 (25%) | 2 (33%) |
| Large | 0 (0%) | 0 (0%) | 2 (50%) | 1 (17%) |
| Surgical extent | ||||
| Intradural | 7 | 9 | 4 | 5 |
| Extradural | 5 | 5 | 0 | 1 |
| Dural violation† | (4) | (2) | (0) |
Abbreviations: LD, lumbar drain; TPFF, temporoparietal fascia flap.
Size classifications are as follows: S ≤ 2.5 cm, M = 2.51-4cm, L ≥ 4.1 cm.
Patients with extradural dissection with dural entry.
The most common tumor type in both the TPFF and adipose tissue groups was paraganglioma (81% and 40%, respectively). Patients with adipose tissue reconstruction had a wide range of other tumor pathologies, including schwannoma and meningioma. There were no significant differences in tumor size between TPFF patients versus adipose-only patients. The percentage of patients requiring intradural dissection was similar between the TPFF and adipose-only groups (69% versus 70%, respectively). Four patients (25%) with TPFF reconstruction and two (10%) with adipose-only reconstruction had an intraoperative violation of the dura in an otherwise extradural case. There was no statistically significant difference between reconstructive groups with regards to extent of dissection.
Four patients from the TPFF group (25%) and six (30%) patients from the adipose-only group had a lumbar drain placed at the time of surgery. The subset of patients who underwent lumbar drain placement had significantly larger tumors than did those without (p = 0.01). There was no significant difference in the extent of surgical dissection between patients with or without lumbar drainage.
Incidence of CSF Leak
The incidence of postoperative CSF leak between the two surgical groups is summarized in Table 2. All leaks occurred within 28 days, and most within 2 weeks, of the original surgery. No patients in the TPFF group had a CSF leak. Six patients (30%) in the adipose tissue group had a CSF leak, four of which were managed medically with observation and lumbar drainage. Two patients required operative intervention; one underwent closure of the eustachian tube and another required a revision craniotomy with fat packing. The characteristics of patients with CSF leak are shown in Table 3. Five patients with a CSF leak underwent intradural dissection; the remaining patient had intraoperative violation of the dura. Four of the six patients had an intraoperative lumbar drain placed prior to having a CSF leak. There was no statistical difference in tumor size or extent of dissection between patients who had an intraoperative lumbar drain and a postoperative CSF leak.
Table 2. Incidence of CSF leak between groups.
| No lumbar drain | Lumbar drain | Total | |
|---|---|---|---|
| TPFF | 0/12 (0%) | 0/4 (0%) | 0/16 (0%) |
| Adipose | 2/14 (13%) | 4/6 (66%) | 6/20 (30%) |
| p value | 0.48 | 0.07 | 0.02 |
Abbreviations: CSF, cerebrospinal fluid; TPFF, temporoparietal fascia flap.
Table 3. Characteristics of CSF leak patients.
| Adipose | Adipose-LD | |
|---|---|---|
| CSF Leak | 2 | 4 |
| Tumor size | ||
| S | 1 | 2 |
| M | 1 | 1 |
| L | - | 1 |
| Surgical extent | ||
| Intradural | 1 | 4 |
| Extradural | 1 | 0 |
| Dural violation | (1) | - |
Abbreviations: CSF, cerebrospinal fluid; LD, lumbar drain.
Statistical Analysis
Statistical significance analysis is shown in Table 2. There was a significant difference in the overall incidence of CSF leak between the TPFF and adipose-only groups (p = 0.02). In the subset of patients with a lumbar drain, the difference between patients with a CSF leak approached significance (p = 0.07). There was no significant difference in the incidence of CSF leak for patients without an intraoperative lumbar drain.
Complications
One patient with a TPFF reconstruction had minor alopecia at the surgical site. All patients with a CSF leak had eventual resolution and no long-term complications.
Discussion
Management of lateral skull base tumors continues to be a surgical challenge. In addition to the risk of damage to neurovascular structures, the surgical reconstruction of the resulting defects requires that one be comfortable with the regional anatomy and tissue coverage options. Options for lateral skull base reconstruction vary and depend on the location and size of the defect and the status of the dura.8 The ultimate goal of any skull base reconstruction is to separate the intracranial contents from the sinuses and outside environment. Adipose tissue has traditionally been used in the past, with the known risks of donor site morbidity and the possibility of eventual absorption. More recently, vascularized tissue has been recognized as the optimal reconstructive option for preventing CSF leaks in skull base surgery, particularly for glomus tumors.9 Whereas free flaps have associated donor site morbidity and a prolonged operative time, local flaps have the advantages of being able to provide a robust skull base closure with minimal morbidity and avoiding the need for an additional donor surgical site.
The use of the TPFF reconstruction for temporal bone surgery is not novel; it was described by Netterville et al10 almost two decades ago as a durable option for skull base reconstruction. The TPFF is an ideal local flap for lateral skull base reconstruction because it has a reliable blood supply, is easily accessed through the operative site, and has adequate tissue area and bulk to provide durable reconstruction. In our series we combined the TPFF with adipose tissue packing, which provided a robust repair with minimal complications. Risks associated with the TPFF include injury to the frontal branch of the facial nerve, which is avoided through careful surgical dissection. Additional complications include alopecia, seroma, or hematoma. One patient in our series had minor postoperative alopecia, and none had injury to the facial nerve. Overall, the TPFF is well tolerated by patients.
The primary purpose of our study was to determine if TPFF with adipose reconstruction could decrease the complication of CSF leak. For our patient cohort, there was a significant decrease in the incidence of CSF leak for patients with TPFF versus those with adipose packing alone. There was no significant difference in tumor size or extent of surgical resection between patients with or without a CSF leak. All patients with a CSF leak had intradural tumor extension or dural violation, though this was not a significant difference from patients who did not have a leak. From our results, the TPFF is effective in decreasing the incidence of postoperative CSF leak when used for reconstruction of lateral skull base defects.
An additional aim of our study was to determine which patients would most benefit from TPFF reconstruction. In this regard, data from the lumbar drainage subset of patients is revealing. Although similar percentages of patients from both reconstructive groups had lumbar drains placed at the time of surgery, there was a trend toward significance showing an increase in the incidence of CSF leak for patients with a lumbar drain in the adipose-only reconstruction group. The decision to place a lumbar drain for both groups of patients was made by the attending surgeon based on the estimated risk of dural violation and subsequent CSF leak. Our data supports this, in that patients with lumbar drainage were significantly more likely to have larger tumors, which could result in a larger postsurgical defect and greater chance of inadvertent dural injury. As such, patients who had a lumbar drain placed are inherently considered at higher risk for CSF leak. Surprisingly, in this high-risk group of patients, none with a TPFF reconstruction had a postoperative CSF leak. Although not statistically significant, the difference in CSF leak rates between the reconstructive groups in this cohort of high-risk patients is worth consideration. A preliminary conclusion is that for patients who are considered at increased risk for CSF leak, based on tumor size or surgeon judgment, a TPFF with adipose tissue is preferred over adipose packing alone.
Similar studies have been done to examine the utility of vascularized tissue reconstruction for skull base defects. Jackson et al11 reviewed patients with skull base tumors and developed a reconstructive algorithm based on dural involvement and tumor size. They concluded that free adipose tissue was adequate for patients with small defects with an intact labyrinth and a well-supported bony margin. For patients who required removal of the labyrinth and/or a large portion of dura, vascularized tissue was the reconstructive method of choice and decreased the rate of postoperative CSF leak by eightfold. Although our results are not as dramatic, they are in keeping with this finding. Cheney et al12 specifically used the TPFF for reconstruction after mastoid, external auditory canal, and temporal bone surgeries. They were able to obtain their reconstructive goals of a fully epithelialized canal, a dry mastoid bowl, and reduction in mastoid bowl volume for all cases. Although our outcome measures were different, we did find the TPFF to be similarly versatile for temporal bone reconstruction. Compared with past investigations, our study is unique in that we evaluated the reconstructive use of a TPFF with adipose packing as an alternative to nonvascularized tissue in a specific subset of temporal bone surgeries. We additionally studied a subset of patients at higher risk for CSF leak and found the TPFF to provide a durable reconstruction. It is this subset of patients that is likely to have the greatest benefit from a local reconstruction with vascularized tissue, and we would recommend consideration of the TPFF for this application.
It should be mentioned that use of the TPFF with adipose reconstruction is limited by the size of the resection cavity. The TPFF has been described as being able to cover up to a 17 cm defect6. However, patients with a larger postresection cavity may benefit more from free flap reconstruction. Additional contraindications follow those for other pedicled local flaps: uncertain blood supply and questionable tissue viability due to previous trauma or radiation. No patients in our study received preoperative radiation therapy and thus we cannot comment on the use of the TPFF in this situation.
There are several important drawbacks to our study. This was a retrospective examination, which resulted in variable sample sizes and data. The resulting sample sizes of the two groups were small in comparison to the initial patient cohort. The outcome measures were determined through review of patient notes and relied on clinician judgment and accurate documentation. No patients in the TPFF group received postoperative radiation, and the durability of this reconstruction for our patient population remains unknown. However, the TPFF has a reliable blood supply and would be preferable to a nonvascularized reconstruction in the setting of radiation therapy. Finally, the decision to use a TPFF was ultimately made by the surgeon either before or after the initial resection and as such made it difficult to accurately determine the patient factors contributing to these decisions. Regardless, this highlights the benefit of being adept in TPFF reconstruction for its ability to be easily employed when there is an intraoperative concern for CSF leak.
Despite these limitations, the advantages of the TPFF for lateral skull base reconstruction are apparent. As compared with adipose packing alone, the TPFF with adipose tissue was used for high-risk patients with larger tumors and resulted in no postoperative CSF leaks. It is an efficient and durable flap with minimal technical difficulty and a low rate of complications.
References
- 1.Brackmann D E, Rodgers G K, Wilkinson E P. Philadelphia: Saunders; 2009. Management of postoperative cerebrospinal fluid leaks; pp. 727–732. [Google Scholar]
- 2.Kaylie D M O'Malley M Aulino J M Jackson C G Neurotologic surgery for glomus tumors Otolaryngol Clin North Am 2007403625–649., x [DOI] [PubMed] [Google Scholar]
- 3.Heth J A Funk G F Karnell L H et al. Free tissue transfer and local flap complications in anterior and anterolateral skull base surgery Head Neck 20022410901–911., discussion 912 [DOI] [PubMed] [Google Scholar]
- 4.Stow N W, Gordon D H, Eisenberg R. Technique of temporoparietal fascia flap in ear and lateral skull base surgery. Otol Neurotol. 2010;31(6):964–967. doi: 10.1097/MAO.0b013e3181e3d33c. [DOI] [PubMed] [Google Scholar]
- 5.Lai A, Cheney M L. Temporoparietal fascial flap in orbital reconstruction. Arch Facial Plast Surg. 2000;2(3):196–201. doi: 10.1001/archfaci.2.3.196. [DOI] [PubMed] [Google Scholar]
- 6.Brent B, Upton J, Acland R D. et al. Experience with the temporoparietal fascial free flap. Plast Reconstr Surg. 1985;76(2):177–188. doi: 10.1097/00006534-198508000-00001. [DOI] [PubMed] [Google Scholar]
- 7.GraphPad Software Available at: http://www.graphpad.com. Accessed June 2, 2013
- 8.Cheney M L, Megerian C A, Brown M T, McKenna M J, Nadol J B. The use of the temporoparietal fascial flap in temporal bone reconstruction. Am J Otol. 1996;17(1):137–142. [PubMed] [Google Scholar]
- 9.Jackson C G, McGrew B M, Forest J A, Netterville J L, Hampf C F, Glasscock M E III. Lateral skull base surgery for glomus tumors: long-term control. Otol Neurotol. 2001;22(3):377–382. doi: 10.1097/00129492-200105000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Netterville J L, Civantos F J. Defect reconstruction following neurotologic skull base surgery. Laryngoscope. 1993;103(11 Pt 2) 60:55–63. doi: 10.1002/lary.1993.103.s60.55. [DOI] [PubMed] [Google Scholar]
- 11.Jackson C G, Netterville J L, Glasscock M E III. et al. Defect reconstruction and cerebrospinal fluid management in neurotologic skull base tumors with intracranial extension. Laryngoscope. 1992;102(11):1205–1214. doi: 10.1288/00005537-199211000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Cheney M L, Megerian C A, Brown M T, McKenna M J, Nadol J B. The use of the temporoparietal fascial flap in temporal bone reconstruction. Am J Otol. 1996;17(1):137–142. [PubMed] [Google Scholar]


