Abstract
Macrolepiota procera, one of edible mushrooms belongs to Agaricaceae of Basidiomycota, has a good taste and good medicinal value. As a preliminary study for the development of artificial cultivation method of edible mushroom, cultural characteristics of M. procera was investigated on various culture media under different environmental conditions. Mycelial growth was compared on culture media composed of various carbon and nitrogen sources, and C/N ratios. The optimal conditions for the mycelial growth were 30℃ and pH 7. M. procera showed the rapid mycelial growth in the PDA media. The optimal carbon and nitrogen sources were maltose and glycine, respectively. The optimum C/N ratio was about 10 : 1 in case that 1% glucose was supplemented to the basal media as carbon source.
Keywords: Cultural conditions, Edible mushroom, Macrolepiota procera, Mycelial growth
Macrorepiota procera (Scop. ex Fr.) Sing., an edible mushroom belongs to Agaricaceae of Agaricales, has been known to be distributed in the northern areas of Asia such as Korea, China and Japan (Park and Lee, 1996). The pileus is 7~20 cm in diameter, oval or spherical in the early stage of its development and gradually opens to a umbonate plane when it matures. The surface color of the pileus ranges from light grayish brown to grayish brown, and becomes reddish-brown and rough scales appears when its epithelial tissues are cleaved. The lamella is white in color and free from the stipe. The stipe is 15~30 × 0.6~1.5 µm in size and empty in its pith. The surface of stipe ranges from brown to grayish brown in its color and is similar to skin of snake in its morphology when its epithelial tissues are cleaved. The spore is oval or ellipsoidal, 15~20 × 10~13 µm in size, and spore print is white. For the duration of summer to fall, M. procera occurs as a fairy ring of a gregarious style on soil surface of the field with rich organic matter such as roadside, pasture, lawn and flower garden. M. procera has been known to contain glycerol, mannitol, glucose, trehalose, lepiotan and about 20 amino acids. Since M. procera has been reported to demonstrate anti-tumor activity to human body and exhibit an antibiotic activity against gram negative bacteria, the fruiting bodies have been extensively used for manufacturing traditional foods and medicines. When M. procera is used for edible purposes, the fruiting body of M. procera which contains the proteins, iron, zinc, chitin, chitosan, fiber, vitamins and minerals have been known to support the health and sustain physiological homeostasis of human body. Therefore, in order to obtain basic data for an artificial cultivation of M. procera, the culture conditions affecting the optimal mycelial growth of M. procera were investigated.
Materials and Methods
The collection and isolation of M. procera
The fruiting body of M. procera was collected at Yungkeonreung, Hwaseong city, Korea in August, 2001. To obtain the pure culture from M. procera, surface sterilized small pieces of fruiting body was transferred to potato dextrose agar (PDA) supplemented with streptomycin (200µg/l), incubated for 15 days at 25℃ and used for an inoculum in the study. The pure culture of M. procera was deposited to the "Culture Collection of Wild Mushroom Species" and acquired accession number, "IUM00103". Unless otherwise stated, all the tests which the strain was used wee performed with 4 replications.
Culture conditions for mycelial growth of M. procera
Effect of pH
A 5 mm diameter plug of an inoculum was removed with cork borer from 10 days old cultures of M. procera grown on PDA, placed in the center of each agar plate of PDA adjusted to the range of pH 4~9 with 1 N NaOH or HCl and incubated for 10 days at 25℃. The measurement of mycelial growth was performed according to the method described by Shim et al. (1997).
Effect of the temperature
To investigated the temperature favorable for the mycelial growth of M. procera, the fungus was incubated for 10 days at 5 different temperature. A 5 mm diameter plug removed from 10 days old cultures of M. procera grown on PDA was placed in the center of each plate filled with PDA. The PDA was adjusted to pH 6 and incubated for 10 days at 15℃, 20℃, 25℃, 30℃ and 35℃, respectively. The measurement of mycelial growth was also performed according to the method described by Shim et al. (1997).
Screening of favorable culture media
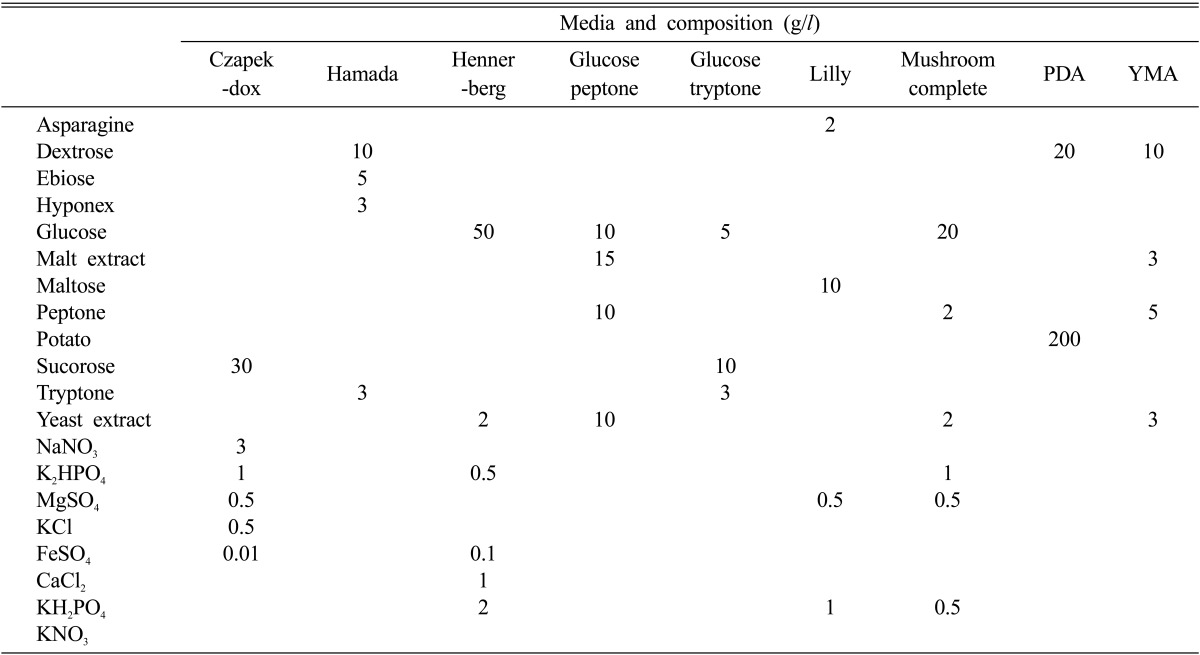
Nine different culture media were prepared to investigate favorable culture media to mycelial growth of M. procera (Table 1). The media were adjusted to pH 6 before autoclave. After autoclave for 15 minutes at 121℃, 20 ml of each medium was aseptically poured into a plate. A 5 mm diameter plug of an inoculum was removed from 10 days old culture of M. procera grown on PDA and placed in the center of each agar plate of 9 different culture media. After 10 days of incubation at 25℃, the mycelial growth and density of M. procera were measured.
Table 1.
Composition of the media used for the growth of Macrolepiota procera
Effect of carbon and nitrogen sources
To screen carbon and nitrogen sources favorable to the mycelial growth of M. procera, the basal medium (Sung et al., 1993) supplemented with each of 10 carbon and 17 nitrogen sources was prepared. The basal medium was composed of MgSO7H2O 0.05 g, KH2PO 0.46 g, K2HPO 1.0 g, Thiamine-HCl 120 µg, agar 20 g and distilled water 1,000 ml. To screen carbon source favorable to the mycelial growth of M. procera, each carbon source was added to the basal medium at the concentration of 0.1M per 1,000 ml and mixed throughly (Shim et al., 1997). The basal medium was adjusted to pH 6 before autoclave for 15 minutes at 121℃ and aseptically poured into a plate. D-glucose was added to the basal medium at the concentration of 2% (w/v) and used as carbon source for expediting the mycelial growth of M. procera. The basal medium which was used for screening a favorable nitrogen source was made of the same additives as those described by Sung et al. (1993). Each nitrogen source was added to the basal medium at the concentration of 0.02 M (Shim et al., 1997). The basal medium was also adjusted to pH 6 before autoclave for 15 minutes at 121℃ and aseptically poured into a plate. To measure colony diameter on the media, M. procera was incubated for 10 days at 25℃. Most of the procedures including the inoculation, incubation and measurement of a mycelial density were carried out according to the method described by Shim et al. (1997).
Effect of C/N ratio
To expedite the mycelial growth of M. procera, D-glucose and NaNOhave been added to the basal medium. The basal media which D-glucose was mixed at the rate of 1, 2, 3 and 4% (w/v) were continually added with NaNO3. Finally, the C/N ratio(D-glucose versus NaNO3) were adjusted to 10 : 1, 20 : 1, 30 : 1 and 40 : 1 in each medium. The basal medium was also adjusted to pH 6 before autoclave for 15 minutes at 121℃ and aseptically poured into a plate. After incubation on the media for 10 days at 25℃, the colony diameter was measured.
Results and Discussions
Culture conditions of M. procera
Effect of pH
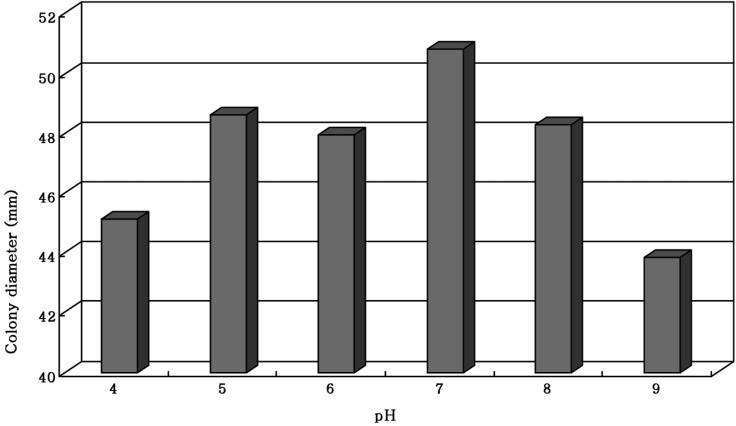
The pH value suitable for a favorable growth of M. procera was obtained in a a broad range of pH 5~8 (Fig. 1). Of 6 pH values, the mycelial growth of M. procera was exceedingly favorable at pH 7 (Fig. 1). Shim et al. (1997) reported that the mycelial growth of Grifola umbellata was exceedingly favorable at pH 4. Chi et al. (1996) suggested that the mycelial growth of Phellinus linteus was favorable in the range of pH 6~7. In case of M. procera, the favorable mycelial growth of seems to be exceedingly suitable at pH 7 in nature and similar to that of Phellinus linteus (Chi et al., 1996).
Fig. 1.
Mycelial growth of Macrolepiota procera on the PDA at different pHs for 10 days of incubation at 25℃.
Effect of temperature
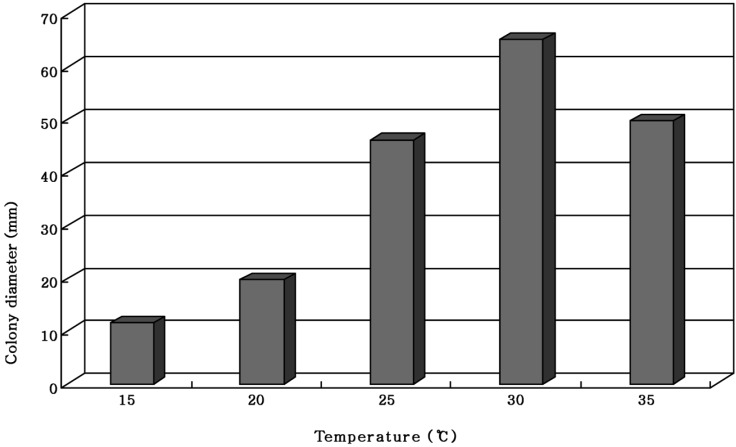
Sung et al. (1999) reported that Pleurotus ostreatus showed the most favorable mycelial growth at 30℃. The mycelial growth of M. procera was most favorable at 30℃ Even though the mycelial growth of M. procera was gradually increased in proportion to the rise of temperature and then exceedingly favorable at 30℃, the mycelial growth was suppressed at 35℃ (Fig. 2). Since most of M. procera occurs in hot and humid summer months of the year, the myceial growth of M. procera might be favorable around 30℃.
Fig. 2.
Mycelial growth of Macrolepiota procera on the PDA for 10 days of incubation at different temperatures.
Screening of favorable culture
Nine different culture media were used to screen the optimal mycelial growth of M. procera. PDA medium showed colony diameter of 67.8 mm, which indicated the rapid growth of M. procera (Table 2). Even though the mycelia of M. procera were spread rapidly on the PDA medium in a short period of time, the mycelial density was poor on the PDA medium. The compact mycelia of filamentous fungi on the medium contained more volume of accumulated hyphae than that of thin hyphae. Since the criterion for screening of suitable culture medium for fungi is based on the size of colony diameter on a given period of time. Therefore, we must have reasonable assaying method to find the media for suitable mycelial growth of M. procera in the future.
Table 2.
Mycelial growth of Macrolepiota procera on various culture media
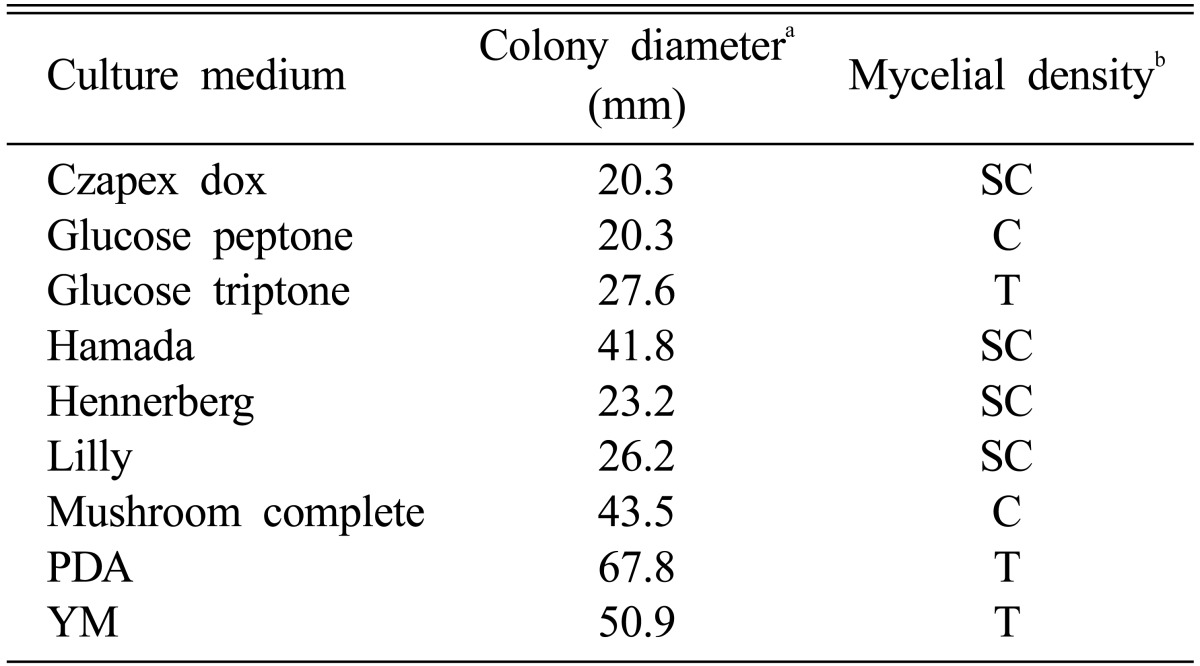
aThe colony diameter was measured at 10 days after incubation.
bMycelial density: C, Compact; SC, Somewhat compact; ST, Somewhat thin; T, Thin.
Effect of carbon and nitrogen sources
Maltose and glycine were screened as carbon and nitrogen sources suitable for the mycelial growth of M. procera (Table 3 and Table 4). After 10 days of incubation, the colony diameters of M. procera recorded 33.1 mm in maltose and 32.6 mm in glycine, respectively. Shim et al. (1998) reported that the mycelial growth of Sparassis crispa was exceedingly favorable on the basal medium supplemented with glycine. Though mycelia of M. procera were spread rapidly on the basal medium supplemented with glycine, the mycelial density of M. procera was higher on the basal medium supplemented with ammonium phosphate than on the basal medium supplemented with glycine (Table 4).
Table 3.
Effect of carbon sources for the mycelial growth of Macrolepiota procera in the basal mediuma
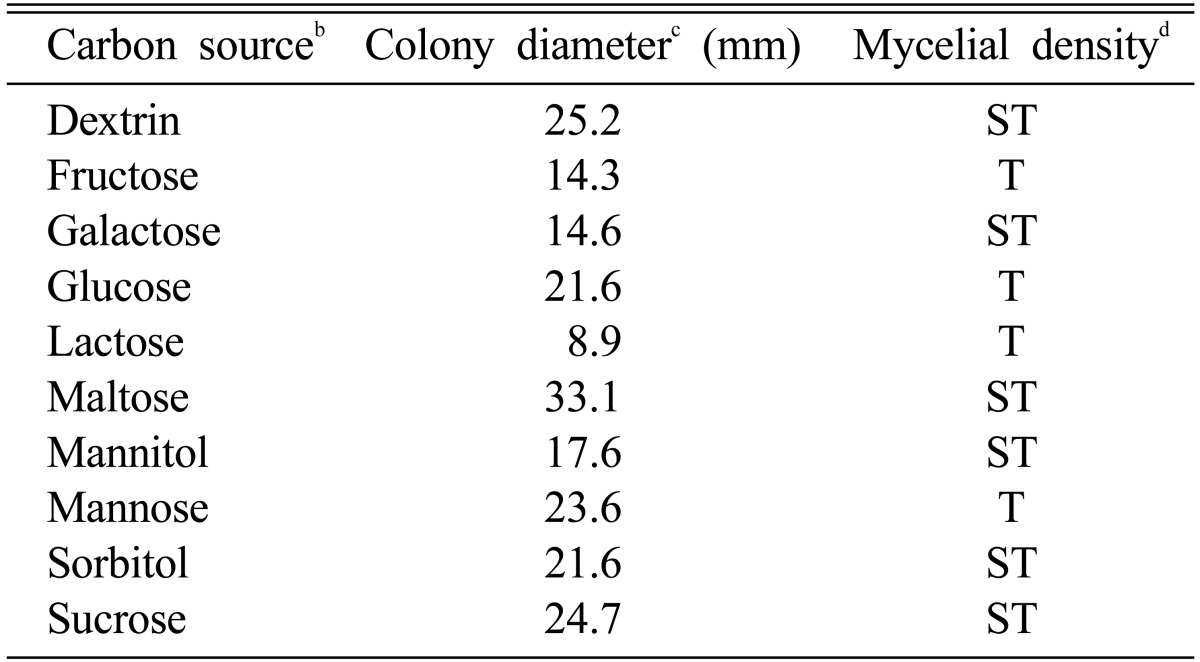
aThe basal medium was composed of peptone 5 g, MgSO4 0.05 g, KH2PO4 0.46 g K2HPO4 1.0 g, Thiamine-HCl 120 µg, agar 20 g and D. W. 1000 ml.
bEach carbon source was added to the basal medium at the concentration of 0.1 M.
cThe colony diameter was measured at 10 days after incubation.
dMycelial density: C, Compact; SC, Somewhat compact; ST, Somewhat thin; T, Thin.
Table 4.
Effect of nitrogen sources for the mycelial growth of Macrolepiota procera in the basal mediuma
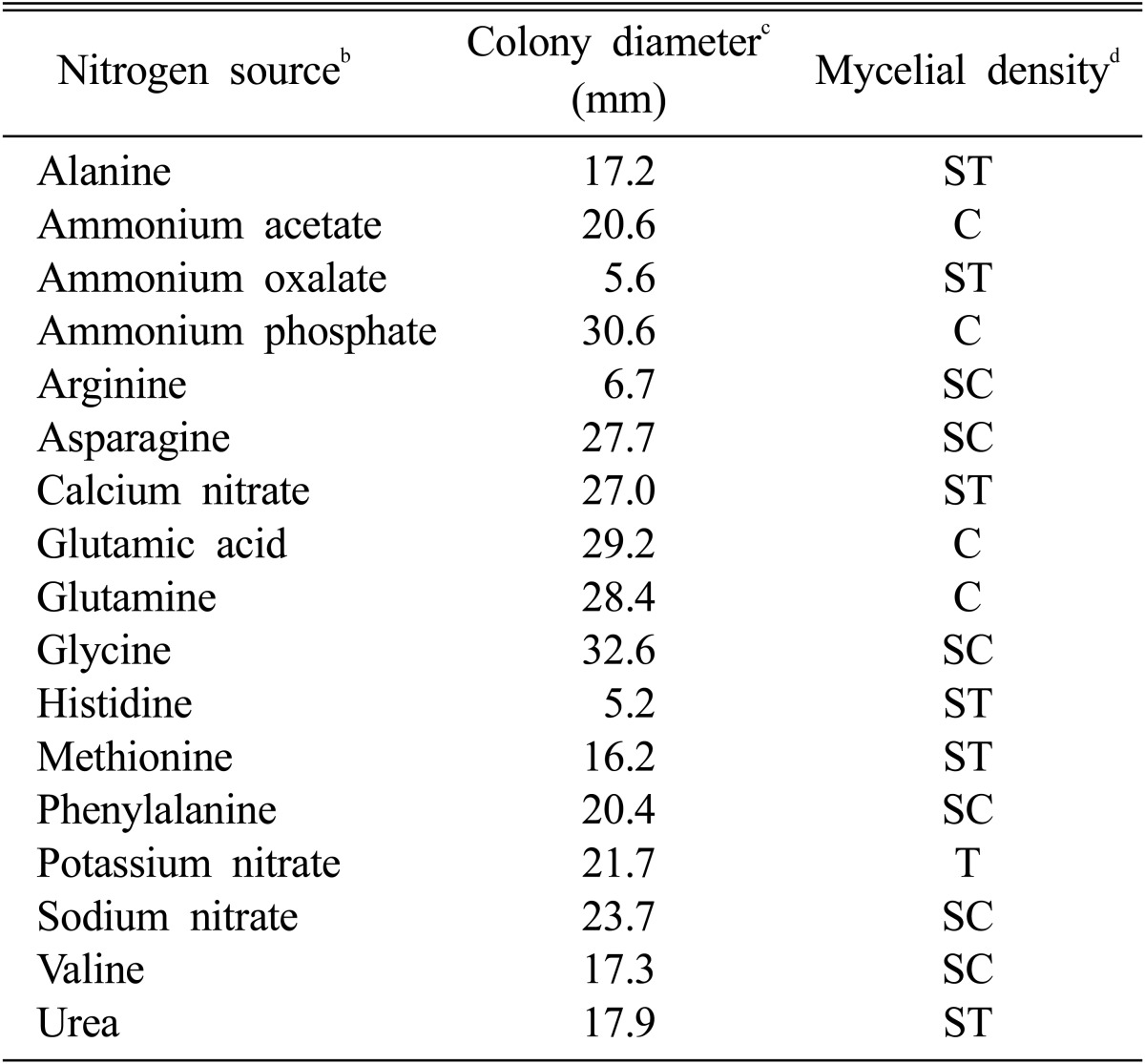
aThe basal medium was composed of MgSO4 0.05 g, KH2PO4 0.46 g K2HPO4 1.0 g, Thiamine-HCl 120 µg, agar 20 g and D. W. 1000 ml.
bEach carbon source was added to the basal medium at the concentration of 0.1 M.
cThe colony diameter was measured at 10 days after incubation.
dMycelial density: C, Compact; SC, Somewhat compact; ST, Somewhat thin; T, Thin.
Effect of C/N ratio
Chi et al. (1996) suggested that optimum C/N ratio suitable for favorable growth of P. linteus was 30 : 1. However, optimum C/N ratio suitable for a favorable growth of M. procera was 10 : 1 (Table 5). The mycelial growth of M. procera was suppressed in proportion to the gradual rise of C/N ratio. Since the suitable C/N ratio for mycelial growth of edible fungi seems to be dissimilar according to their indigenous characteristics, the mycelial growth of M. procera was favorable in the high nitrogen content of the medium.
Table 5.
Mycelial growth of Macrolepiota procera on various C/N ratio in the basal mediuma
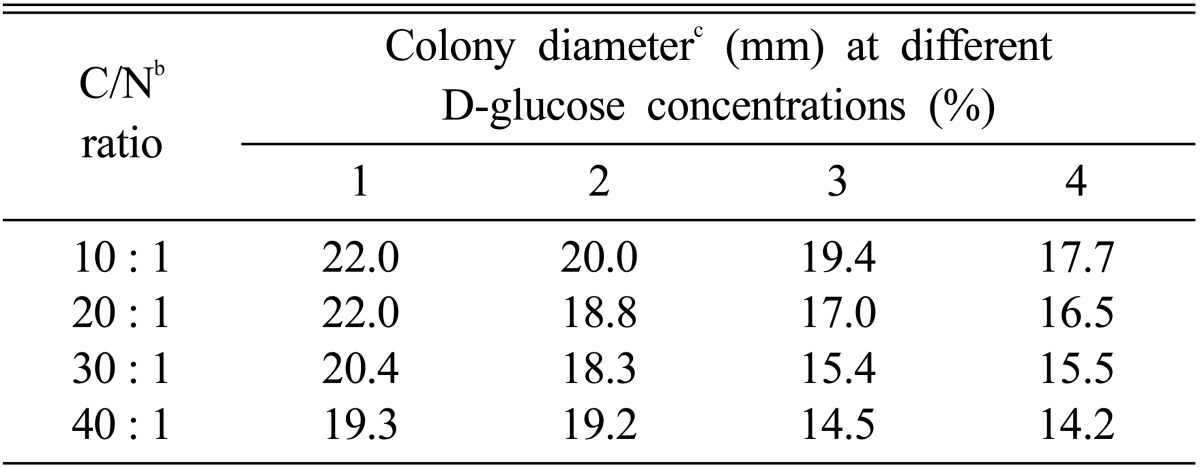
aThe basal medium was composed of MgSO4 0.05 g, KH2PO4 0.46 g K2HPO4 1.0 g, Thiamine-HCl 120 µg, agar 20 g and D. W. 1000 ml.
bThe ratio of NaNO3 versus D-glucose was adjusted to the ratio of 10 : 1, 20 : 1, 30 : 1 and 40 : 1, respectively.
cThe colony diameter was measured at 10 days after incubation.
Acknowledgement
This work was supported in part by a 2004 research grant from University of Incheon and KOSEF through Cuture Collection of Wild Mushroom Species in the University of Incheon.
References
- 1.Chi JH, Ha TM, Kim YH, Rho YD. Studies on the main factors affecting the mycelial growth of Phellinus linteus. Korean J Mycol. 1996;24(3):214–222. [Google Scholar]
- 2.Kim SW, Hwang HJ, Xu C, Na YS, Song SK, Yun JW. Influence of nutritional conditions on the mycelial growth and exo-biopolymer production in Paecilomyces sinclairii. Lett Appl Microbiol. 2002;34(6):389–393. doi: 10.1046/j.1472-765x.2002.01105.x. [DOI] [PubMed] [Google Scholar]
- 3.Park WH, Lee HD. Wild fungi of Korea. 6th edition. Kyo-Hak Publishing Co., Ltd.; 1996. pp. 1–508. [Google Scholar]
- 4.Shim JO, Son SG, Kim YH, Lee YS, Lee JY, Lee TS, Lee SS, Lee MW. The cultural conditions affecting the mycelial growth of Grifola umbellata. Korean J Mycol. 1997;25(3):209–218. [Google Scholar]
- 5.Shim SM, Lee KL, Im KH, Lee UY, Lee MW, Lee TS. The characteristics of cultural conditions for the mycelial growth and fruiting body formation of Paecilomyces sinclairii. Korean J Mycol. 2003;31(1):8–13. [Google Scholar]
- 6.Shim SM, Lee KL, Kim SH, Im KH, Kim JW, Lee UY, Shim JO, Lee MW, Lee TS. The optimal culture conditions affecting the mycelial growth and fruiting body formation of Paecilomyces fumosoroseus. Mycobiology. 2003;31(4):214–220. [Google Scholar]
- 7.Song CH, Cho KY. A synthetic medium for the production of submerged cultures of Lentinula edodes. Mycologia. 1987;79(6):866–876. [Google Scholar]
- 8.Sung JM, Kim CH, Yang KJ, Lee HK, KIim YS. Studies on distribution and utilization of Cordyceps militaris and C. nutans. Korean J Mycol. 1993;21(2):94–105. [Google Scholar]
- 9.Sung JM, Moon HW, Park DS. Growth condition of liquid culture by Pleurotus ostreatus. Korean J Mycol. 1999;27(1):1–9. [Google Scholar]