Abstract
Attachment of DOTA to a novel monofluoro-cyclooctyne facilitates bioconjugation to an azide-modified peptide via Cu-free click chemistry. The resulting conjugate was radiolabeled with 111In to afford a potential targeted molecular imaging agent with high specific activity and in excellent radiochemical purity.
Keywords: DOTA, copper-free, click chemistry, peptides, chelators, bioconjugates
Numerous synthetic approaches have been applied to add radionuclide chelators, such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), to peptides and other molecular targeting vectors to produce bifunctional molecular targeting agents for molecular imaging and therapy.1-5 Reactive groups present in DOTA derivatives that have proven useful for these applications include active esters, para-isothiocyanato derivatives, and terminal amine functional groups. One particularly advantageous approach for chemical conjugations is the use of so-called “click” chemistry, which has received remarkable attention over the past several years for use in numerous applications in bioconjugate chemistry.6 The most widely-used click reaction is based on [3 + 2] cycloaddition reactions between azides and alkynes to form triazoles as first described by Huisgen.7 Copper(I) catalyzed variants of this cycloaddition have been subsequently developed that lead to triazole products in high yields under mild conditions.8-11 For example, El-Sagheer and Brown highlight the advantages of the reaction for preparation of a modified DNA for numerous applications.7 Significantly, very few publications have reported the use of copper catalyzed click reactions for the coupling of metal chelators (e.g., DOTA) to bioactive molecules such as peptides and aptamers.12 This is primarily due to the high affinity of the Cu catalyst for the macrocyclic chelator moiety, which can interfere with subsequent radiolabeling reactions, thereby reducing the achievable specific radioactivity of the desired biconjugate. Recently, however, Lebedev et al., described the facile preparation of DOTA – peptide conjugates by Cu(I) catalyzed azide-alkyne click chemistry.13 Copper was removed from the bioconjugated chelators by precipitation with Na2S and this work represents a promising route for preparation of bioactive radiolabeled agents. We have also attempted to utilize Cu(I)-catalyzed click reactions for the preparation of various DOTA-biomolecule conjugates. While the cycloaddition reactions themselves were successful, the ability to remove the copper catalyst from our reaction mixtures proved troublesome. This was particularly true in the case of the preparation of DOTA-RNA aptamer constructs in which the conditions for the copper sulfide precipitation were corrosive to our nucleic acids. Further, we found that the presence of Cu inhibited radiolabeling of DOTA-modified bioconjugates, reducing the achieveable specific radioactivity for imaging applications. These experiences led us to examine the feasibility of employing Cu-free click reactions.14 Specifically, it has been demonstrated that ring strain inherent in cyclooctynes promotes spontaneous cycloaddition with organic azides under mild conditions and in the absence of copper catalysts.15-18 We thus considered construction of a DOTA derivative functionalized with a modified cyclooctyne moiety that could then be attached to molecular targeting vectors via copper-free click chemistry. Numerous apparently suitable cyclooctyne ligands for bioconjugate applications have been advanced. Notably however, as pointed out by Jewett and Bertozzi, preparation of each of these functional moieties presents problematic steps.14 Our investigations along these lines resulted in development of an efficient, tractable, high-yielding three-step synthesis of a versatile monoflouro-substituted cyclooctyne (MFCO) that potentially could be used to facilitate a variety of bioconjugation processes (Scheme 1).19
Scheme 1.
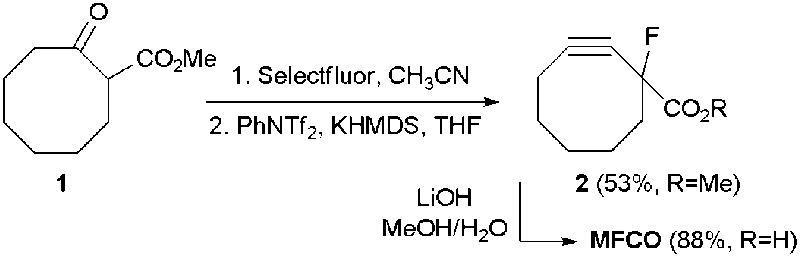
Synthesis of MFCO
In this communication, we present the use of MFCO to prepare a DOTA derivative (DOTA-MFCO) for copper-free click chemical addition at an internal azide-modified Lys residue of a neuropeptide Y (NPY) analog, which is under examination for use in molecular imaging of childhood neuroblastoma.20 This approach provides a straightforward route to a targeted molecular imaging agent that exhibits high specific activity and excellent radiochemical purity when reacted with single-photon emitting radionuclide indium-111 (111In). Notably, this study also highlights the potentially general utility of DOTA-MFCO reagents such as 4 for assembly of bioconjugates relevant to targeted molecular imaging.
The synthesis of MFCO was achieved using a route that we described previously.19 Briefly, methyl-2-oxo-cyclooctane carboxylate 1 was fluorinated with Selectfluor (Scheme 1). The resulting mono-fluoro cyclooctanone was then converted to the cyclooctyne 2 in one pot via elimination of an in situ-generated vinyl triflate. Finally, simple saponification of the methyl ester afforded MFCO. Incorporation of the fluoro substituent serves two purposes. First, fluorination of 1 produces a quaternary center adjacent to both carbonyl groups, thereby facilitating chemo- and regioselective enolization of the ketone in the next step. Second, the presence of electron-withdrawing fluorine atoms adjacent to the strained alkyne is known to further increase the rate of dipolar cycloaddition with azides.16-19
With MFCO in hand, a DOTA-MFCO-NPY conjugate was prepared by first coupling an amine-modified DOTA 3 to MFCO, then conjugating the DOTA-MFCO 4 to the azide on Lys4 of the NPY peptide 5. Briefly, MFCO (1 equiv) was dissolved in dichloromethane (DCM), and triethylamine (1 equiv) and benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate (BOP; 1 equiv) were added. To this mixture was added an amine modified and t-butyl protected DOTA derivative 3 (1.1 equiv), and more triethylamine (1.1 equiv). The reaction mixture was mixed gently at 25°C for 24 hours, at which time the solvent was removed by rotary evaporation. The product (DOTA-MFCO 4) was characterized by mass spectrometry, and used in the following steps without further purification.
Neuropeptide Y derivative 5 [Lys(N3)4, Ahx5-24]NPY‡,21 was synthesized on a 0.10 mmol scale on Rink resin using standard Fmoc procedures.† For peptide conjugation, the lysine at position 4 (Lys4) was replaced with an azide derivative for reaction with 4 by “click” chemistry. DOTA-MFCO was conjugated to the azide-modified side chain of Lys4 using an approximate 10:1 molar ratio of DOTA-MFCO:NPY in DMF for 24 hours at 25°C. The DOTA-MFCO-NPY bioconjugate 6 was simultaneously cleaved from the resin and deprotected by 95:2.5:2.5 trifluoroacetic acid (TFA)/triisopropylsilane (TIS)/water (v/v/v) and then precipitated with ice cold ether and purified by semipreparative high performance liquid chromatography (HPLC).
Radiolabeling experiments were carried out using the DOTA-MFCO-NPY bioconjugates and single-photon emitting radionuclide 111In. Serial dilutions of the DOTA-MFCO-NPY conjugate 6 were prepared from 12 to 1.5 nmoles of DOTA-peptide in 200 μL of sodium acetate/acetic acid buffer (pH 4.53). To these solutions was added 111In aliquots of approximately 135 MBq each, dissolved in identical buffer. The reaction mixtures were incubated at 99°C for 30 minutes with continuous mild agitation. These samples were then cooled and analyzed sequentially without further purification by radioHPLC (Figure 1). Radiolabeling produced excellent results at each concentration of DOTA-peptide conjugate leading to specific activity as high as 88 MBq nmole-1 and radiochemical purity in excess of 98% without additional purification.
Figure 1.
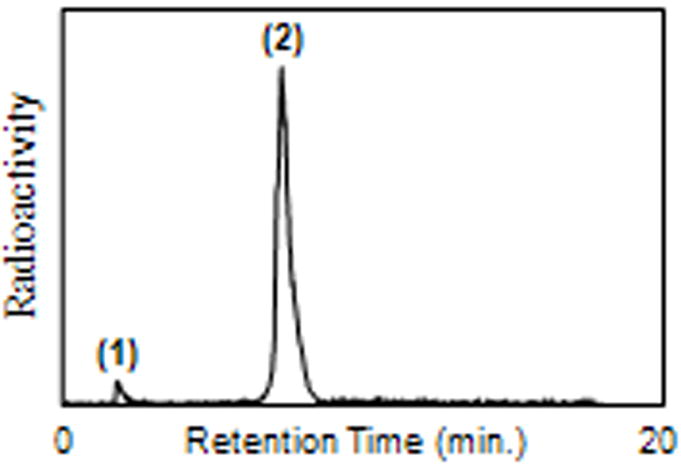
Radio-HPLC analysis of 111In-labeled DOTA-MFCO-NPY (1.5 nmole) conjugate; (1) retention time of “free” (unlabeled) 111In; (2) retention time of 111In-labeled DOTA-MFCO-NPY; >98% radiochemical purity; and specific activity of 88 MBq nmole-1.
In summary, through this proof of concept study we have successfully conjugated a novel DOTA derivative to a neuropeptide Y analog using copper-free click chemistry. Subsequent radiolabeling of this assembly with 111In was achieved in high radiochemical purity, and the radiolabeled material displayed specific activity approaching 90 MBq nmole-1. While the peptide targeting agent employed in this study is under investigation for molecular imaging of childhood neuroblastoma, the approach described above should be compatible with the preparation of a wide range of peptide-DOTA bioconjugates. Indeed, it is envisioned that key intermediate 4 may serve as the prototype for a family of versatile reagents for the introduction of macrocyclic chelators to a variety of biomolecules via Cu-free click chemistry. The ready availability of cyclooctyne MFCO using a straightforward three-step synthesis should facilitate these efforts for this general approach. Optimization of the reaction conditions for the copper-free click chemical addition using the DOTA-MFCO and other −MFCO functionalized chelators for preparing peptide conjugates suitable for molecular imaging is a subject of continuing research in our laboratories.
Supplementary Material
Scheme 2.
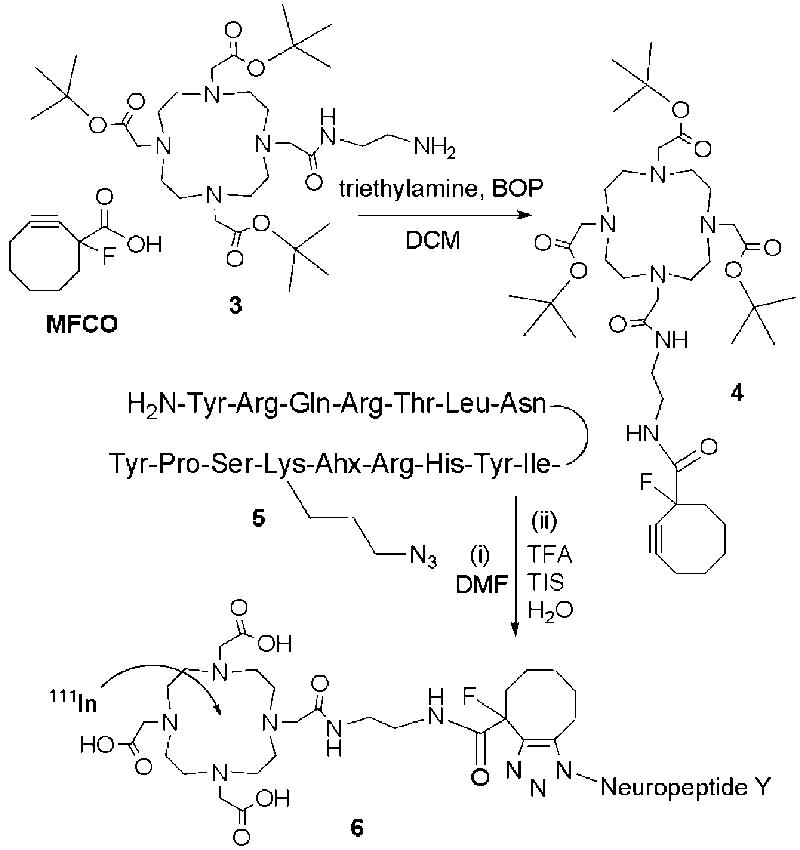
Synthesis of DOTA-MFCO (4); and DOTA-MFCO-NPY bioconjugate (6, 13% overall yield) and radiolabeling with 111In.
Acknowledgments
The authors acknowledge the support of the University of Iowa Holden Comprehensive Cancer Center (MSO, MKS, MEM) and the American Cancer Society (Young Investigator Seed Grant, MKS) for partial financial support of this work. FCP, SGP, and MKS thank the Roy J. Carver Charitable Trust. MEM is supported by the Iowa Cardiovascular Interdisciplinary Research Fellowship (HL07121). The authors kindly thank Lynn Teesch and Vic Parcell of the University of Iowa Department of Chemistry Mass Spectrometry Facility for spirited assistance with mass spectrometric analysis.
References
- 1.Anderson CJ, Wadas TJ, Wong EH, Weisman GR. Q J Nucl Med Mol Imaging. 2008;52:185. [PMC free article] [PubMed] [Google Scholar]
- 2.Boswell CA, Regino CA, Baidoo KE, Wong KJ, Bumb A, Xu H, Milenic DE, Kelley JA, Lai CC, Brechbiel MW. Bioconjug Chem. 2008;19:1476. doi: 10.1021/bc800039e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brechbiel MW. Q J Nucl Med Mol Imaging. 2008;52:166. [PMC free article] [PubMed] [Google Scholar]
- 4.De Leon-Rodriguez LM, Kovacs Z. Bioconjug Chem. 2008;19:391. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 5.Hausner SH, Kukis DL, Gagnon MK, Stanecki CE, Ferdani R, Marshall JF, Anderson CJ, Sutcliffe JL. Mol Imaging. 2009;8:111. [PMC free article] [PubMed] [Google Scholar]
- 6.Sletten EM, Bertozzi CR. Angew Chem Int Ed Engl. 2009;48:6974. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Sagheer AH, Brown T. Chem Soc Rev. 2010;39:1388. doi: 10.1039/b901971p. [DOI] [PubMed] [Google Scholar]
- 8.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed Engl. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Kolb HC, Sharpless KB. Drug Discov Today. 2003;8:1128. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 10.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed Engl. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Wangler C, Schirrmacher R, Bartenstein P, Wangler B. Curr Med Chem. 2010;17(11):1092. doi: 10.2174/092986710790820615. [DOI] [PubMed] [Google Scholar]
- 12.Knor S, Modlinger A, Poethko T, Schottelius M, Wester HJ, Kessler H. Chemistry. 2007;13:6082. doi: 10.1002/chem.200700231. [DOI] [PubMed] [Google Scholar]
- 13.Lebedev AY, Holland JP, Lewis JS. Chem Commun (Camb) 2010;46:1706. doi: 10.1039/b924784j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jewett JC, Bertozzi CR. Chem Soc Rev. 2010;39:1272. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc Natl Acad Sci U S A. 2007;104:16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Proc Natl Acad Sci U S A. 107:1821. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. J Am Chem Soc. 2008;130:11486. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JA, Baskin JM, Bertozzi CR, Koberstein JT, Turro NJ. Chem Commun (Camb) 2008:3064. doi: 10.1039/b803043j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz MK, Parameswarappa SG, Pigge FC. Org Lett. 2010 doi: 10.1021/ol100774p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwanziger D, Khan IU, Neundorf I, Sieger S, Lehmann L, Friebe M, Dinkelborg L, Beck-Sickinger AG. Bioconjug Chem. 2008;19:1430. doi: 10.1021/bc7004297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


