Intrahepatic inflammation and liver T cell dysfunction due to obstructive jaundice are both ameliorated through abrogation of PD-1 expression.
Keywords: Treg, obstructive jaundice, intrahepatic immunity
Abstract
Biliary obstruction is a common clinical problem that is associated with intrahepatic inflammation and impaired immunity. PD-1 is well known to mediate T cell dysfunction but has been reported to promote and attenuate acute inflammation in various injury models. With the use of a well-established murine model of BDL, we studied the effects of intrahepatic PD-1 expression on LTC function, inflammation, and cholestasis. Following BDL, PD-1 expression increased significantly among LTCs. Increased PD-1 expression following BDL was associated with decreased LTC proliferation and less IFN-γ production. Elimination of PD-1 expression resulted in significantly improved proliferative capacity among LTC following BDL, in addition to a more immunostimulatory cytokine profile. Not only was LTC function rescued in PD-1−/− mice, but also, the degrees of biliary cell injury, cholestasis, and inflammation were diminished significantly compared with WT animals following BDL. PD-1-mediated acute inflammation following BDL was associated with expansions of intrahepatic neutrophil and Th17 cell populations, with the latter dependent on IL-6. PD-1 blockade represents an attractive strategy for reversing intrahepatic immunosuppression while limiting inflammatory liver damage.
Introduction
Obstructive jaundice is an important cause of intrahepatic inflammation, arising as a consequence of numerous clinical disorders, including stone disease and pancreatobiliary malignancies. Jaundiced patients suffer from significant metabolic derangements and are at increased risk of complications following surgical intervention [1, 2]. The induction of inflammation within the liver can disrupt T cell homeostasis and limit the ability of the host to respond to infection or malignancy [3, 4]. Resident intrahepatic T cells play a unique role in maintaining the balance between tolerance and immunity in response to inflammatory insults [5, 6]. With the use of an established BDL model to study obstructive jaundice [7], we previously demonstrated suppression of LTCs as a result of an increase in the intrahepatic Treg population. Treg depletion restored LTC responsiveness to stimulation but worsened intrahepatic inflammation and cholestasis [8]. Herein, we investigated alternative mechanisms through which BDL affects liver inflammation and LTC function in an effort to restore intrahepatic immune function without exacerbating tissue damage.
Expression of PD-1 (CD279) on T cells has been associated with impaired proliferation and depressed cytokine production [9–12]. PD-1 has been shown to be up-regulated in multiple models of liver injury, including biliary cirrhosis, ischemia/reperfusion, and viral infection [13–15]. Overexpression of PD-1 in these models was associated with T cell exhaustion and loss or blockade of PD-1-restored T cell function [10, 15, 16]. Whereas generally linked to suppressing inflammatory responses [9, 17], PD-1 has also been shown to actually promote tissue injury in models of sepsis and airway inflammation [10, 18]. Importantly, PD-1 modulation of inflammatory responses occurs, in part, via regulation of T cell subsets that may contribute to the pathogenesis of obstructive jaundice [19]. We sought to test whether PD-1 inhibition may represent a viable approach for rescuing T cell function under immunosuppressive conditions without undue collateral inflammatory tissue damage.
IL-17-producing CD4 T cells, or Th17 cells, exist in a balance with immunosuppressive Tregs sharing a common, naïve CD4 T cell progenitor [20, 21]. Intrahepatic Th17 cells have been implicated recently as mediators of inflammatory liver injury [22]. The role of Th17 cells in the pathogenesis of obstructive jaundice has not been defined clearly. The present study was designed to determine the contributions of LTC PD-1 expression and the intrahepatic Treg:Th17 balance to the immunologic and inflammatory effects of BDL. Collectively, our results document previously unrecognized effects of PD-1 in promoting intrahepatic neutrophil and Th17 cell expansion and as a consequence, the inflammatory response to obstructive jaundice. PD-1 blockade may offer the potential for intrahepatic immunostimulation without unacceptable collateral tissue damage.
MATERIALS AND METHODS
Mice
Adult, 6- to 10-week-old male C57Bl/6 (B6, H-2Kb, WT), IL-6−/− (B6.129S2-Il6tm1Kopf/J), and BALB/c (H-2Kb) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). PD-1−/− mice were bred in-house (from Dr. Tasuku Honjo, Kyoto University, Kyoto, Japan, via A.A. at Rhode Island Hospital). All animals were maintained in a pathogen-free environment at Roger Williams Medical Center and received humane care, according to the criteria outlined by the U.S. NIH (NIH Publication 86–23, revised 1985). Procedures were approved by the Institutional Animal Care and Use Committee.
Surgical procedures
Mice were anesthetized with inhaled 2% isoflurane. After being shaved and sterilely prepped, an upper-midline laparotomy incision was made, and the common bile duct ligated with 6–0 silk suture (Ethicon, Somerville NJ, USA). The fascia was closed with 3–0 vicryl suture (Ethicon), followed by skin clips. SH animals underwent all preceding steps, except for the ligation of the bile duct. Animals that had jaundiced tissues, a distended biliary tree, and patchy liver discoloration were deemed to have successful BDL (>95% of our operations met these criteria).
Cell preparation
Liver NPCs were isolated as described previously with modifications [6, 8]. Briefly, following euthanization, the portal vein was injected with 3 ml 1% (w/v) collagenase IV (Sigma, St. Louis, MO, USA) in PBS (Cellgro, Manassas, VA, USA). Blood for serum chemistry analysis was taken via cardiac puncture. Livers were homogenized and then incubated for 20 min in 1% collagenase at 37°C. The cell suspension was passed through a 100-μm nylon mesh filter (Falcon; BD Biosciences, San Jose, CA, USA) and spun at 30 g for 5 min. The resulting NPCs were spun at 300 g for 7 min and red cells lysed and washed with RPMI (RPMI 1640; Cellgro). To enrich the NPC, OptiPrep (Sigma) was used. Splenocytes were suspended in RPMI after homogenization and filtration through a 70-μm filter (Falcon; BD Biosciences). Liver NPC or splenocytes were incubated with 1 μg/1×106 cells anti-FcγRIII/II mAb2.4G2 (AbD Serotec, Raleigh, NC, USA) and fractionated based on Thy1.2 (CD90.2), CD45, or CD11c expression using immunomagnetic beads (Miltenyi Biotec, Auburn, CA, USA). The following definitions were used: bulk T cells (Thy 1.2+), CD4 T cells (Thy1.2+CD4+CD8−), Tregs (Thy1.2+CD4+CD25+ for functional studies and Thy1.2+CD4+FoxP3+ for phenotype). PMNs were analyzed as CD11b+Gr1HICD11c− from Thy1.2−CD45+ NPCs.
Flow cytometry
Flow cytometry was performed on a CyAn (Beckman Coulter, Brea, CA, USA) machine. Samples were labeled with antibodies against CD3 (145-2C11), CD4 (RM45), CD25 (PC61), CD11b (M1/70), CD11c (HL3), and Gr1 (RB6-8C5), all from BD Biosciences. Anti-PD-1 (MIH5) and anti-PD-L1 (10F.9G2) were purchased from BioLegend (San Diego, CA, USA). For intracellular staining, T cells were stimulated with leukocyte-activating cocktail [PMA, ionomycin, BD GolgiPlug (brefeldin A)], fixed, and permeabilized as per the manufacturer's protocol before staining (BD Biosciences). Prior to intracellular staining of Thy1.2−CD45+ cells, stimulation was carried out overnight with 5 μg/200 μl CpG (ODN1826 tlr1-modn-1; InvivoGen, San Diego, CA, USA), and brefeldin A was added for the final 6 h. Intracellular staining was used to identify FoxP3 (MF23), IL-10 (JES5-16E3), IL-6 (MP5-20F3), RORγT (Q31-378), STAT3 (4/P-STAT3), and IL-17 (TC11-18H10), with all antibodies from BD Biosciences. Anti-TGF-β (TW7-16B4) was obtained from BioLegend. Data analysis was carried out using FlowJo software (Tree Star, Ashland, OR, USA). The University of Massachusetts, Worchester Medical Center Experimental Pathology Service Core, performed H&E staining of liver specimens.
T cell cultures and suppression assays
MLR was performed, as described previously, by culturing splenic DCs (CD11c+) from BALB/c mice with bulk T cells from WT or PD-1−/− mice [6, 8]. Bulk T cells were stained with CFSE (Invitrogen, Carlsbad, CA, USA) prior to culture. The DCs were added at a 1:2 ratio with bulk LTCs. In several experiments, anti-PD-1 (10 μg/ml) was added to the MLR (rat PD-1 mAb 1–14; BioLegend). Cell proliferation was measured by flow cytometry after 5–6 days. Supernatant was harvested from triplicate wells for cytokine measurement by CBA (BD Biosciences) or ELISA (eBioscience, San Diego CA, USA). For suppression assays, Tregs (CD4+CD25+) were isolated using FACS (MoFlo; Dako, Carpinteria, CA, USA). Tregs were added at a 1:10 ratio to responders in MLR assays. CD4+ T cell SHP-1 inhibition was performed with SSG (10 μg/ml; EMD Millipore, Billerica, MA, USA), following stimulation with anti-CD3 (145-2C11; 1 μg/ml) and anti-CD28 (37.51; 1 μg/ml; BioXcell, West Lebanon, NH, USA) in the presence of IL-6 (0.5 ng/ml) and TGF-β (5 μg/ml; PeproTech, Rocky Hill, NJ, USA).
Statistics
Statistical analyses were performed using a two-tailed t-test (Prism 5.00 for Windows; GraphPad Software, San Diego, CA, USA), and values are expressed ±sem, with P < 0.05 deemed statistically significant.
RESULTS
Increased expression of PD-1 among LTCs as a result of obstructive jaundice
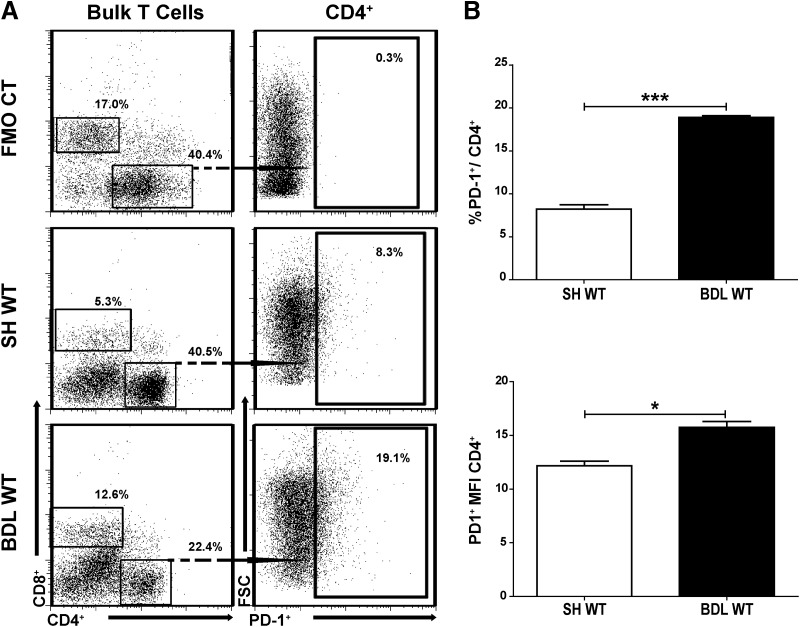
We reported previously that LTC function is suppressed following BDL [8]. As PD-1 activity has been associated with impaired T cell function, we analyzed changes in PD-1 expression following BDL [10, 11, 23, 24]. Cholestatic injury and the associated T cell responses are most prominent by the end of the 1st week after BDL [25], and we therefore studied LTC 7 days following BDL and stained for PD-1 (Fig. 1A). Our analysis of CD4+ T cells showed a twofold increase in the expression of PD-1 after BDL compared with SH (P=0.0005; Fig. 1B). CD8+ LTCs also up-regulated PD-1 significantly following BDL (43.6% vs. 0.9% on SH, P<0.001; data not shown). Given our previously published data [8] on the importance of CD4+ liver Tregs in mediating LTC dysfunction following BDL and the unique properties of liver CD4+ T cells [6], further experiments focused on CD4+ LTCs.
Figure 1. Increased expression of PD-1 among LTCs.
BDL was performed in WT C57Bl/6 mice, and 7 days later, LTCs were collected using immunomagnetic beads specific for Thy1.2. PD-1 (CD279) expression among CD4+ LTCs was measured by flow cytometry (A). PD-1 expression among CD4+ LTCs is shown (B). CT, Control; FSC, forward-scatter. Means ± sem are shown, based on triplicate samples, with each sample pooled from two to three livers. Data shown are representative of greater than three independent experiments, ***P ≤ 0.001; *P ≤ 0.05.
PD-1 mediates LTC dysfunction in jaundiced mice
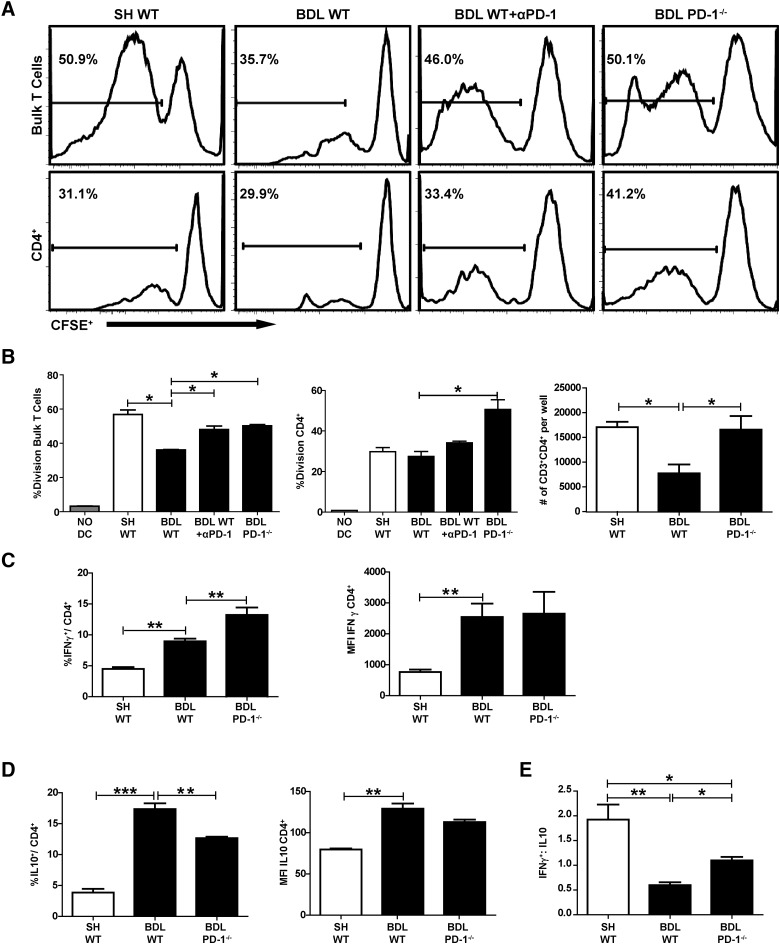
As reported previously [8], LTCs were less responsive to DC stimulation following BDL (Fig. 2A). Addition of anti-PD-1 restored LTC responsiveness to DCs after T cell isolation from jaundiced livers (Fig. 2A). This effect was even more pronounced with LTCs isolated from BDL PD-1−/− mice. Both bulk and CD4+ LTCs from the PD-1−/− mice proliferated more than WT after BDL (Fig. 2A). The improved responsiveness was also reflected by a twofold increase in the absolute number of CD4+ T cells from PD-1−/− following stimulation compared with WT mice following BDL (P=0.03; Fig. 2B). The limited responsiveness of the WT CD4+ T cell fraction in bulk T cell cultures from SH liver is consistent with our prior results [6]. SH PD-1−/− LTCs did not proliferate as robustly as SH WT LTCs (data not shown).
Figure 2. Loss of PD-1 signaling restores LTC proliferation.
LTCs were labeled with CFSE and stimulated with allogeneic CD11c+ splenocytes. Division of bulk and CD4+ LTC was assessed using flow cytometry to determine the degree of CFSE loss. Gates were drawn based on the division of unstimulated T cell controls (A). Absolute numbers of CD4+ T cells calculated from experimental wells at the end of the assay (B). LTCs, isolated 7 days following BDL, were stimulated overnight, and IFN-γ expression among CD4+ T cells was measured by intracellular staining and flow cytometry (C). Intracellular production of IL-10 was measured by the same method (D), and the ratio of IFN-γ:IL-10 expressing T cells was calculated (E). The histograms are single samples, each comprised of two to three pooled livers. The data in A–E are representative of a minimum of three independent experiments. αPD-1, Anti-PD-1; MFI, mean fluorescence intensity. Means ± sem derived from greater than or equal to three samples/experiment, *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
The predominance of a suppressive cytokine milieu has been associated with limited responsiveness of intrahepatic T cells [6]. As such, we measured expression of IFN-γ and IL-10 among CD4+ T cells. Loss of PD-1 expression led to a greater expansion of IFN-γ+CD4+ LTC compared with WT LTCs after BDL (13.3% vs. 9.7%, P=0.004; Fig. 2C). In contrast, an increase in IL-10-producing LTCs was limited when BDL was performed in PD-1−/− mice (12.7% vs. 17.4%, P=0.003; Fig. 2D). IL-10 expression among CD4+ LTCs was not significantly different when SH WT and SH PD-1−/− were compared (6.9% vs. 3.9%, P=0.07; data not shown). The IFN-γ:IL-10 ratio was significantly lower following BDL in WT mice (1.1 vs. 0.6, P=0.002; Fig. 2E).
PD-1 is not essential for liver Treg expansion and suppressive function following BDL
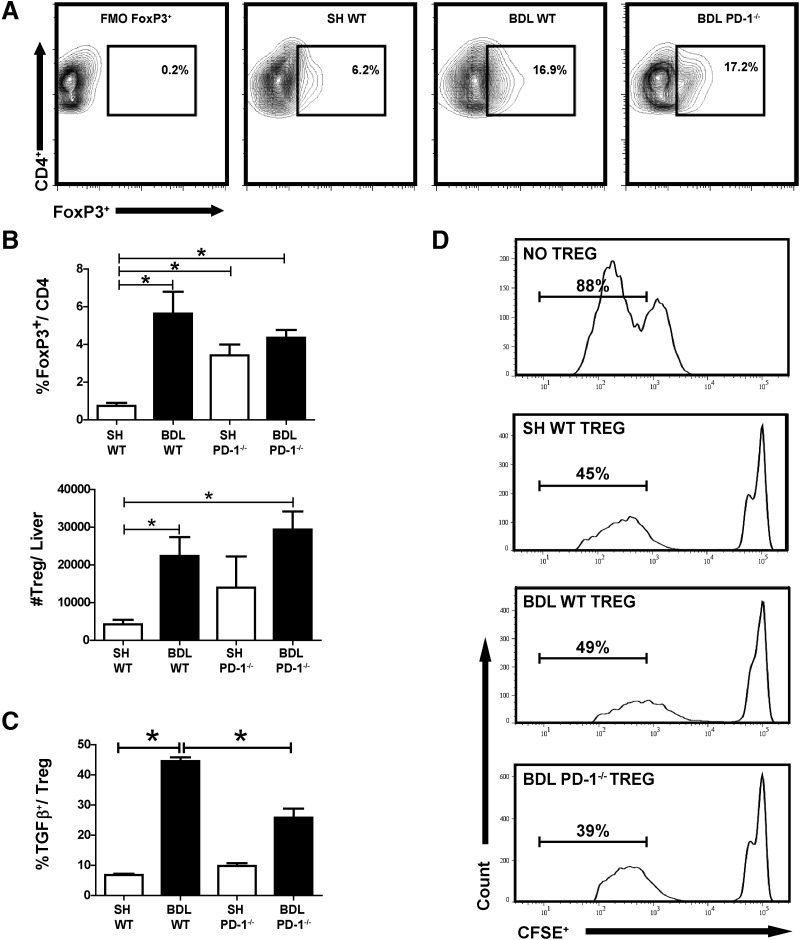
We speculated that the improved function of LTC following BDL in PD-1−/− mice might be accompanied by a lower frequency of intrahepatic Tregs. The expansion of liver Tregs as a result of BDL was unaffected by PD-1−/− (Fig. 3A and B). As reported previously [8], BDL in WT mice led to greater than a fourfold (P=0.005) expansion in liver Tregs, which was similar in PD-1−/− animals (P<0.0001). Given the importance of TGF-β for Treg function, we measured TGF-β expression among CD4+CD25+ LTCs. Following BDL, PD-1−/− CD4+CD25+ expressed lower levels of TGF-β compared with WT (2.9% vs. 5.8%, P=0.04; Fig. 3C). Despite decreased TGF-β production, we found that PD-1−/− Tregs retained their suppressive capacity following BDL. Tregs from WT and PD-1−/− livers limited T cell alloresponsiveness (Fig. 3D). Across all experimental groups, liver Tregs were found to express PD-L1 (10–20%; data not shown).
Figure 3. PD-1 is not essential for liver Treg expansion and suppressive function following BDL.
Treg populations (CD3+CD4+FoxP3+) in Thy1.2+ LTCs were measured by flow cytometry from WT and PD-1−/− animals (A). The absolute number of Tregs was calculated (B). Treg expression of TGF-β was measured by intracellular staining (C). FACS-sorted purified liver CD3+CD4+CD25+ cells from WT and PD-1−/− were added to MLR reactions consisting of WT CFSE-labeled splenic T cell responders and allogeneic CD11c+ stimulators. Proliferation was measured by flow cytometry on Day 6, based off of stimulated control wells that lacked suppressors. Data are representative of at least two separate repetitions with comparable results (D). The contour plot in A is a representative example compiled from two to three pooled livers. FoxP3 and TGF-β graphs were compiled from three separate, independent experiments with comparable results, with ≥10 samples/group, *P ≤ 0.05.
PD-1 up-regulation is associated with expansion of liver Th17 cells following BDL
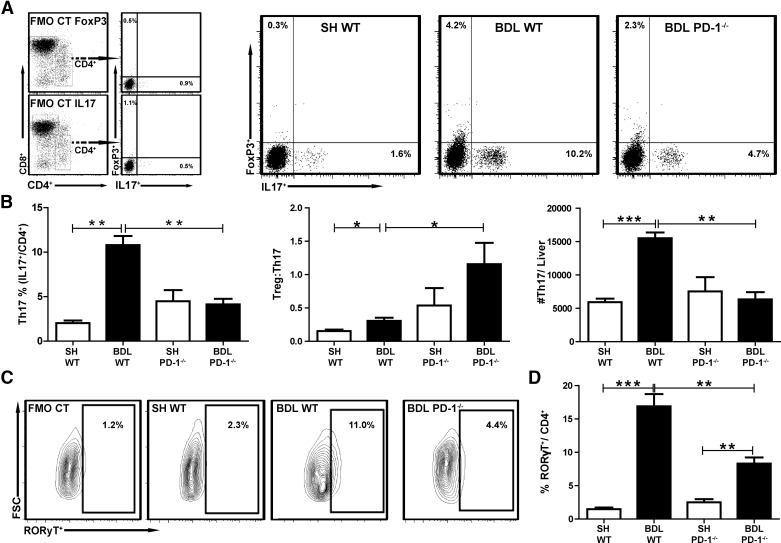
Having found that PD-1−/− did not alter intrahepatic Treg frequencies, we next examined how BDL impacted the proinflammatory Th17 population. After BDL, a significant expansion of CD4+IL-17+, LTC was noted in WT animals (10.2% vs. 1.6%, P=0.0002; Fig. 4A). In contrast, the CD4+IL-17+ population in BDL PD-1−/− mice was reduced by more than twofold compared with BDL WT (10.2% vs. 4.7%, P=0.0014; Fig. 4A). Liver Th17 cell frequencies were similar in SH WT and SH PD-1−/− groups (P=0.07; Fig. 4B). SH WT and PD-1−/− had comparable absolute numbers of Th17 cells (P=0.49; Fig. 4B). The ratio of Treg:Th17 after BDL was greater in the PD-1−/− mice than the WT (P=0.024; Fig. 4B). We also examined CD4+ LTC expression of RORγT, a transcription factor critical for Th17 development. As with IL-17 expression, RORγT+CD4+ LTC increased significantly in frequency following BDL in WT mice compared with SH (P<0.0001) and was diminished significantly in PD-1−/− animals (P=0.0005; Fig. 4C and D). To further examine the role of PD-1 signaling in promoting Th17 expansion following BDL, we targeted the SHP-1/SHP-2 phosphatases under Th17-polarizing conditions [26]. SSG, a SHP inhibitor, was added to naive CD4 T cells in culture with IL-6 and TGF-β, which led to a decrease in RORγT expression among CD4+ T cells from BDL livers (5.1% vs. 3.5%, P=0.02; not shown).
Figure 4. Loss of PD-1 signaling prevents expansion of liver Th17 cells following BDL.
Liver Thy1.2+ cells were isolated, and the Treg (CD3+CD4+FoxP3+IL-17−) or Th17 (CD3+CD4+FoxP3−IL-17+) populations were measured by flow cytometry. Gates were drawn based on FMO controls (A). Treg and Th17 cells were quantified following BDL in WT and PD-1−/− mice (B). The Treg:Th17 ratio was also calculated from percent Treg and Th17 of CD4+ T cells. Absolute counts of Th17 cells were calculated/experimental liver. Intracellular RORγT (RORyT+) production was also measured (C and D). All data are representative of at least three separate repetitions with comparable results. Bar graphs illustrate means ± sem, *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Intrahepatic PD-1 expression promotes biliary cell injury and cholestasis
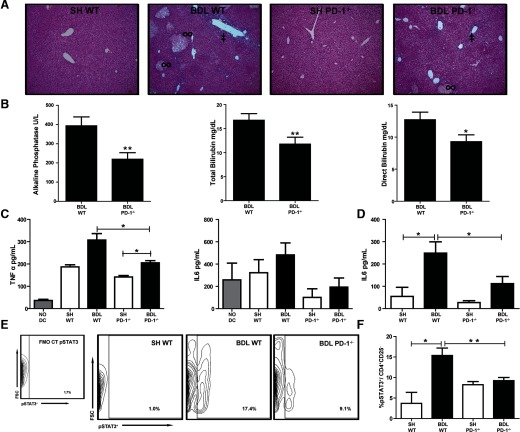
The diminished expansion of Th17 cells in PD-1−/− mice led us to speculate that biliary epithelial cell injury and cholestasis would also be less severe in the absence of PD-1 following BDL. The degrees of periportal inflammation, cellular infiltration, bile duct proliferation, and necrosis were less in the PD-1−/− mice compared with WT following BDL (Fig. 5A). A decrease in biliary epithelial cell damage among PD-1−/− mice following BDL was reflected by 44% lower alkaline phosphatase level compared with WT mice (P=0.004; Fig. 5B). Total and direct bilirubin concentrations were also significantly lower in PD-1−/− mice compared with WT (P=0.02, P=0.04, respectively; Fig. 5B). Alanine aminotransferase levels were not significantly different among groups at the 7-day time-point (data not shown) [25].
Figure 5. Loss of PD-1 limits hepatocyte and biliary cell injury.
Liver tissue, taken 7 days after SH or BDL operations, was processed by routine H&E staining and assessed for periportal inflammation (‡) and necrosis (∞) under 10× magnification (A). Blood was taken at time of killing to measure alkaline phosphatase and total and direct bilirubin (B). Bulk LTC cytokine production was measured by CBA from the supernatant of the allogeneic stimulation assays (C). Serum IL-6 levels were measured by ELISA 7 days after surgical procedures (D). pSTAT3 frequency was measured from CD4+CD25− T cells (E and F). Each data point in D represents serum pooled from at least two mice and an aggregate of three independent experiments with similar results. Serum liver tests are compiled from individual mice from a minimum of three separate experiments with comparable results (greater than or equal to eight mice/group), *P ≤ 0.05; **P ≤ 0.01.
We next attempted to correlate the above findings with inflammatory cytokine production. PD-1−/− LTC production of TNF-α was significantly less than that of WT mice following BDL (205.2 pg/ml, 307.7 pg/ml, P=0.03; Fig. 5C). IL-6 in the LTC supernatant did not decrease consistently but did demonstrate a trend toward a decrease in PD-1−/− mice (Fig. 5C). The serum collected from PD-1−/− mice following BDL compared with that of WT did show significantly lower levels of IL-6 (P=0.05; Fig. 5D).
Along with TGF-β, IL-6 is an important driver of Th17 expansion [27]. We speculated that the decreased levels of IL-6 observed in mice that lacked PD-1 limited the Th17 cell development. We then looked at pSTAT3, a known inducer of RORγT transcription and differentiation of uncommitted CD4+ Th0 cells into Th17 lymphocytes [28, 29]. Following BDL, we demonstrated a significant increase in pSTAT3 levels among CD4+CD25− T cells. In the absence of PD-1 signaling, the BDL-induced pSTAT3 increase was reduced twofold (Fig. 5E and F).
Intrahepatic Th17 cell accumulation and inflammation are driven by PD-1 and IL-6
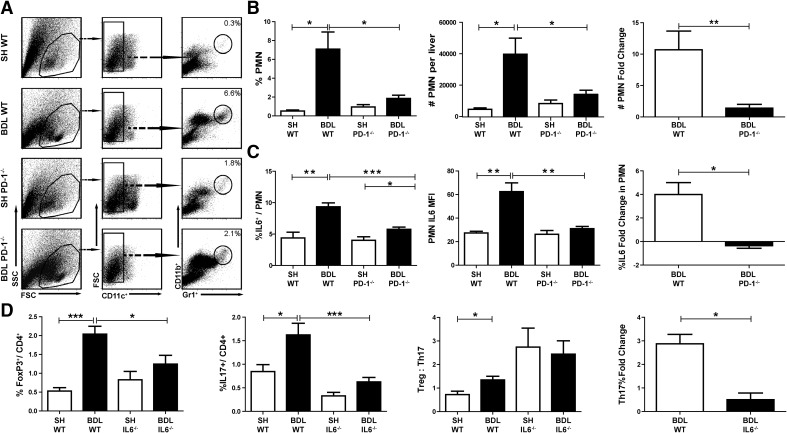
To extend our findings that intrahepatic inflammation is attenuated in PD-1−/− mice, we measured the extent of intrahepatic PMN expansion following BDL in WT and PD-1−/− mice. In WT mice, PMNs increased, on average, from 0.5% to 7.1% of CD45+ NPCs (P=0.02; Fig. 6A and B). Following BDL in mice lacking PD-1, neutrophils accounted for only 1.9% of liver leukocytes (P=0.02; Fig. 6A and B). In addition, neutrophils from PD-1−/− mice expressed 50% less IL-6 than WT following BDL (P=0.0003; Fig. 6C).
Figure 6. Intrahepatic Th17 cell accumulation and inflammation are driven by PD-1 and IL-6.
CD45+ liver NPCs were purified, and neutrophils (CD11b+Gr1HICD11c−) were quantified by flow cytometry (A). The percent and absolute numbers of neutrophils among liver leukocytes were measured. The fold change from SH livers was also calculated (B). The percentages of neutrophils expressing IL-6 and the IL-6 mean fluorescence intensity were determined by intracellular cytokine staining following stimulation with CpG. The fold change in neutrophil IL-6 production following BDL for each strain is shown (C). The effect of IL-6−/− on liver Treg and Th17 population dynamics following BDL was measured. The ratio of Treg:Th17 cells and the Th17 fold change in WT and IL-6−/− mice are shown (D). SSC, Side-scatter. The flow data are pooled from two to three livers/experimental sample, with three to six samples/group. Bar graphs are representative of a single representative experiment repeated at least three times, *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Having seen both diminished Th17 expansion with decreased serum levels of IL-6 following BDL in PD-1−/− mice, we speculated that Th17 accumulation in jaundiced livers was dependent on IL-6. To test this hypothesis, we measured the frequency of liver Th17 and Tregs in IL-6−/− mice after BDL. In contrast to the two- to threefold Th17 expansion in WT mice following BDL, the intrahepatic Th17 population did not expand in IL-6−/− mice (Fig. 6D).
DISCUSSION
Our current study demonstrates that increased PD-1 expression is a maladaptive response to obstructive jaundice, contributing to inflammation and T cell dysfunction. The expansion of liver neutrophil and Th17 cell populations following BDL was partially dependent on PD-1 expression, as demonstrated by significantly diminished liver neutrophil and Th17 responses to cholestasis in PD-1−/− animals. At the same time, PD-1 up-regulation among LTCs, suppressed responsiveness to DC stimulation and reduced IFN-γ production. Elimination of PD-1 signaling restored LTC function, while concomitantly leading to less biliary inflammatory injury and reduced cholestasis. Taken together, PD-1 blockade in the setting of obstructive jaundice has the potential to reverse LTC dysfunction and also minimize intrahepatic inflammation and tissue injury.
The restoration of LTC proliferative capacity following PD-1 elimination is consistent with the well-described role of PD-1 as a negative regulator of T cell function [9, 11, 30]. The higher levels of PD-1 expression among LTCs coincided with a decrease in their ability to proliferate in response to stimulation by DCs. LTC dysfunction following BDL was dependent on PD-1, as LTC responsiveness to DCs was restored in PD-1−/− mice. As reported previously, LTC suppression following BDL may also be mediated by other factors, such as expansion of suppressive cell types, including Tregs [8]. Our data demonstrate that BDL resulted in a decreased IFN-γ:IL-10 ratio and increased IL-10 production among CD4+ LTCs, suggesting that a perturbed cytokine milieu may have also contributed to LTC dysfunction in the setting of obstructive jaundice. The increased IL-10 production by LTC in association with PD-1 activation is in accord with a report by Dong et al. [31]. Whereas it is intriguing to speculate that the lower levels of IL-10 production in PD-1−/− mice contributed to the improvement in LTC function, further work is needed to definitively establish this relationship. In addition, an increase in CD4+ LTC IFN-γ production has been associated with decreased neutrophil burden, as demonstrated in our model [32].
The expansion of liver Th17 cells in response to BDL has been documented in a recent report by Meng et al. [33]. In their report, Meng and colleagues [33] demonstrated that intrahepatic IL-17 was responsible for increased levels of other inflammatory cytokines, including IL-6, TNF-α, and TGF-β. Similarly, we found increased levels of IL-6, TNF-α, and TGF-β in association with Th17 cell expansion following BDL. Loss of PD-1 resulted in a significantly diminished Th17 expansion following BDL, which was confirmed by measuring RORγT expression among LTCs. The decreased Th17 expansion in PD-1−/− mice resulted in an increased Treg:Th17 ratio in jaundiced mice, which was associated with lower levels of inflammation, biliary cell injury, cholestasis, and neutrophil infiltration. A significant attenuation of pSTAT3 levels accompanied the decrease in Th17 expansion following BDL in the absence of PD-1 signaling. This finding led us to speculate that increased levels of IL-6 following BDL may be driving Th17 expansion in a STAT3-dependent manner. In vitro inhibition of SHP-1/SHP-2 phosphatase, which is critical for PD-1 signaling, also mediated a decrease in RORγT expression by CD4+ T cells under Th17-polarizing conditions. Further experiments are needed to determine whether PD-1 and SHP-1/SHP-2 directly drive RORγT expression following BDL or whether Th17 expansion is driven by PD-1 through IL-6 signaling.
An increase in neutrophils following BDL has also been reported by Heymann et al. [34], who illustrated that decreased levels of CD11b+Gr1HI cells led to a significant reduction in intrahepatic inflammation and injury. Neutrophils have been implicated as important mediators of acute cholestatic liver injury in a CCR8-dependent manner [32]. Gujral and colleagues [32] demonstrated that CCR8 knockout reduced intrahepatic neutrophils following BDL, and our work also implicates PD-1 in the acute cellular inflammatory response to BDL. Our results do not allow us to determine if PD-1 directly influenced the accumulation of liver neutrophils or if PD-1 acted via specific chemokine axes or by affecting IL-17 production. Based on the reduced levels of liver Th17 cells following BDL in IL-6−/− mice, we speculate that IL-6 derived from expanded myeloid cells, including neutrophils, contributed to the increased levels of Th17 in jaundiced mice. DCs were also found to be a significant source of IL-6 in our model (not shown), as reported previously [7]. The increased TGF-β production by liver Tregs in our model may have worked in concert with heightened levels of IL-6 from neutrophils to promote Th17 conversion. We also recognize that immunologic dialogue between Th17 cells and neutrophils is likely to be bidirectional. Whereas we posit that IL-6 from expanded myeloid cells promoted Th17 cell accumulation in the liver, IL-17 may, in turn, drive neutrophil expansion following BDL.
The improved proliferative response among bulk LTCs from PD-1−/− mice following BDL was surprising, given that liver Treg expansion occurred at a level comparable with WT animals. We demonstrated that liver Tregs from WT and PD-1−/− mice expressed PD-L1, and this may be an important contributor to their suppressive function. Absence of cognate PD-1 on LTCs from PD-1−/− mice used in the bulk T cell stimulation assays may have diminished the suppressive influence of PD-L1+ Tregs. In our in vitro suppression assay, we used PD-1+ responder cells from WT spleens, which may have allowed Tregs from PD-1−/− mice to exert a suppressive effect not seen in the proliferation assays. Thus, we conclude that following BDL, the liver Treg frequency is similar to that seen in WT mice, but their suppressive impact is limited, as a result of lack of PD-1 expression on other T cell populations. We did note a decreased Treg expansion in PD-1−/− livers following BDL, but the final liver Treg populations were comparable in WT and PD-1−/−, as a result of higher baseline levels of liver Tregs in PD-1−/− animals. The suppressive influence of other PD-L1+ cells that we did not examine, including myeloid suppressor cells [35, 36], hepatocytes [37], and endothelial cells [38], may have been negated in PD-1−/− mice as well.
Our data resonate well with what has been reported in a model of acute lung injury, in that PD-1−/− mice were shown to experience less-severe inflammatory damage [39]. A previous report indicated that inflammation and tissue damage were decreased in PD-1−/− mice in a model of sepsis [10]. In the setting of viral hepatitis, the extent of inflammation within the liver was correlated with increased levels of PD-1 expression [40, 41]. Inhibition of PD-1 signaling appears to limit inflammatory tissue damage in multiple murine models, making it an attractive target for rescuing T cell function. It is important to note that other groups [42–44] have reported that in other models, the presence of PD-1 limited inflammation by preventing Th17 cell expansion, in contrast to our findings. The discrepant findings are likely a result of biologic differences between models, particularly with respect to the predominant cell types driving the inflammatory processes. In CD8+ T cell -driven inflammation models, PD-1 blockade worsened the extent of tissue injury [45, 46]. In contrast, in the setting of asthma, which is a Th2-driven process, PD-1 blockade was protective in terms of the degree of inflammation [18]. This is consistent with our data, in that PD-1 was up-regulated among CD4+ LTCs, and PD-1 blockade resulted in less inflammation and cholestasis following BDL. PD-1 up-regulation has also been reported among CD11b+ and Gr1+ cells in another model of inflammation [39]. As PD-1 has been shown to mediate apoptosis [47, 48], we speculate that the absence of PD-1 following BDL may promote survival of immunomodulatory cell types, such as myeloid-derived suppressor cells, that limit inflammation. Inhibition of PD-L1, which was expressed by liver Tregs in our model (not shown), was not tested and may represent another strategy for modulating intrahepatic immune function and inflammation.
Several limitations of our study are important to consider. Other immune cells in the liver, including NKT cells [6], DCs, Kupffer cells, and liver sinusoidal endothelial cells [49], may be modulating LTC function and inflammation following BDL. In addition, NK cells have been demonstrated to be important mediators of BDL-induced liver damage [50]. Whereas our data support the importance of PD-1 signaling, IL-6, and TGF-β for Th17 expansion, we did not measure IL-21 or IL-23, both of which have been reported to support liver Th17 development [51]. We also did not measure PD-1 expression on Th17 cells directly, which may have impacted their expansion following BDL. Finally, whereas we suspect that the expansion of the Treg and Th17 populations is, in part, a result of intrahepatic conversion [8], we have not excluded the possibility that there is a migration into the liver from other sites.
Inhibition of PD-1 signaling is of significant clinical interest and has potentially broad therapeutic applications. Blockade of PD-1 and its coreceptor PD-L1 has been used to restore T cell function [9, 17, 52], and blocking PD-1 decreases T cell exhaustion, allowing for more effective pathogen clearance and better healing [14, 17]. Taken together, our data indicate that the immunosuppressive and inflammatory effects of obstructive jaundice are regulated by intrahepatic Tregs and Th17 cells, in association with PD-1. We found that inhibition of PD-1 signaling is an approach for restoring LTC function without promoting tissue damage or increased levels of inflammation. Manipulation of Treg:Th17 homeostasis through PD-1 inhibition represents a therapeutic strategy for modulating intrahepatic T cell function and inflammation.
ACKNOWLEDGMENTS
Support for this work was provided by the National Center for Research Resources (5P20RR018757-10) and the National Institute of General Medical Sciences (8 P20 GM103414-10) from the U.S. National Institutes of Health.
We thank Dr. Tasuku Honjo (Kyoto University, Kyoto, Japan) for the generous provision of mice via our collaborator's (A.A.) facility at Rhode Island Hospital.
Footnotes
- −/−
- deficient
- BDL
- bile duct ligation
- FMO
- fluorescence minus one
- FoxP3
- forkhead box P3
- LTC
- liver T cell
- MLR
- mixed leukocyte reaction
- NPC
- nonparenchymal cells
- PD-1
- programmed death-1
- PD-L1
- programmed death ligand 1
- PMN
- polymorphonuclear neutrophil (CD11b+Gr1HI)
- pSTAT
- STAT phosphorylation
- ROR
- retinoic acid receptor-related orphan receptor
- SH
- sham
- SHP
- Src homology-2-containing tyrosine phosphatase
- SSG
- sodium stibogluconate
- Treg
- regulatory T cell
- WT
- wild type
AUTHORSHIP
L.A.L. coordinated the study design and execution, analyzed data, created figures, and wrote the manuscript. C.T.N. and R.A.B. performed animal surgery and tissue processing and assisted in data accumulation and figure production. V.F. assisted with study design and critically reviewed the manuscript and figures. N.J.E. and R.P.J. assisted with study design, execution, and the writing and editing of the manuscript and figures. A.A. provided PD-1−/− mice and assisted with study design, execution, and the writing and editing of the manuscript and figures. M.T. coordinated study design, data analysis, and figure production and assisted with the writing and editing of the manuscript and figures. S.C.K. conceived of the study and coordinated its design and execution, analyzed data, created figures, and wrote the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Grande L., Garcia-Valdecasas J. C., Fuster J., Visa J., Pera C. (1990) Obstructive jaundice and wound healing. Br. J. Surg. 77, 440–442 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong C. P., Dixon J. M., Duffy S. W., Elton R. A., Davies G. C. (1984) Wound healing in obstructive jaundice. Br. J. Surg. 71, 267–270 [DOI] [PubMed] [Google Scholar]
- 3. Kahraman A., Barreyro F. J., Bronk S. F., Werneburg N. W., Mott J. L., Akazawa Y., Masuoka H. C., Howe C. L., Gores G. J. (2008) TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology 47, 1317–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wintermeyer P., Cheng C. W., Gehring S., Hoffman B. L., Holub M., Brossay L., Gregory S. H. (2009) Invariant natural killer T cells suppress the neutrophil inflammatory response in a mouse model of cholestatic liver damage. Gastroenterology 136, 1048–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crispe I. N. (2009) The liver as a lymphoid organ. Ann. Rev. Immunol. 27, 147–163 [DOI] [PubMed] [Google Scholar]
- 6. Katz S. C., Pillarisetty V. G., Bleier J. I., Kingham T. P., Chaudhry U. I., Shah A. B., DeMatteo R. P. (2005) Conventional liver CD4 T cells are functionally distinct and suppressed by environmental factors. Hepatology 42, 293–300 [DOI] [PubMed] [Google Scholar]
- 7. Bleier J. I., Katz S. C., Chaudhry U. I., Pillarisetty V. G., Kingham T. P., 3rd, Shah A. B., Raab J. R., DeMatteo R. P. (2006) Biliary obstruction selectively expands and activates liver myeloid dendritic cells. J. Immunol. 176, 7189–7195 [DOI] [PubMed] [Google Scholar]
- 8. Katz S. C., Ryan K., Ahmed N., Plitas G., Chaudhry U. I., Kingham T. P., Naheed S., Nguyen C., Somasundar P., Espat N. J., Junghans R. P., Dematteo R. P. (2011) Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J. Immunol. 187, 1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Souza M., Fontenot A. P., Mack D. G., Lozupone C., Dillon S., Meditz A., Wilson C. C., Connick E., Palmer B. E. (2007) Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J. Immunol. 179, 1979–1987 [DOI] [PubMed] [Google Scholar]
- 10. Huang X., Venet F., Wang Y. L., Lepape A., Yuan Z., Chen Y., Swan R., Kherouf H., Monneret G., Chung C. S., Ayala A. (2009) PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. USA 106, 6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharpe A. H., Wherry E. J., Ahmed R., Freeman G. J. (2007) The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 8, 239–245 [DOI] [PubMed] [Google Scholar]
- 12. Carambia A., Herkel J. (2010) CD4 T cells in hepatic immune tolerance. J. Autoimmun. 34, 23–28 [DOI] [PubMed] [Google Scholar]
- 13. Ji H., Shen X., Gao F., Ke B., Freitas M. C., Uchida Y., Busuttil R. W., Zhai Y., Kupiec-Weglinski J. W. (2010) Programmed death-1/B7-H1 negative costimulation protects mouse liver against ischemia and reperfusion injury. Hepatology 52, 1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franceschini D., Paroli M., Francavilla V., Videtta M., Morrone S., Labbadia G., Cerino A., Mondelli M. U., Barnaba V. (2009) PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J. Clin. Invest. 119, 551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Francisco L. M., Salinas V. H., Brown K. E., Vanguri V. K., Freeman G. J., Kuchroo V. K., Sharpe A. H. (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown K. E., Freeman G. J., Wherry E. J., Sharpe A. H. (2010) Role of PD-1 in regulating acute infections. Curr. Opin. Immunol. 22, 397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watanabe T., Bertoletti A., Tanoto T. A. (2010) PD-1/PD-L1 pathway and T-cell exhaustion in chronic hepatitis virus infection. J. Viral Hepatitis 17, 453–458 [DOI] [PubMed] [Google Scholar]
- 18. Singh A. K., Stock P., Akbari O. (2011) Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy 66, 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan C., Ramaswamy M., Shi G., Vistica B. P., Siegel R. M., Gery I. (2011) Inflammation-inducing Th1 and Th17 cells differ in their expression patterns of apoptosis-related molecules. Cell. Immunol. 271, 210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weaver C. T., Harrington L. E., Mangan P. R., Gavrieli M., Murphy K. M. (2006) Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24, 677–688 [DOI] [PubMed] [Google Scholar]
- 21. Chaudhry A., Rudra D., Treuting P., Samstein R. M., Liang Y., Kas A., Rudensky A. Y. (2009) CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326, 986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lan R. Y., Salunga T. L., Tsuneyama K., Lian Z. X., Yang G. X., Hsu W., Moritoki Y., Ansari A. A., Kemper C., Price J., Atkinson J. P., Coppel R. L., Gershwin M. E. (2009) Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J. Autoimmun. 32, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riley J. L. (2009) PD-1 signaling in primary T cells. Immunol. Rev. 229, 114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamazaki T., Akiba H., Koyanagi A., Azuma M., Yagita H., Okumura K. (2005) Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-γ-induced nitric oxide production. J. Immunol. 175, 1586–1592 [DOI] [PubMed] [Google Scholar]
- 25. Georgiev P., Jochum W., Heinrich S., Jang J. H., Nocito A., Dahm F., Clavien P. A. (2008) Characterization of time-related changes after experimental bile duct ligation. Br. J. Surg. 95, 646–656 [DOI] [PubMed] [Google Scholar]
- 26. Chemnitz J. M., Parry R. V., Nichols K. E., June C. H., Riley J. L. (2004) SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 173, 945–954 [DOI] [PubMed] [Google Scholar]
- 27. Afzali B., Mitchell P., Lechler R. I., John S., Lombardi G. (2010) Translational mini-review series on Th17 cells: induction of interleukin-17 production by regulatory T cells. Clin. Exp. Immunol. 159, 120–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kimura A., Naka T., Kishimoto T. (2007) IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc. Natl. Acad. Sci. USA 104, 12099–12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mauldin I. S., Tung K. S., Lorenz U. M. (2012) The tyrosine phosphatase SHP-1 dampens murine Th17 development. Blood 119, 4419–4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Penna A., Pilli M., Zerbini A., Orlandini A., Mezzadri S., Sacchelli L., Missale G., Ferrari C. (2007) Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 45, 588–601 [DOI] [PubMed] [Google Scholar]
- 31. Dong H., Zhu G., Tamada K., Chen L. (1999) B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5, 1365–1369 [DOI] [PubMed] [Google Scholar]
- 32. Gujral J. S., Farhood A., Bajt M. L., Jaeschke H. (2003) Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology 38, 355–363 [DOI] [PubMed] [Google Scholar]
- 33. Meng F., Wang K., Aoyama T., Grivennikov S. I., Paik Y., Scholten D., Cong M., Iwaisako K., Liu X., Zhang M., Osterreicher C. H., Stickel F., Ley K., Brenner D. A., Kisseleva T. (2012) Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143, 765–776.e1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heymann F., Hammerich L., Storch D., Bartneck M., Huss S., Russeler V., Gassler N., Lira S. A., Luedde T., Trautwein C., Tacke F. (2012) Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology 55, 898–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cripps J. G., Wang J., Maria A., Blumenthal I., Gorham J. D. (2010) Type 1 T helper cells induce the accumulation of myeloid-derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology 52, 1350–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim Y. J., Park S. J., Broxmeyer H. E. (2011) Phagocytosis, a potential mechanism for myeloid-derived suppressor cell regulation of CD8+ T cell function mediated through programmed cell death-1 and programmed cell death-1 ligand interaction. J. Immunol. 187, 2291–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wahl C., Bochtler P., Chen L., Schirmbeck R., Reimann J. (2008) B7-H1 on hepatocytes facilitates priming of specific CD8 T cells but limits the specific recall of primed responses. Gastroenterology 135, 980–988 [DOI] [PubMed] [Google Scholar]
- 38. Eppihimer M. J., Gunn J., Freeman G. J., Greenfield E. A., Chernova T., Erickson J., Leonard J. P. (2002) Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 9, 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monaghan S. F., Thakkar R. K., Heffernan D. S., Huang X., Chung C. S., Lomas-Neira J., Cioffi W. G., Ayala A. (2012) Mechanisms of indirect acute lung injury: a novel role for the coinhibitory receptor, programmed death-1. Ann. Surg. 255, 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen J., Wang X. M., Wu X. J., Wang Y., Zhao H., Shen B., Wang G. Q. (2011) Intrahepatic levels of PD-1/PD-L correlate with liver inflammation in chronic hepatitis B. Inflamm. Res. 60, 47–53 [DOI] [PubMed] [Google Scholar]
- 41. Xie Z., Chen Y., Zhao S., Yang Z., Yao X., Guo S., Yang C., Fei L., Zeng X., Ni B., Wu Y. (2009) Intrahepatic PD-1/PD-L1 up-regulation closely correlates with inflammation and virus replication in patients with chronic HBV infection. Immunol. Invest. 38, 624–638 [DOI] [PubMed] [Google Scholar]
- 42. Lazar-Molnar E., Chen B., Sweeney K. A., Wang E. J., Liu W., Lin J., Porcelli S. A., Almo S. C., Nathenson S. G., Jacobs W. R., Jr (2010) Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc. Natl. Acad. Sci. USA 107, 13402–13407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luz-Crawford P., Noel D., Fernandez X., Khoury M., Figueroa F., Carrion F., Jorgensen C., Djouad F. (2012) Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PloS One 7, e45272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dulos J., Carven G. J., van Boxtel S. J., Evers S., Driessen-Engels L. J., Hobo W., Gorecka M. A., de Haan A. F., Mulders P., Punt C. J., Jacobs J. F., Schalken J. A., Oosterwijk E., van Eenennaam H., Boots A. M. (2012) PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J. Immunother. 35, 169–178 [DOI] [PubMed] [Google Scholar]
- 45. Tarrio M. L., Grabie N., Bu D. X., Sharpe A. H., Lichtman A. H. (2012) PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J. Immunol. 188, 4876–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kroner A., Schwab N., Ip C. W., Leder C., Nave K. A., Maurer M., Wiendl H., Martini R. (2009) PD-1 regulates neural damage in oligodendroglia-induced inflammation. PloS One 4, e4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dong H., Strome S. E., Salomao D. R., Tamura H., Hirano F., Flies D. B., Roche P. C., Lu J., Zhu G., Tamada K., Lennon V. A., Celis E., Chen L. (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 [DOI] [PubMed] [Google Scholar]
- 48. Wu Y. Y., Lin C. W., Cheng K. S., Lin C., Wang Y. M., Lin I. T., Chou Y. H., Hsu P. N. (2010) Increased programmed death-ligand-1 expression in human gastric epithelial cells in Helicobacter pylori infection. Clin. Exp. Immunol. 161, 551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Katz S. C., Pillarisetty V. G., Bleier J. I., Shah A. B., DeMatteo R. P. (2004) Liver sinusoidal endothelial cells are insufficient to activate T cells. J. Immunol. 173, 230–235 [DOI] [PubMed] [Google Scholar]
- 50. Cheng C. W., Duwaerts C. C., Rooijen N., Wintermeyer P., Mott S., Gregory S. H. (2011) NK cells suppress experimental cholestatic liver injury by an interleukin-6-mediated, Kupffer cell-dependent mechanism. J. Hepatol. 54, 746–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao L., Qiu de K., Ma X. (2010) Th17 cells: the emerging reciprocal partner of regulatory T cells in the liver. J. Digestive Dis. 11, 126–133 [DOI] [PubMed] [Google Scholar]
- 52. Amarnath S., Costanzo C. M., Mariotti J., Ullman J. L., Telford W. G., Kapoor V., Riley J. L., Levine B. L., June C. H., Fong T., Warner N. L., Fowler D. H. (2010) Regulatory T cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS Biol. 8, e1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]