Abstract
Objectives To present and validate a chicken wing model for endoscopic endonasal microsurgical skill development.
Setting A surgical environment was constructed using a Styrofoam box and measurements from radiological studies. Endoscopic visualization and instrumentation were utilized in a manner to mimic operative setting.
Design Five participants were instructed to complete four sequential tasks: (1) opening the skin, (2) exposing the main artery in its neurovascular sheath, (3) opening the neurovascular sheath, and (4) separating the nerve from the artery. Time to completion of each task was recorded.
Participants Three junior attendings, one senior resident, and one medical student were recruited internally.
Main Outcome Measures Time to perform the surgical tasks measured in seconds.
Results The average time of the first training session was 48.8 minutes; by the 10th training session, the average time was 22.4 minutes. The range of improvement was 25.7 minutes to 72.4 minutes. All five participants exhibited statistically significant decrease in time after 10 trials. Kaplan-Meier analysis revealed that an improvement of 50% was achieved by an average of five attempts at the 95% confidence interval.
Conclusions The ex vivo chicken wing model is an inexpensive and relatively realistic model to train endoscopic dissection using microsurgical techniques.
Keywords: chicken wing dissection, endoscopic surgical skills, training model
Introduction
The endoscopic endonasal approach (EEA) has revolutionized surgery of the skull base. Use of the endoscope maximizes the operative visualization through direct ventral corridors to midline tumors, minimizing the need to manipulate neural structures when compared with lateral skull base approaches.1,2 EEA has been reported to reduce postoperative morbidity and recovery time, shorten hospitalization, and decrease cost of care.2
One of the greatest difficulties of EEA is the long learning curve.3,4,5,6,7 Acquisition of endoscopic dissection techniques is a difficult task. The endonasal route requires the use of long and/or pistol-grip surgical instruments with which the surgeon must perform microsurgical-like dissection under the nonstereoscopic visualization provided by the endoscope.8 For these reasons, a surgical training model for endoscopic endonasal surgery is needed.9,10,11
A variety of different training models for endoscopic neurosurgery have been recently described in the literature.12,13,14 However, very few models have been developed that adequately simulate endoscopic intradural dissection using microsurgical-like techniques.15,16 In a previous publication, we reported the use of an animal training model for endoscopic neurosurgery. Although this model attempted to closely simulate the surgical environment, it has the obvious disadvantage of requiring the use of live animals, which adds significant animal and labor costs and limits overall availability. The goal of this study is to develop and demonstrate the efficacy of an ex vivo endoscopic surgical training model using chicken wings. Here we aim to validate the proposed training model by providing chronometric evidence of improvement in surgical skills. Secondary objectives are the analysis of reproducibility, availability, cost effectiveness, and feasibility.
Methods
Construction of a Surgical Environment
To build the training model, a study of the patient's head positioning intraoperatively was performed to indentify the angle between the palatal plane and the floor level for a typical transplanum approach. This step provided important information to simulate the position of the surgeon's hands during the transplanum approach to the suprasellar cistern and infrachiasmatic space. The transplanum angle was chosen due to its prevalence in neuroendoscopic practice and associated requirement for intradural neurovascular dissection.
The depth of dissection was assessed with a retrospective radiological analysis on 30 fine-cut computed tomography (CT) scans of patients with no previous history of trauma and no bone disruption. The angles and distances of the surgical tools according to the transcribriform, transplanum, transsellar, and transclival EEA were obtained. Using the midsagittal slice, the distance between the nasal columella and the planum sphenoidale was measured. These measurements are summarized in Table 1. A Student t-test analysis was performed verifying that there were no statistically significant differences between females and males (p > 0.05). A recent study on human anatomic specimens, as well as patients, has shown that the working area provided by the human nostrils (considering their elasticity) averaged 28 mm (± 3 mm) × 31 mm (± 3 mm).17
Table 1. Head positioning computed tomography study.
| Average +/− standard deviation (n = 30) |
|
|---|---|
| Distance to foramen magnum parallel to palate (mm) | 126.5 |
| Distance to transition between lower and middle clivus (mm) | 117.4 |
| Angle from midclivus to hard palate (degree) | 10.6 |
| Distance to dorsum sellae to posterior clinoid (mm) | 116.1 |
| Angle from posterior clinoid to hard palate (degree) | 21.5 |
| Distance to tuberculum sellae (planum) (mm) | 103.4 |
| Angle from planum to hard palate (degree) | 25.1 |
| Distance to midpoint cribriform (mm) | 78.9 |
| Angle from midpoint of cribriform to hard palate (degree) | 34.3 |
A simple Styrofoam box was used to emulate the sinonasal cavity (Fig. 1A, B). Based on the data above, two holes were made using a manual bur in the roof of the box to simulate the nares. The anterior edges of the holes were covered with tape to provide elasticity and mimic the flexibility experienced in surgery. Precise lines were drawn on the box to indicate proper specimen position to keep the same distance and angle from the simulated nostrils and have the same hand and instrument positioning as in surgery.
Fig. 1.
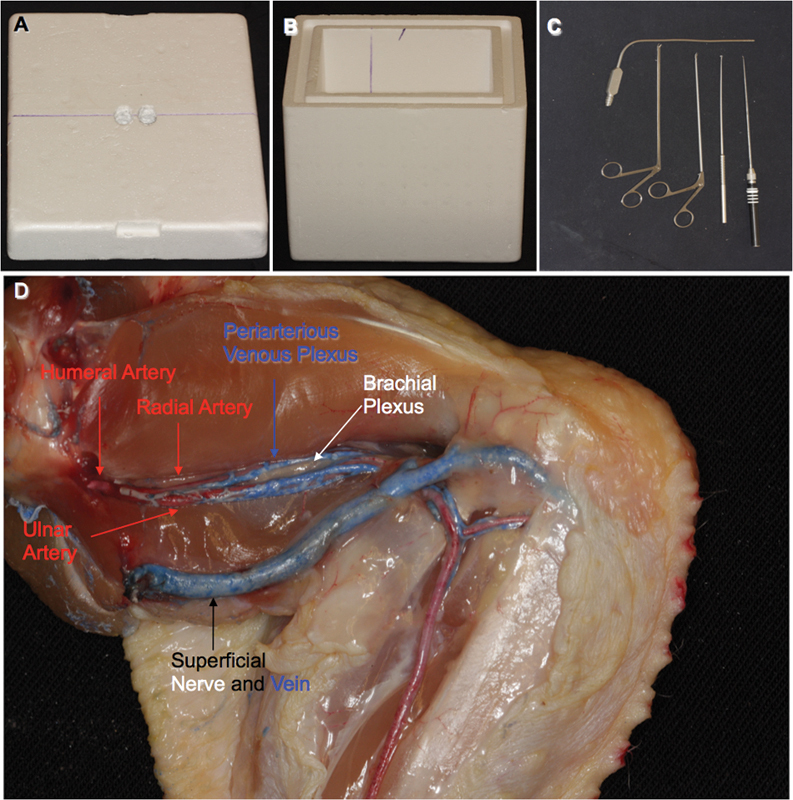
(A) Lid. (B) Box interior illustrating line used to align chicken wings. (C) Instrumentation. (D) Normal chicken wing anatomy.
Training Model
All dissections were conducted using a 0-degree rod lens endoscope HD 2 (Karl Storz, Culver City, California, USA) and a xenon light source. Appropriate endoscopic microsurgical instrumentation included pistol-grip endoscopic angled scissors (Karl Storz), rod-style endoscopic microdissectors (KLS Martin, Pittsburgh Black and Gold set; Jacksonville, Florida, USA), and long suctions (Fig. 1C). Video and still images of the training sessions were recorded using an AIDA video recording system (Karl Storz).
Chicken wings (all right sided) were obtained from a local grocery store and cut at the shoulder. The arteries and veins in one chicken wing specimen were injected with red and blue silicone, respectively (Fig. 1D). This was done to enhance understanding of the surgical anatomy prior to initiating the training exercises.
All procedures were performed using the two-surgeons and three-four hand technique9 with the endoscope at the 12 o'clock position in the right simulated nostril, the surgical instruments at the 6 o'clock position in the right and left nostrils, and the chicken wing placed unsecured in the transplanum position at the bottom of the box using the reference marks described above. The weight of the chicken wing provided ample stability while maintaining realistic malleability.
Four tasks were designed to provide increasing difficulty. The goal of task one was opening the skin from joint-to-joint. The task was considered successfully completed when the entire length of muscle was visible (Fig. 2A). Task two involved connective tissue dissection with separation of the two main groups of muscles and exposure of the perivasculonervous sheath. Upon opening the skin a large superficial vein and its superficial nerve are visible. This was not our target bundle. Task two is completed after exposing the artery from the shoulder to the level of the superficial vein and nerve (Figs. 2B, C). Task three was defined as opening of the perivascular sheath and exposure of the dorsal wall of the vessel in its entire length (Fig. 2D). In task four the trainee was directed to completely dissect the nerve from the artery while trying not to injure either of them. The timing was completed when vessel and nerve were completely separated (Fig. 2E, F). Participants were given advice on dissection and instrumentation when requested; however, all participants employed the same dissection technique. The main outcome recorded was time of completion of each individual task, using lap timing and total time of the four tasks combined (from skin to neurovascular dissection).
Fig. 2.
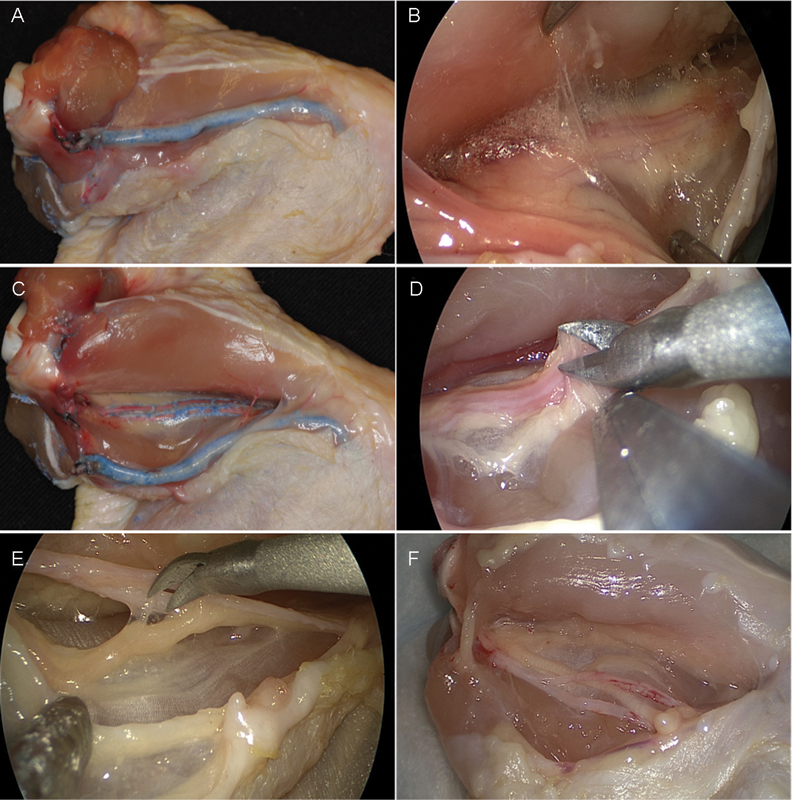
(A) Task 1, initial skin incision. (B, C) Task 2, intramuscular dissection. (D) Task 3, opening of the perivascular sheath. (E, F) Task 4, arteriovenous dissection.
Participants for the study were recruited internally. Three junior attendings, one senior resident, and one medical student (5 total) completed 10 trials of the four surgical tasks. Junior attendings were defined as having graduated from residency within the past 10 years. Two junior surgeons had previous experience (fewer than 100 cases) with neuroendoscopy. Two endoscopic operators performed and supervised all trails, thus reducing variability. A junior attending with extensive endoscopic surgery experience (more than 500 cases) did not participate in the study but supervised the construction of the surgical environment and training model.
All quantitative variables were expressed in average and standard deviation and analyzed with a nonparametric Mann-Whitney U test due to the small study group.
Results
The results from 10 training sessions from each participant are depicted in Fig. 3. The time of the first training session averaged 2,926 ± 810 seconds (48 min, 46 sec ± 13 min, 30 sec) with the longest operation taking 4,020 seconds (1 min, 7 sec) and the fastest operation lasting 1,646 seconds (27 min, 26 sec). By the 10th trial, average operative time decreased to 1,342 ± 579 seconds (22 min, 22 sec ± 9 min, 39 sec). The final trial, however, was not the fastest for most surgeons. The average of the best procedures for each surgeon was calculated to be 1,062 ± 317 seconds (17 min, 42 sec ± 5 min, 17 sec). The most improvement observed was 4,345 seconds (1 h, 12 min, 24 sec), and the least improvement observed was 1,541 seconds (25 min, 41 sec). Participants improved most in task 2, averaging 74.1% decrease in time, and improved the least in task 3, averaging 51.8% decrease in time (Fig. 3B).
Fig. 3.
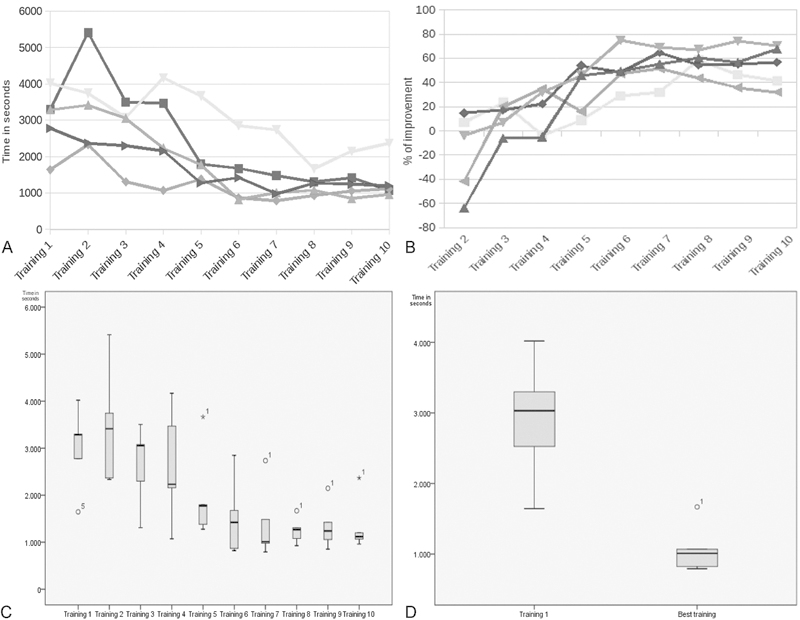
Descriptive and graphical analysis of data. (A) Graphical representation of training time for all four tasks for participants across trials. (B) Box and whisker plot showing percentage of improvement between first and fastest training for every surgeon. (C) Box and whisker plot illustrating operative time for each trial. (D) Box and Whisker plot comparing first trial against fastest trial. (p = 0.001).
Difference in average time between the first training and the best training was statistically significant (p = 0.001). That difference is also demonstrated in the box and whisker plot shown in Fig. 3. This plot also illustrates a threefold decrease in trainee variability in the shortening of the gap between first and third quartile. The same analysis was performed on the difference between the first and last trial, which was seen to be not significant. The difference in variance between 1st and 10th (final) trail, though convincing, was not found to be statistically significant.
Kaplan-Meier analysis revealed that an improvement of 50% is achieved by an average of five attempts at the 95% confidence interval.
Discussion
Here we introduce an ex vivo surgical training model for endoscopic skull base surgery. Importantly, we show the benefits of the proposed training model by demonstrating a threefold improvement in procedural time and a threefold decrease in trainee variability. Although the improvement from the first trial to the last trial was not statistically significant, the improvement from the first trial to the best trial was. Nevertheless, Fig. 3 demonstrates—at least visually—a large difference in total operative time between Trials 1 and 10. Combined effects of intrasurgeon and intersurgeon variability probably explain the former. This is to say that there are ups and downs in everyone's training, and that each surgeon progresses at a different rate.
During the first few trials, large variability in operative times was noted; however, as the study progressed, the gap between participants decreased. We also observed that all participants improved their quality of dissection. During the first few trials, most participants were unable to keep the muscle intact while performing task two. By the end of the study, cutting into the muscle was a rare event. Although not objectively measured in this study, we can state that operative technique also improved greatly; trainees progressively made more purposeful gestures and reduced the percentage of time performing unnecessary movements (operative efficiency). These data illustrate the effective acquisition of endoscopic dissection skills provided by the training model presented here.
The use of experimental animals for surgical training is well documented in the literature. Besides the obvious advantage of animal models to simulate real surgery, such as hemostasis, those models are much more resource intensive when compared with the chicken wing model. The associated stresses and regulations of animal work are enough for some surgeons to avoid using live animals as models. More specifically, animal work requires an internally approved animal laboratory and use of anesthesia. In some cases, a veterinarian is required. Acquisition and upkeep of the animals adds significant cost. Ethical issues also arise when practical alternatives to animal models exist.
For any training model, realistic simulation of a surgical environment is paramount. Although there is no accurate method to objectively measure this, all surgeons with previous experience with endoscopy subjectively stated that this neurovascular dissection model is highly representative of actual surgery. In fact, in comparing video from a chicken wing dissection and actual surgery, many of the same motions, maneuvers, and techniques can be seen.
Another important consideration when implementing training models is time. The setup time for the box and endoscopic workstation is less than 10 minutes once the chicken wings are thawed. Most of the participants were able to initially perform the entire procedure in less than an hour. With practice, all were completed within half an hour. All of these factors combined make the chicken wing training model an inexpensive and time-efficient training experience.
Some ideas for future consideration include the use of different angles to simulate different endonasal approaches and changing the orientation of the chicken wing. This may not only increase the difficulty of the four tasks but can also emulate specific anatomical conditions (for example the vertebrobasilar junction). Also, instead of chronometric assessment, other criteria could be used to illustrate improvement, such as number of errors. Importantly, although the training model presented here is effective in improving the laboratory exercise designed for this study, we cannot prove that this provides any clinical benefit during surgery. Further studies are needed to evaluate the impact of laboratory training on the clinical performance of trainees.
Finally, we believe that the use of currently existing endoscopic models should be thoroughly investigated. For example, we tested the Phacon model (www.phacon.de; Leipzig, Germany) by placing the chicken wing in the bone cavity in lieu of the digital sinus compartment (Fig. 4). We believe that the Phacon optimally simulated operative conditions. The curvature of the face provides for better and more accurate hand positioning when compare with the box model. The sinonasal cavity in more realistically recreated, thereby better simulating the actual dissection environment. Additionally, the malleability of the face material allows for optimal endoscopic positioning. In sum, these elements result in both better “driving” of the endoscope and more realistic dissection technique.
Fig. 4.
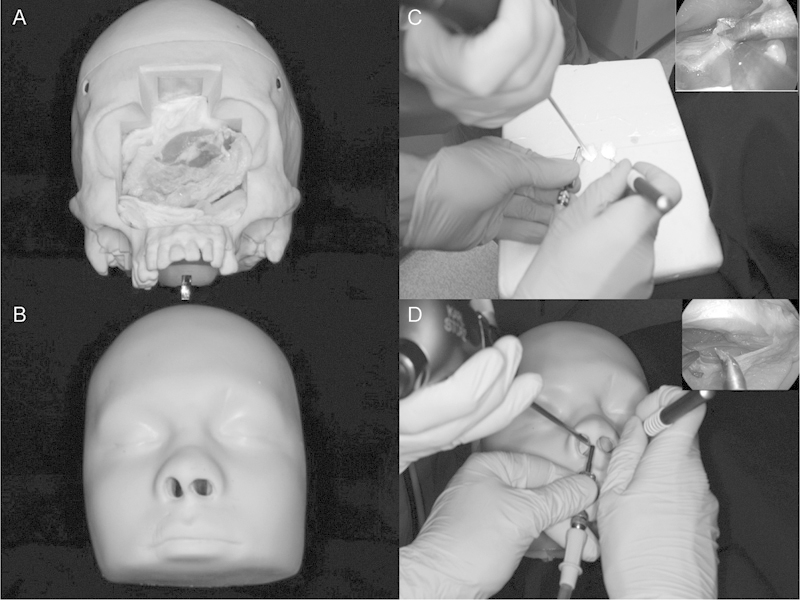
Phacon training model. (A) Phacon training module with face mask removed to show placement of chicken wing in the bony cavity. (B) Phacon training module with face mask. (C) Hand positioning using constructed box and endoscopic visualization. (D) Hand positioning using Phacon and endoscopic visualization.
Conclusions
The ex vivo chicken wing model is an inexpensive and relatively realistic model to train endoscopic dissection using microsurgical techniques. The benefits of such training are reflected in the statistically significant improvement in procedural time. Moreover, the variability between surgeons also decreased regardless of previous endoscopic experience. For these reasons we highly recommend implementing this model in the training of endoscopic skull base surgeons.
Acknowledgments
The authors wish to thank Eugenio Cardenas, MD; Cristina Hostalot, MD; Pablo Barcelo, MD; Cristobal Blanco, MD; Milton Manrique Rastelli Jr., MD; Mary Jo Tutchko; and Wendy Fellows-Mayle, PhD for performing the surgical tasks and for their irreplaceable, careful, and immediate technical assistance.
Footnotes
Note The authors have no conflicts of interest or financial disclosures related to this study.
References
- 1.Maroon J C. Skull base surgery: past, present, and future trends. Neurosurg Focus. 2005;19(1):E1. doi: 10.3171/foc.2005.19.1.2. [DOI] [PubMed] [Google Scholar]
- 2.Prevedello D M, Kassam A B, Gardner P A, Carrau R L, Snyderman C H. New York: Springer; 2010. Expanded endoscopic endonasal approaches to the skull base; pp. 239–251. [Google Scholar]
- 3.Sonnenburg R E, White D, Ewend M G, Senior B. The learning curve in minimally invasive pituitary surgery. Am J Rhinol. 2004;18(4):259–263. [PubMed] [Google Scholar]
- 4.Koc K, Anik I, Ozdamar D, Cabuk B, Keskin G, Ceylan S. The learning curve in endoscopic pituitary surgery and our experience. Neurosurg Rev. 2006;29(4):298–305, discussion 305. doi: 10.1007/s10143-006-0033-9. [DOI] [PubMed] [Google Scholar]
- 5.O'Malley B W Jr, Grady M S, Gabel B C. et al. Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus. 2008;25(6):E10. doi: 10.3171/FOC.2008.25.12.E10. [DOI] [PubMed] [Google Scholar]
- 6.Leach P, Abou-Zeid A H, Kearney T, Davis J, Trainer P J, Gnanalingham K K. Endoscopic transsphenoidal pituitary surgery: evidence of an operative learning curve. Neurosurgery. 2010;67(5):1205–1212. doi: 10.1227/NEU.0b013e3181ef25c5. [DOI] [PubMed] [Google Scholar]
- 7.Smith S J, Eralil G, Woon K, Sama A, Dow G, Robertson I. Light at the end of the tunnel: the learning curve associated with endoscopic transsphenoidal skull base surgery. Skull Base. 2010;20(2):69–74. doi: 10.1055/s-0029-1238214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyderman C H, Pant H, Carrau R L, Prevedello D, Gardner P, Kassam A B. What are the limits of endoscopic sinus surgery?: the expanded endonasal approach to the skull base. Keio J Med. 2009;58(3):152–160. doi: 10.2302/kjm.58.152. [DOI] [PubMed] [Google Scholar]
- 9.Snyderman C, Kassam A, Carrau R, Mintz A, Gardner P, Prevedello D M. Acquisition of surgical skills for endonasal skull base surgery: a training program. Laryngoscope. 2007;117(4):699–705. doi: 10.1097/MLG.0b013e318031c817. [DOI] [PubMed] [Google Scholar]
- 10.Prevedello D M, Kassam A B, Snyderman C. et al. Endoscopic cranial base surgery: ready for prime time? Clin Neurosurg. 2007;54:48–57. [PubMed] [Google Scholar]
- 11.Yasargil M G. New York: Thieme Medical Publishers, Inc; 1996. Laboratory training; pp. 26–28. [Google Scholar]
- 12.Chen G, Ling F. A new plastic model of endoscopic technique training for endonasal transsphenoidal pituitary surgery. Chin Med J (Engl) 2010;123(18):2576–2579. [PubMed] [Google Scholar]
- 13.Olabe J, Olabe J. Microsurgical training on an in vitro chicken wing infusion model. Surg Neurol. 2009;72(6):695–699. doi: 10.1016/j.surneu.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Filho F V, Coelho G, Cavalheiro S, Lyra M, Zymberg S T. Quality assessment of a new surgical simulator for neuroendoscopic training. Neurosurg Focus. 2011;30(4):E17. doi: 10.3171/2011.2.FOCUS10321. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Miranda J C, Barges-Coll J, Prevedello D M. et al. Animal model for endoscopic neurosurgical training: technical note. Minim Invasive Neurosurg. 2010;53(5-6):286–289. doi: 10.1055/s-0030-1269927. [DOI] [PubMed] [Google Scholar]
- 16.Okuda T, Kataoka K, Kato A. Training in endoscopic endonasal transsphenoidal surgery using a skull model and eggs. Acta Neurochir (Wien) 2010;152(10):1801–1804. doi: 10.1007/s00701-010-0728-0. [DOI] [PubMed] [Google Scholar]
- 17.Muyamida Y Fresh cadaveric dissection as a training system of endoscopic endonasal surgery Skull Base 2009190136 (abstr) [Google Scholar]


