Abstract
Introduction Complete or partial removal of the pterygoid process provides lateral extension of the endonasal corridor necessary to approach the Meckel cave, infrapetrous skull base, and medial infratemporal fossa. This paper provides the anatomical foundations for the endoscopic endonasal transpterygoid approach with preservation of all neurovascular structures inside the pterygopalatine fossa.
Methods Eight endoscopic transpterygoid approaches were performed in fresh cadaveric specimens. In all dissections the vidian nerve and the periosteal sac enclosing the pterygopalatine fossa were preserved.
Results We reliably transposed the pterygopalatine fossa to approach the Meckel cave, infrapetrous skull base, and medial infratemporal region, preserving the neurovascular structures inside the pterygopalatine fossa in all specimens.
Conclusions The transposition of the pterygopalatine fossa neurovascular structures for endoscopic endonasal approaches to the skull base is an alternative technique that is both feasible and desirable. The transposition requires no additional technical skills but requires comprehensive knowledge of its anatomy. The anatomical preservation of the neurovascular structures is potentially beneficial to the quality of life of patients. Clinical studies are necessary to prove the real benefits of this technique.
Keywords: endoscopy, endonasal, cranial base, pterygopalatine fossa, transpterygoid approaches
Introduction
A lateral extension of the endonasal corridor is necessary for expanded endonasal approaches (EEAs) to the Meckel cave, infrapetrous skull base, and medial infratemporal fossa. Removal of the base of the pterygoid process is imperative to adequately expose these areas.1,2 During the approach, neurovascular structures are commonly sacrificed to achieve adequate exposure.
The vidian nerve runs lateral to the junction of the petrous and paraclival segments of the internal carotid artery (ICA) and travels along the sphenoid sinus floor to exit through the vidian foramen located on the anteromedial aspect of pterygoid base. Parasympathetic fibers travel along the vidian nerve and pass through the sphenopalatine ganglion to stimulate lacrimation. Its canal is often used as an anatomical landmark to the petrous ICA; thus, the vidian nerve and artery are dissected and can be sacrificed in the process.3,4 Sacrifice of the vidian nerve can result in clinically significant dryness of the eye, though patients often refer to decreased emotional lacrimation. Nonetheless, it is not infrequent that patients with middle fossa tumors present with concomitant V1 deficits with its consequent reduction or loss of corneal sensation (i.e., corneal reflex). In such cases, a vidian nerve or pterygopalatine ganglion injury could compound the magnitude of the problem, as loss of corneal sensation associated with a dry eye is likely to produce corneal ulcers and ocular complications.9 Similarly, patients with an ipsilateral facial palsy are at increased risk due to the lack of adequate protective mechanisms and lagophthalmos.
If additional inferior exposure is needed during the transpterygoid approach, the medial and/or the lateral pterygoid plates can be removed. This consistently involves the ligation of the descending palatine artery and sacrifice of the greater palatine nerve to achieve optimal exposure of pterygoid plates. Injury of the greater palatine nerve results in anesthesia of the ipsilateral hard palate.6 Patients are somewhat tolerant of this deficit; however, it adds to the morbidity. Injuries to the descending palatine artery are of lesser clinical importance, since the rich blood supply of the palate guarantees an adequate contralateral irrigation.7,8
We believe that every effort toward the preservation of anatomical structures in the pterygopalatine fossa is of value. This paper describes an endoscopic transpterygoid approach in which the medial and lateral pterygoid plates and their base are removed with preservation of all neurovascular structures inside the pterygopalatine fossa. This technique describes an en bloc transposition of the neurovascular structures of the pterygopalatine fossa.
Methods
We used four fresh cadaveric specimens that were dissected bilaterally. All dissections included endonasal endoscopic transpterygoid approaches to the Meckel cave, infrapetrous skull base, and medial infratemporal fossa. In every dissection, we preserved the vidian nerve and the periosteal sac enclosing the pterygopalatine fossa. This comprises the periosteum that covers the posterior aspect of the back wall of the maxillary sinus anteriorly and the periosteum that covers the pterygoid base posteriorly. All heads were previously evaluated for the absence of craniofacial trauma/surgery, sinonasal neoplasms, skull base tumors, or any other condition that could modify the anatomy of our region of interest. All specimens were prepared with the intravascular injection of colored liquid silicone using a previously described technique.10
Dissection Technique
The exposure was initiated using a 0-degree rod lens endoscope (Karl Storz Endoscopy, Culver City, California, USA). For the purpose of standardization, we dissected the left side first. Ipsilateral middle and inferior turbinectomies, anterior and posterior ethmoidectomies, and a large sphenoidotomy were performed to expand the nasal corridor before starting the transpterygoid approach. All the mucoperiosteum covering the perpendicular plate of the palatine bone was removed to identify the sphenopalatine foramen. This corridor was then expanded laterally, removing the medial wall of the maxillary sinus, thus exposing the entire height of the posterior wall of maxillary sinus.
For the transpterygoid approach, we carefully removed the entire posterior wall of the maxillary sinus preserving its posterior periosteum, which covers the contents of the pterygopalatine fossa. Dissection of the inferomedial aspect of the posterior wall of the maxillary sinus deserves special attention, as it contains the greater palatine canal. This canal is formed by the oblique groove of the medial wall of the maxillary bone and the greater palatine groove deep to the lateral surface of the perpendicular plate of the palatine bone.11,12 Both the descending palatine artery and the greater palatine nerve travel through this canal (Fig. 1).
Fig. 1.
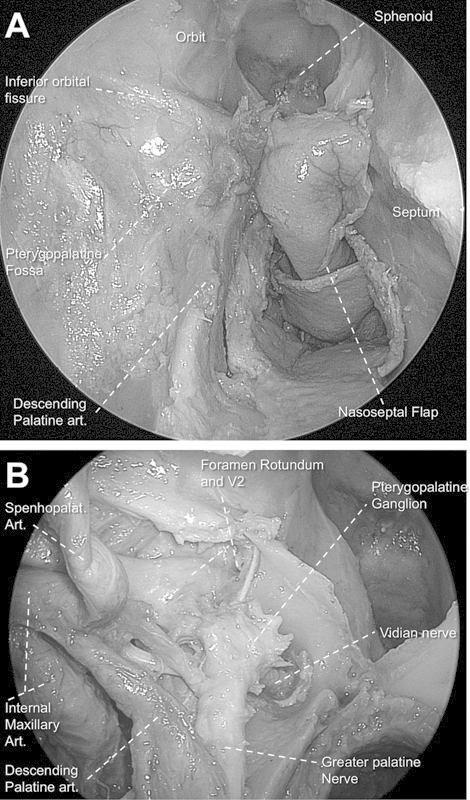
(A) Endoscopic view of right pterygopalatine fossa still covered by periosteum (0-degree endoscope). Note the descending palatine artery after removal of the surrounding bony sheath. (B) View after opening periosteum and removal of fat tissue of pterygopalatine fossa. Note the communication between vidian nerve, pterygoid ganglion, V2, and greater palatine nerve.
An adequate exposure involved removal of all the posterior wall of the antrum, from the posterior aspect of the orbital floor (inferior orbital fissure) to the floor of the maxillary sinus. The infraorbital nerve was identified as it leaves the pterygopalatine fossa and enters the infraorbital canal.
After complete exposure of the anterior pterygopalatine fossa, its medial aspect was approached. The orbital process and the perpendicular plate of the palatine bone were removed, thus exposing the medial part of the pterygopalatine fossa. Inferiorly, the bone of the greater palatine canal was removed until the descending palatine artery and the greater palatine nerve were completely free. Inferiorly, it was important to verify that the dissection reached the greater palatine foramen. This will guarantee the possibility of an adequate lateral mobilization of the pterygopalatine fossa in ensuing steps of the dissection.
Subsequently, the sphenoid process of the palatine bone was removed to expose the pharyngeal branch of the internal maxillary artery running along the palatovaginal canal. This canal is found inferomedially on the posterior wall of the pterygopalatine fossa and ruins, in the roof of the nasopharynx.13,14 The artery was sectioned to allow subsequent lateralization of the contents of the pterygopalatine fossa and identification of the opening of the vidian canal at the base of the pterygoid plates.
At this point of the exposure, if the pterygopalatine fossa contents were mobilized laterally, the vidian foramen and nerve could be seen along the base of the pterygoid plates. If the descending palatine artery and the greater palatine nerve were pushed laterally, the anterior aspect of the pterygoid plates could be exposed inferiorly. However, as the vidian nerve was still in its canal, it tethered the entire fossa, and significant resistance could be experienced during the lateral mobilization.
The floor of the sphenoid sinus was then drilled using a 4-mm hybrid cutting/diamond bur until it was reduced back to be in the same plane as the clival recess. Once the vidian canal was identified, drilling can then proceed cautiously along its inferior and medial aspect. If the vidian canal is considered a clock, drilling of the left canal should be initiated from the 6 to 9 o'clock positions. Once the bone was removed along the inferior and medial aspect of the vidian canal, and once the depth was clearly understood, determining the position of the petrous ICA, the bone superior to the vidian canal was then drilled from the 9 to 3 o'clock positions. Once the superior border of the vidian canal was unroofed, the vidian nerve could be transposed from its canal and retracted superiorly, thus allowing the drill to come inferiorly and remove the bone from the 3 to 6 o'clock positions and finalizing the 360 degrees of freedom around the vidian nerve.
After the identification of the anterior genu of the ICA, we verified that all bone surrounding the vidian nerve was entirely drilled until eggshell thin. A dissector can be used to fracture and remove the thin residual bone, preserving the periosteum of the vidian canal. It was important to ensure that the nerve is completely freed from its bone canal to allow adequate mobilization. Preservation of the pterygoid canal periosteum helps to protect the nerve from manipulation during the exposure. Once free, the nerve and the contents of the pterygopalatine fossa can be easily mobilized laterally, providing a better exposure of the surgical field and facilitating instrumentation during the dissection. Approaches to the Meckel cave and infrapetrous area were possible, preserving the connection between the vidian nerve and the pterygopalatine fossa.
Finally, the vidian nerve, descending palatine artery, greater palatine nerve, and pterygopalatine fossa were transposed laterally en bloc. After the mobilization, it was feasible to achieve sufficient exposure to completely drill the medial and lateral pterygoid plates (Fig. 2). Once the bone removal was finished, the medial aspect of the infratemporal fossa was approached, exposing the Eustachian tube with preservation of the pterygopalatine fossa structures.
Fig. 2.
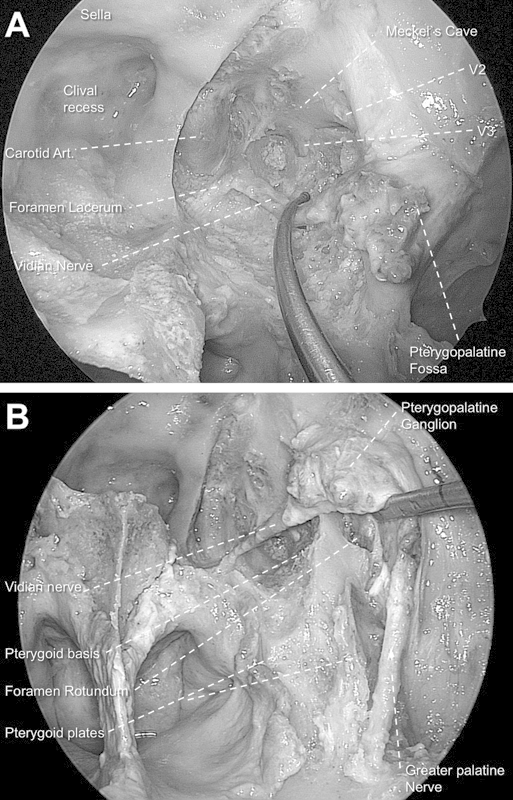
(A) Endoscopic transpterygoid approach to the Meckel cave in the left side (0-degree endoscope). Note that the vidian nerve is preserved. (B) View after unlocking the greater palatine nerve from its bony sheath. Note that the lateral mobilization of the neural structures of the pterygopalatine fossa allows an optimal exposure of all pterygoid base (partially drilled) and plates.
Results
We reliably transposed the pterygopalatine fossa to approach the Meckel cave, infrapetrous skull base, and medial infratemporal region, preserving the neurovascular structures inside the pterygopalatine fossa in all specimens. Dissection in the Meckel cave and infrapetrous skull base was easily achieved with the space provided by the approach. The dissection of the medial aspect of the infratemporal fossa was also feasible; however, we encountered some limitation to dissect the lateral infratemporal fossa.
Discussion
In the last decade, skull base surgery has undergone dramatic advances, including the evolution of endoscopic techniques. During early stages, the endoscopic approaches were limited by the frequent failure of reconstruction of the resultant skull base defects. Following the introduction of the nasoseptal flap (Hadad-Bassagasteguy flap) and other pedicled flaps, the incidence of postoperative cerebrospinal fluid (CSF) leak decreased to a level that is comparable to that of traditional craniofacial approaches.15,16,17,18,19 Currently, tumors localized in the Meckel cave, infrapetrous skull base, or infratemporal regions are amenable to resection and reconstruction through EEAs.1,2,5,20,21
The sacrifice of the vidian nerve during a traditional transpterygoid approach may cause loss of lacrimal secretion and resultant desiccation of the cornea.1,2,3,4,10 Another complaint that may arise is nose dryness. These consequences are primarily caused by loss of postganglionic parasympathetic secretomotor fibers innervating the lacrimal gland and nasal mucosa.22,23 Some patients have complained of a lack of tears under emotional circumstances, associated eye discomfort, and redness.24
Prevedello et al published the transposition of the vidian nerve during the Meckel cave approach.25 That technique spares the vidian nerve during drilling the pterygoid base. However, if the intent is to remove the pterygoid plates, the transposition of the vidian nerve alone is not sufficient for a good exposure. In such cases, the greater palatine nerve and artery are consistently transected to guarantee that exposure.
During the transpterygoid approach to the infratemporal fossa, the pterygopalatine fossa is opened and its structures are dissected, retracted. and/or sectioned until the surgeon reaches the infratemporal region. This kind of dissection carries an increased risk of injury of the neurovascular elements of the pterygopalatine fossa.1,2 Injury of the greater palatine nerve may result in anesthesia of the palate.6
Considering the complications and sequelae caused by injuries to the neurovascular structures of the pterygopalatine fossa, every effort to preserve those structures should be attempted. The transposition of the pterygopalatine fossa contents demonstrated to be a reliable and efficient technique to preserve those structures and reach the Meckel cave, infrapetrous skull base, and infratemporal region (Fig. 3). Dissection within the infratemporal fossa seemed to be more limited when compared with the customary endoscopic approach through the pterygopalatine fossa. As the structures are mobilized laterally, however, the transposition provided an adequate exposure of the medial aspect of the infratemporal fossa. Exposure of the lateral infratemporal fossa, however, was limited. Patients requiring dissection of the lateral aspect of the infratemporal fossa are best served by opening of the pterygopalatine fossa.
Fig. 3.
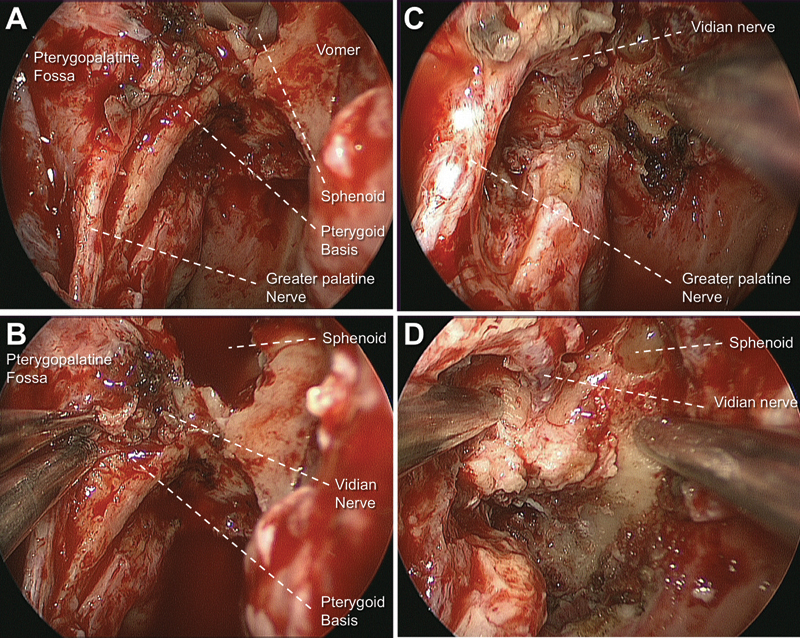
Surgical pictures of endoscopic transpterygoid approach (right side with a 0-degree endoscope). (A) After exposure of pterygopalatine fossa and greater palatine nerve. (B) Note the vidian canal opening with lateral mobilization of pterygopalatine fossa. (C, D) Vidian and greater palatine nerve preserved after the pterygoid base and the medial pterygoid plate were partially removed.
Conclusions
The transposition of the pterygopalatine fossa neurovascular structures for endoscopic endonasal approaches to the skull base is an alternative technique that is both feasible and desirable. The transposition requires no additional technical skills but does require comprehensive knowledge of its anatomy. The anatomical preservation of the neurovascular structures is potentially beneficial to the quality of life of patients. Clinical studies are necessary to prove the real benefits of this technique.
References
- 1.Kassam A B, Gardner P, Snyderman C, Mintz A, Carrau R L. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19(1):E6. [PubMed] [Google Scholar]
- 2.Zanation A M, Snyderman C H, Carrau R L, Gardner P A, Prevedello D M, Kassam A B. Endoscopic endonasal surgery for petrous apex lesions. Laryngoscope. 2009;119(1):19–25. doi: 10.1002/lary.20027. [DOI] [PubMed] [Google Scholar]
- 3.Vescan A D, Snyderman C H, Carrau R L. et al. Vidian canal: analysis and relationship to the internal carotid artery. Laryngoscope. 2007;117(8):1338–1342. doi: 10.1097/MLG.0b013e31806146cd. [DOI] [PubMed] [Google Scholar]
- 4.Kassam A B, Vescan A D, Carrau R L. et al. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg. 2008;108(1):177–183. doi: 10.3171/JNS/2008/108/01/0177. [DOI] [PubMed] [Google Scholar]
- 5.Hofstetter C P, Singh A, Anand V K, Kacker A, Schwartz T H. The endoscopic, endonasal, transmaxillary transpterygoid approach to the pterygopalatine fossa, infratemporal fossa, petrous apex, and the Meckel cave. J Neurosurg. 2010;113(5):967–974. doi: 10.3171/2009.10.JNS09157. [DOI] [PubMed] [Google Scholar]
- 6.Snyderman C H, Carrau R L. Endoscopic ligation of the sphenopalatine artery for epistaxis. Oper Tech Otolaryngol--Head Neck Surg. 1997;8:85–89. [Google Scholar]
- 7.Maher W P. Distribution of palatal and other arteries in cleft and non-cleft human palates. Cleft Palate J. 1977;14(1):1–12. [PubMed] [Google Scholar]
- 8.Gullane P J, Arena S. Extended palatal island mucoperiostealflap. Arch Otolaryngol Head Neck Surg. 1985;1:330–332. doi: 10.1001/archotol.1985.00800070082013. [DOI] [PubMed] [Google Scholar]
- 9.Bhatti M T Patel R Neuro-ophthalmic considerations in trigeminal neuralgia and its surgical treatment Curr Opin Ophthalmol 2005166334–340. Review [DOI] [PubMed] [Google Scholar]
- 10.Fortes F S, Sennes L U, Carrau R L. et al. Endoscopic anatomy of the pterygopalatine fossa and the transpterygoid approach: development of a surgical instruction model. Laryngoscope. 2008;118(1):44–49. doi: 10.1097/MLG.0b013e318155a492. [DOI] [PubMed] [Google Scholar]
- 11.Daniels D L, Mark L P, Ulmer J L. et al. Osseous anatomy of the pterygopalatine fossa. AJNR Am J Neuroradiol. 1998;19(8):1423–1432. [PMC free article] [PubMed] [Google Scholar]
- 12.Mellema J W Tami T A An endoscopic study of the greater palatine nerve Am J Rhinol 200418299–103. An endoscopic study of the greater palatine nerve. [PubMed] [Google Scholar]
- 13.Rumboldt Z, Castillo M, Smith J K. The palatovaginal canal: can it be identified on routine CT and MR imaging? AJR Am J Roentgenol. 2002;179(1):267–272. doi: 10.2214/ajr.179.1.1790267. [DOI] [PubMed] [Google Scholar]
- 14.Borden N M, Dungan D, Dean B L, Flom R A. Posttraumatic epistaxis from injury to the pterygovaginal artery. AJNR Am J Neuroradiol. 1996;17(6):1148–1150. [PMC free article] [PubMed] [Google Scholar]
- 15.Hadad G, Bassagasteguy L, Carrau R L. et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 16.Pinheiro-Neto C D, Prevedello D M, Carrau R L. et al. Improving the design of the pedicled nasoseptal flap for skull base reconstruction: a radioanatomic study. Laryngoscope. 2007;117(9):1560–1569. doi: 10.1097/MLG.0b013e31806db514. [DOI] [PubMed] [Google Scholar]
- 17.Fortes F S, Carrau R L, Snyderman C H. et al. The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope. 2007;117(8):1329–1332. doi: 10.1097/mlg.0b013e318062111f. [DOI] [PubMed] [Google Scholar]
- 18.Fortes F S, Carrau R L, Snyderman C H. et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope. 2007;117(6):970–976. doi: 10.1097/MLG.0b013e3180471482. [DOI] [PubMed] [Google Scholar]
- 19.Zanation A M, Snyderman C H, Carrau R L, Kassam A B, Gardner P A, Prevedello D M. Minimally invasive endoscopic pericranial flap: a new method for endonasal skull base reconstruction. Laryngoscope. 2009;119(1):13–18. doi: 10.1002/lary.20022. [DOI] [PubMed] [Google Scholar]
- 20.Kassam A B Prevedello D M Carrau R L et al. The front door to meckel's cave: an anteromedial corridor via expanded endoscopic endonasal approach- technical considerations and clinical series Neurosurgery 2009643, Supplons71–ons82., discussion ons82-ons83 [DOI] [PubMed] [Google Scholar]
- 21.Cohen N A, Kennedy D W. Endoscopic sinus surgery: where we are-and where we're going. Curr Opin Otolaryngol Head Neck Surg. 2005;13(1):32–38. doi: 10.1097/00020840-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Robinson S R, Wormald P J. Endoscopic vidian neurectomy. Am J Rhinol. 2006;20(2):197–202. [PubMed] [Google Scholar]
- 23.Tubbs R S Salter E G Vidius Vidius (Guido Guidi): 1509-1569 Neurosurgery 2006591201–203., discussion 201-203 [DOI] [PubMed] [Google Scholar]
- 24.Osawa S Rhoton A L Jr Seker A Shimizu S Fujii K Kassam A B Microsurgical and endoscopic anatomy of the vidian canal Neurosurgery 200964502385–411., discussion 411-412 [DOI] [PubMed] [Google Scholar]
- 25.Prevedello D M Pinheiro-Neto C D Fernandez-Miranda J C et al. Vidian nerve transposition for endoscopic endonasal middle fossa approaches Neurosurgery 201067(2, Suppl Operative):478–484. [DOI] [PubMed] [Google Scholar]


