Abstract
Selected isolates of Pseudomonas fluorescens (Pf4-92 and PfRsC5) and P. aeruginosa (PaRsG18 and PaRsG27) were examined for growth promotion and induced systemic resistance against Fusarium wilt of chickpea. Significant increase in plant height was observed in Pseudomonas treated plants. However, plant growth was inhibited when isolates of Pseudomonas were used in combination with Fusarium oxysporum f. sp. ciceri (FocRs1). It was also observed that the Pseudomonas spp. was colonized in root of chickpea and significantly suppressed the disease in greenhouse condition. Rock wool bioassay technique was used to study the effect of iron availability on the induction of systemic resistance to Fusarium wilt of chickpea mediated by the Pseudomonas spp. All the isolates of Pseudomonas spp. showed greater disease control in the induced systemic resistance (ISR) bioassay when iron availability in the nutrient solution was low. High performance liquid chromatography (HPLC) analysis indicated that all the bacterial isolates produced more salicylic acid (SA) at low iron (10µM EDDHA) than high iron availability (10µFe3+ EDDHA). Except PaRsG27, all the three isolates produced more pseudobactin at low iron than high iron availability.
Keywords: Fusarium oxysporum f. sp. ciceri, Fusarium wilt, Pseudobactin, Pseudomonas spp., Salicylic acid
Infection of plants with necrotizing pathogens induces systemic resistance to subsequent attacks by pathogens. This resistance is called as systemic acquired resistance (SAR; Ryals et al., 1996). The capacity of plants to express broad-spectrum, SAR after primary infection with a necrotizing pathogen is well known. Some rhizosphere microorganisms, which do not cause necrosis, were also reported to induce systemic resistance (ISR; Pieterse et al., 1996). Phenotypically, ISR resembles pathogen-induced SAR, which is effective against a broad spectrum of plant pathogens. This form of induced disease resistance is commonly referred to as rhizosphere bacteria mediated ISR (van Loon et al., 1998). Some other rhizosphere bacteria e.g. P. fluorescens and P. aeruginosa are also present on the root surface, where plant exudates provide nutrients and caused ISR for different plant diseases. Several mechanisms have been suggested for disease control by these bacteria, viz. siderophore - mediated competition for iron, competition for carbon, production of HCN, ammonia, antibiotics, volatile compounds etc. or by competing with pathogens for nutrients or colonization space (Glick, 1995).
Some Pseudomonas spp. can also produce a non-fluorescens siderophore called pyochelin, which consist of a salicylic-substitute cysteinyl peptide (Cox et al., 1981). Salicylic acid considered to be an intermediate in pyochelin synthesis (Bucheuer and Cox, 1988), which is produced under iron limiting condition (Visca et al., 1993). Buysens et al. (1996 )demonstrated that P. aeruginosa 7NSK2 showed plant growth promotion and suppressed Pythium induced disease though the production of pyochelin and pyoverdin. In iron-limiting conditions P. aeruginosa strain produced three siderophores-pyochelin (Hofte et al., 1990), pyoverdin and salicylic acid (Buysens et al., 1996).
Pseudobactins, a yellow-green, fluorescent siderophore are also produced under iron limiting conditions by some Pseudomonas spp. (Leong, 1986). They are consisting polypeptide chain containing hydroxamate groups linked to a fluorescent quinoline chromophore (Hofte, 1993). It was demonstrated that the pseudobactin for the plant growth promoting strain P. putida WCS358 highly specific for the uptake of iron. It was reported that ISR is influenced by iron availability (Leeman et al., 1996; Press et al., 2001). Leeman et al. (1996) reported ISR induced by P. fluorescens WCS374 against Fusarium wilt of radish is inversely related to iron availability of the plant substrate. Similarly, Press et al. (1997) also reported that ISR caused by Serratia marcescens strain 90-166 against cucumber anthracnose was reduced when iron is made available in the planting medium. ISR mediated by rhizobacteria was improved significantly when external iron availability to the host plant was reduced through addition of iron chelator EDDHA (Press et al., 2001). They also reported that when the iron concentration of a planting mixture decreased suppression of cucumber anthracnose significantly improved by S. marcescens strain 90-166. It had also been reported that several strains of rhizobaceria produced salicylic acid in environment when iron is not abundant (Meyer and Hofte, 1997; Press et al., 1997).
We isolated some strains of P. fluorescens and P. aeruginosa from chickpea and rice rhizosphere soil and they were assessed for their effect on charcoal rot and Fusarium wilt of chickpea (Srivastava et al., 2001; Saikia et al., 2003). Some of these strains were found effective and exhibited systemic resistance against charcoal rot (Srivastava et al., 2001) and Fusarium wilt of chickpea (Saikia et al., 2003) as well as sheath blight of rice (data not publish). Our previous studies indicated that shoot and root length was significantly increased in P. fluorescens treated chickpea plants and the reduction in disease severity was more pronounced when chemical inducers were applied with P. fluorescens (Saikia et al., 2003). It was exhibited that exogenously supplied salicylic acid stimulated systemic resistance against Fusarium wilt and reduced the disease severity significantly. We also reported that P. fluorescens could produce SA and induced systemic resistance to chickpea against F. o. f. sp. ciceri. Here, we determined whether induction of systemic resistance by Pseudomonas spp. against Fusarium wilt of chickpea was dependent on iron availability or not and observed the effect of iron availability on siderophores production (SA and pseudobactin) by Pseudomonas isolates.
Materials and Methods
Plant material, bacterial isolates and pathogen
Seeds of chickpea (Cicer arietinum L.) cultivar JG-62 highly susceptible to F. o. f. sp. ciceri were surface sterilized as described earlier (Saikia et al., 2003). Isolates of P. fluorescens and P. aeruginosa were isolated from the rhizosphere soil of chickpea and rice from different area of Varanasi and Guwahati (India) and grown on KB medium. Isolation and identification was done according to the method as described earlier by Yeole and Dube (1997) and isolates of P. fluorescens and P. aeruginosa were designated as Pf4-92, PfRsC5, PaRsG18 and PaRsG27. These isolates were grown in KB medium in 500 ml Erlenmeyer flasks on a rotary shaker (50 rpm) for 24 h at 28±2℃ and cell concentration was adjusted to 108 cells ml-1. The pathogen, F. o. f. sp. ciceri Rs1 (FocRs1) was obtained from the Laboratory of Applied Mycology, Department of Botany, Banaras Hindu University, Varanasi, India.
Plant growth promotion, disease induction and root colonization
Surface sterilized chickpea seeds were sown in earthen pots (18 cm dia., 3 seeds pot-1) filled with FocRs1 infested and P. fluorescens inoculated soil. Inoculum of FocRs1 was prepared in sterile sand: maize meal medium (50 g + 1.5 g maize meal + 10 ml water) incubated for 15 days at 28±2℃. The inoculum (5% w/w) was mixed thoroughly in double autoclaved sandy loam chickpea field soil, the isolates of Pseudomonas spp. were poured separately (30 ml pot-1, ca. 108 cells ml-1) to the pots as per following treatments. (i) control - pots containing sterilized soil, (ii) FocRs1 control - pots containing soil infested with inoculum of the pathogen (1.0 g pot-1, 103 cfu g soil-1), (iii) Pseudomonas spp. treatment - the seeds sown in pots containing either of 4 isolates of Pseudomonas spp. and (iv) the soil containing isolates of Pseudomonas spp. + FocRs1. Experiment was carried out as complete randomized designs (CRD) in a greenhouse. Disease severity was examined for the next 27 days by the formula (Saikia et al., 2003); % of DS = (Infection length in control - infection length in treatment) × 100/Infection length in control. Shoot and root length of plants was measured before the plants were removed at 45 days and population levels of strains of P. fluorescens were also determined using five randomly selected plantlets 1 week after challenge inoculation with FocRs1.
Iron availability on ISR
Rock wool bioassay technique was used for this experiment. Surface sterilized seeds of chickpea were sown in sand; after 7 days, seedlings were transferred to locally made rock wool cubes (4 cm2 × 4.5 cm deep; one seed per cube) in such a way that the root system was divided over two cubes (Leeman et al., 1995). Pseudomonas spp. were inoculated on the lower part of the root system, at the root tips and 3 days later, inoculum of the pathogen, F. o. f. sp. ciceri prepared in sand: maize meal medium (Saikia et al., 2003) was applied to the part of the root system near the stem. The populations of the bacteria and the pathogen remained spatially separated throughout the experiment to avoid direct interaction between the bacteria and pathogen. These plants were grown in green house and watered with sterile deionised water. Half-strength Hoagland's nutrient solution (pH 7; Hoagland and Arnon, 1938) with iron (Fe-EDDHA 10 µM) was applied in the root base compartment where pathogen was inoculated. Ten µM EDDHA (low iron availability) or F3+-saturated EDDHA (12 µM FeCl3 + 10 uM EDDHA; for high iron availability) was substituted for the iron source of nutrient solution for study of the effect of iron availability on Pseudomonas spp. mediated ISR. This nutrient solution was applied to the root tip compartment of bacterized zone. Three week after pathogen inoculation, plants were harvested and percentage of disease severity was recorded (Saikia et al., 2003).
Iron availability on production of pseudobaction and SA
To study the in vitro growth and production of the siderophore, Pseudomonas isolates were grown in standard succinate medium (SSM) (pH 7.0) for 48 h at 28±2℃ on a rotary shaker. To observe the effect of iron on the production of pseudobaction and SA, different concentration (0~30 µM) of filter-sterilized FeCl3 was added to SSM medium. Bacterial growth was measured by UV-spectrophotometer (Thermospectronic, USA) at 650 nm. In control, no bacterial isolates was inoculated. Pseudobactin was extracted from ammonium sulphate saturated supernatant with phenol-chloroform (1 : 1, w/v) followed by column chromatography as described by Raaijmakers et al. (1994). The concentration of pseudobactin was determined spectrophotometrically using a molar extinction coefficient of 14,000M-1cm-1 at 400 nm and pH 7.1.
For measurement of SA, above liquid culture was centrifuged at 2800 g for 20 min at 4℃ and then supernatant was acidified to pH 2. The solution was filtered through nylon membrane under vacuum and partitioned twice with 2 ml CHCl3, finally dried under nitrogen stream at 40℃. Each sample was re-suspended in 1 ml of 23% methanol in 20 mM sodium acetate buffer (pH 5) and then the samples were analyzed with HPLC (Yalpani et al., 1991) at 280 nm with a Bondapak C18 column (3.9 mm × 30 cm), with a mobile phase flow rate at 0.5 ml min-1. SA was separated isocratically with 23% methanol (v/v) in 20 mM sodium acetate buffer (pH 5). Ten µl of each sample was injected in to the column. Retention time of isolated SA was compared with standard SA (Sigma). The SA was estimated µg ml-1 bacterial culture. All the experiments were repeated twice with 5 replicates for each treatment.
Results
Plant growth promotion, disease induction and root colonization
A marked increase in plant height was observed in Pseudomonas treated plants (Table 1). For example, 15.6% increase in plant height was obtained in the plants treated with Pf4-92. PaRsG24 was found the least effective in growth promotion activity among the isolates. In general, FocRs1 inhibited the plant growth when used in combination with Pseudomonas isolates or alone (Table 1). For example, 37.5% and 16.6% reduction of plant height was recorded when the plants were grown in FocRs1 infested and FocRs1 plus Pf4-92 inoculated pots, respectively. The isolates of Pseudomonas systemically induced resistance against Fusarium wilt of chickpea and suppressed the wilt disease by 34~45% compared to control. Maximum reduction (45%) in disease was observed with Pf4-92, followed by PfRsC5 (41.4%) and PaRsG18 (36.4%; Table 1).
Table 1.
Effect of Pseudomonas isolates on chickpea growth and Fusarium wilt disease
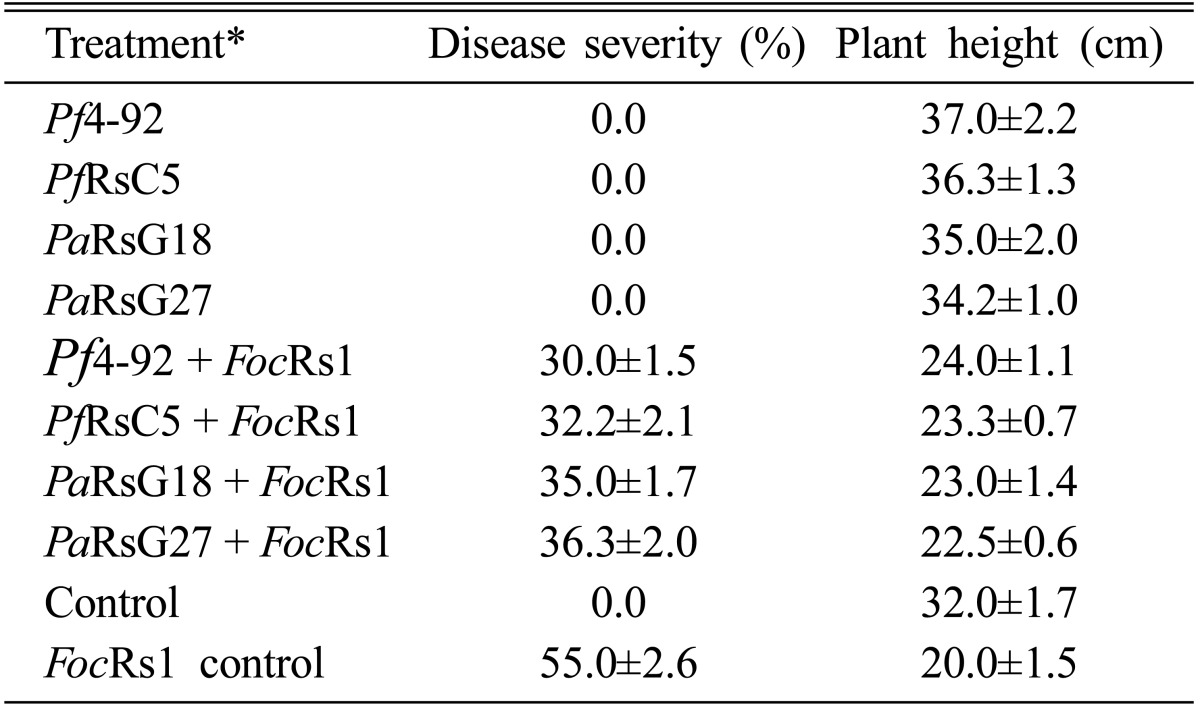
Critical difference (C.D.) = 3.43 for disease severity; 5.25 for plant height; C.D. computed at P = 0.05. *Pf4-92: Pseudomonas fluorescens 4-92; PfRsC5: Pseudomonas fluorescens RsC5; PaRsG18: Pseudomonas aeruginosa RsG18; PaRsG27: Pseudomonas aeruginosa RsG127; FocRs1: Fusarium oxysporum f. sp. ciceri Rs1.
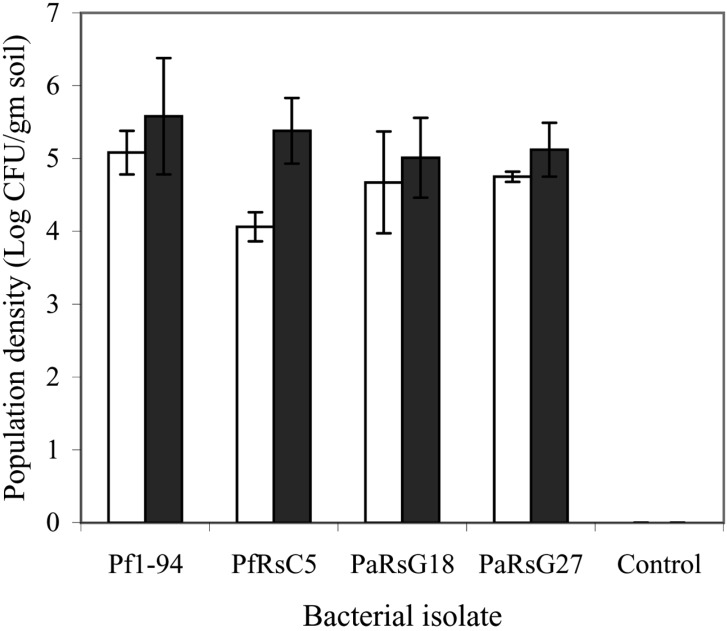
All the isolates of Pseudomonas colonized roots of chickpea, however, bacterial populations were not significantly different before and after challenge with FocRs1. Population densities were from 4.06 to 5.08 log CFU gm-1 when determined before challenge with FocRs1 and bacterial densities determined 1 week after challenge with FocRs1 were from 5.01 to 5.58 log CFU gm-1. In both the cases, population density was highest in isolate Pf4-92 than others (Fig. 1). No bacteria were isolated in blank control.
Fig. 1.
Chickpea root colonization by Pseudomonas spp. Bars represent: □ = before inoculation and ▪ = after inoculation; I=S.D.
Iron availability on ISR
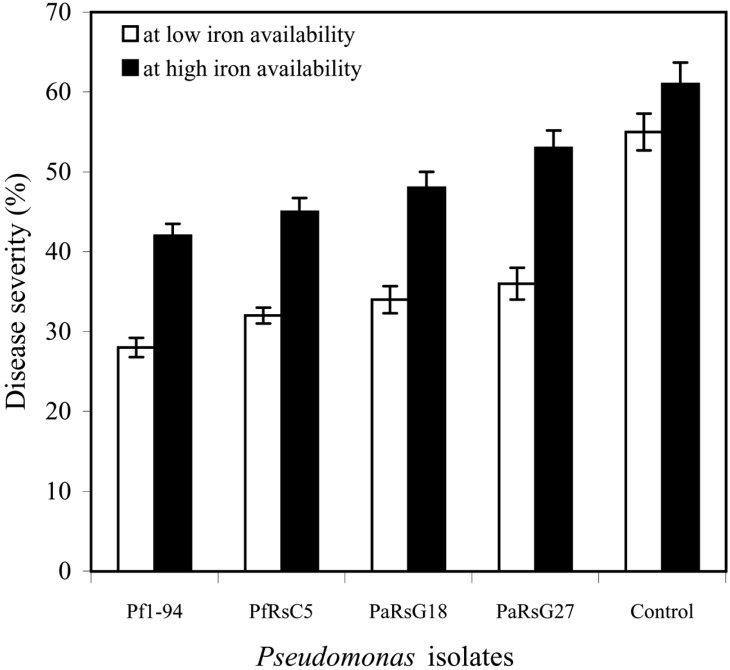
Except PaRsG27, all other isolates suppressed the disease significantly (P = 0.05) both at low (10 µM EDDHA) and high iron (10 µM Fe3+ EDDHA) availability (Fig. 2), however, more disease reduction was observed at low iron than high iron availability. PaRsG27 suppress the disease significantly (P = 0.05) only at low iron availability.
Fig. 2.
Percentage of disease severity in the ISR at low (10 µM EDDHA) and high 10 µM (Fe-EDDHA) iron availability.
Iron availability on production of pseudobaction and SA
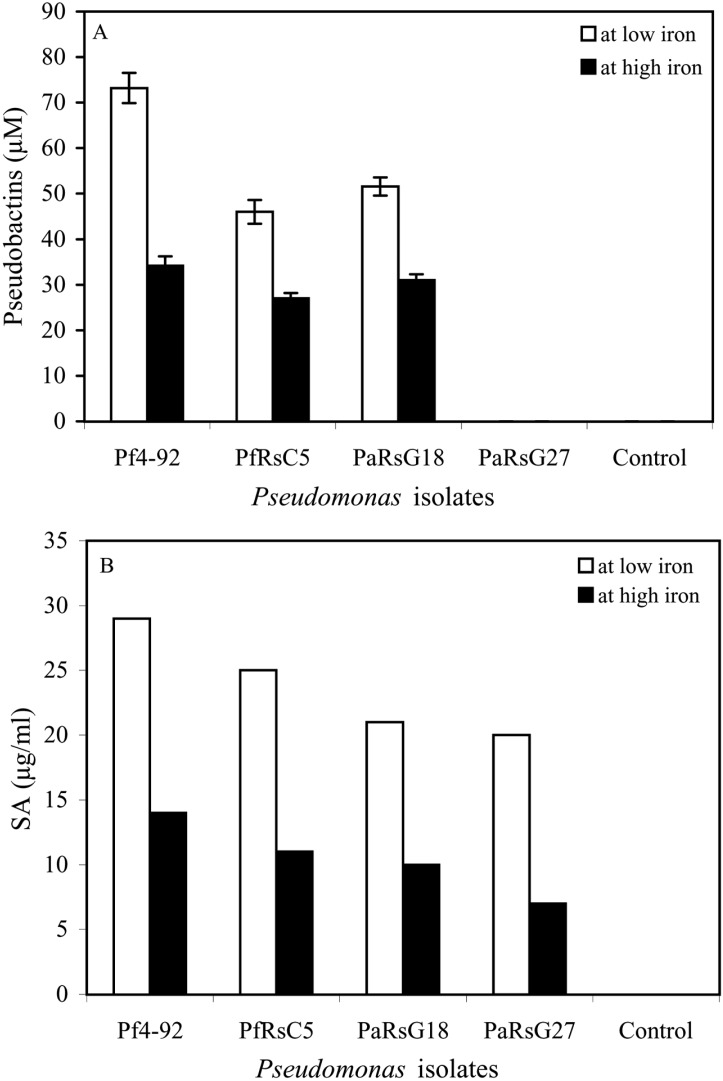
Pseudobactin was produced by all the isolates of Pseudomonas except PaG27. Isolate Pf4-92 produced more pseudobactin than other isolates, as detected at 400 nm (Fig. 3a). No pseudobactin was detected in PaG27 culture. All the isolates of Pseudomonas produced SA in SSM medium. More pseudobactin and salicylic acid (SA) was produced at low iron (10 µM EDDHA) than high iron availability (10 µFe3+ EDDHA). HPLC analysis also indicated that Pf4-92 produced comparatively more SA (29 µg ml-1) than the other isolates (Fig. 3b). In control, where no bacterial strain was inoculated, neither pseudobactin nor SA was detected (Fig. 3a & 3b).
Fig. 3.
Production of pseudobactins (A), salicylic acid (B) by Pseudomonas spp.
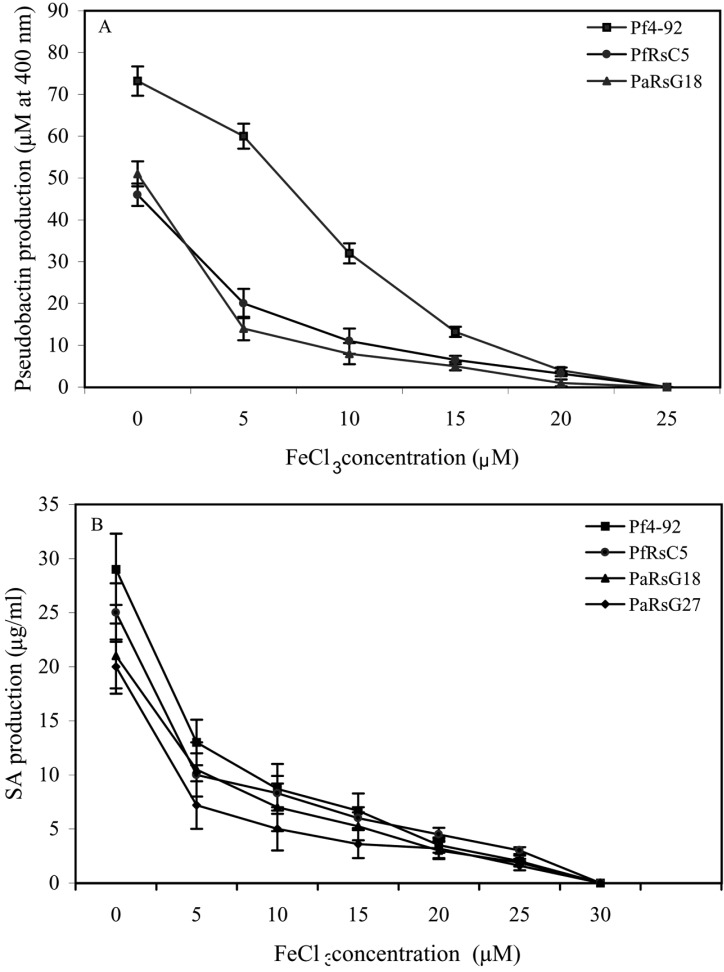
The production of pseudobactin as well as SA production was affected by iron availability in the medium. The production of these siderophore decreased with increasing iron (FeCl3) concentration. More pseudobactin and SA was produced by the isolates when iron was not present in the medium (Fig. 4a & 4b). A rapid reduction of pseudobactin production was observed between the concentrations of FeCl2 from 5 µM to 15µM. No pseudobaction production was observed after 20 µM concentration of FeCl3. SA production was also progressively decreased with the increasing of FeCl3 concentration. SA production was recorded up to 25 µM concentration of FeCl3. However, after this concentration of FeCl3 no SA was detected.
Fig. 4.
Effect of FeCl3 concentration on pseudobactin production (A) and SA production (B).
Discussion
Pseudomonas spp. have been widely used for plant growth promotion as well as for the control of plant diseases (Kloepper, 1992; Meena et al., 2000; Saikia et al., 2003; Siddiqui and Shaukat, 2004). The results demonstrate that the isolates of Pseudomonas increased the growth and controlled the severity of wilt disease of chickpea. Plant growth promotion by Pseudomonas spp. is studied in detail and several mechanisms such as phosphate solublization, production of siderophore and plant hormones, greater rhizosphere competence etc. has been suggested for their growth promotion activity (Glick, 1995; van Loon et al., 1998). The phenomenon of induction of disease resistance may be dependent on SA accumulation or jasmonic acid pathway (van Loon et al., 1998). Significant level of disease control was observed by the isolates of Pseudomonas inoculation (Table 1). These results are in agreement with the work of Meyer and Hofte (1997), Meena et al. (2001), Saikia et al. (2003).
In this study, we observed that the isolates of Pseudomonas colonized in root system of chickpea (Fig. 1). Plant growth promoting bacteria including Pseudomonas spp. have been reported to stimulate the development of healthy root system and rapidly colonized root system of plant was also reported by Bolton et al. (1990). The inductions of physiological changes by application of Pseudomonas spp. have been demonstrated in several plants, which reduced the disease symptoms of a wide range of pathogens (Leeman et al., 1995; Liu et al., 1995; Hoffland et al., 1996). Our results also observed that isolates of Pseudomonas spp. enhanced chickpea growth promotion and suppressed the disease.
Rock wool bioassay for ISR study, both P. fluorescens and P. aeruginosa could reduce more disease severity when the iron availability in the nutrient solution was low (Fig. 2). The results indicate that Pseudomonas mediated ISR is dependent on iron availability. In presence of high iron availability i.e. addition of Fe-EDDHA to the nutrient solution reduced the disease suppressive activity of the Pseudomonas isolates. This observation was in agreement with those reported by Leeman et al. (1996) and Press et al. (2001). Leeman et al. (1996) observed that when radish plant was treated with P. putida strain WCS358 did not show ISR under conditions of high iron availability. Similarly, the other strain P. fluorescens WC374 was more effective in suppressing disease through ISR under low iron availability.
The amount of production of SA and pseudobactin differed among the isolates. Pf4-92 which showed maximum ISR produced more SA and pseudobactin than other strains. It indicates that production of the siderophore is correlated with ISR activity. Leeman et al. (1996) also observed positive correlation between siderophore production and ISR activity. Many other workers have showed role of SA in ISR with different plants (Meyer and Hofte, 1997; Chen et al., 1999; Audenaert et al., 2002; Saikia et al., 2003). The findings indicate that SA and pseudobactin production is negatively regulated by iron under culture conditions and depends upon the concentration FeCl3.
Production of SA was recorded in microbial decomposed of corn and rye residues (Chou and Patrick, 1976) and also in rhizosphere soil of mung bean and corn, but, SA was not detected in non-rhizosphere soil (Pareek and Gour, 1973). It suggests that saprophytic and rhizosphere microorganisms are involved in SA biosynthesis. In present study, apparently, it seems that at low iron availability more salicylic acid was produced and it may be responsible for the additional reduction of disease in the ISR rock wool bioassay under low iron. However, the possibility cannot be denied that the pseudobactin was involved in the ISR as well. Although, the pseudobactin was not detected in PaRsG27 strain, it also significantly (P = 0.05) suppressed the disease. It is not clear by which disease-suppressive mechanisms the PaRsG27 strain was able to suppress the disease. In some bioassay, pseudobactin negative mutant of P. putida WCS358 could suppress Fusarium wilt disease of radish (Leeman et al., 1996). However, Boer et al. (2003) reported that the pseudobactin negative strains P. putida was no longer suppressed disease significantly. We think that effect of iron availably on ISR caused by Pseudomonas spp. against Fusarium wilt of chickpea is a new finding of this paper.
Overall findings suggest that - (i) Pseudomonas spp. can control the Fusarium wilt of chickpea as well as promote the growth and colonized the roots of chickpea; (ii) ISR caused by Pseudomonas spp. against Fusarium wilt of chickpea is related with iron availability; (iii) though both SA and pseudobactin taking part in ISR, Pseudomonas isolate which do not produce pseudobactin can also exihibit ISR activity.
Acknowledgement
The study was supported by the grants from DST-Fast Track Project (SR/FT/L-18-2003), Government of India, New Delhi. The first author is grateful to the funding agency.
References
- 1.Audenaert K, Pattery T, Cornelis P, Hofte M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: role of salicylic acid, pyochelin and pyocyanin. Mol Plant-Microbe Interact. 2002;15:147–1156. doi: 10.1094/MPMI.2002.15.11.1147. [DOI] [PubMed] [Google Scholar]
- 2.Bolton H, Jr, Elliott LF, Turco RF, Kennedy AC. Rhizosphere colonization of pea seedlings by Rhizobium leguminosarum and a deleterious root colonizing Pseudomonas spp. and effects on plant growth. Plant Soil. 1990;123:121–124. [Google Scholar]
- 3.de Boer M, Bom P, Frodo K, Joost JBK, van der Ientse S, Van Loon LC, Peter AHMB. Control of Fusarium wilt of radish by combining Pseudomonas putida strains that have different disease-suppressive mechanisms. Phytopathology. 2003;93:626–632. doi: 10.1094/PHYTO.2003.93.5.626. [DOI] [PubMed] [Google Scholar]
- 4.Bucheuer RG, Cox CD. Isolation and characterization of Pseudomonas aeruginosa mutants requiring salicylic acid for pyrochelin biosynthesis. J Bacterol. 1988;170:5364–5367. doi: 10.1128/jb.170.11.5364-5367.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buysens S, Heungens K, Poppe J, Hofte M. Involvement of pyochelin and pyroverdin in suppression of Pythium-induced damping-off tomato by Pseudomonas aeruginosa 7NSK2. Appl Environ Microbiol. 1996;62:865–871. doi: 10.1128/aem.62.3.865-871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Richard RB, Benhamou N, Paulitz TC. Role of salicylic acid in systemic resistance induced by Pseudomonas spp. against Pythium aphanidermatum. Eur J Pl Pathol. 1999;105:477–486. [Google Scholar]
- 7.Chou CH, Patrick ZA. Identification and phytotoxic activity of compounds produced during decomposition of corn and rye residues soil. J Chem Ecol. 1976;2:369–387. [Google Scholar]
- 8.Cox CD, Rinehart KL, Moore ML, Cook JK. Pyochelin: Novel structure of an iron-chelating growth promoter of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1981;78:4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glick BR. The enhancement of plant growth by free-living bacteria. Can J Microbiol. 1995;41:109–117. [Google Scholar]
- 10.Hoagland DR, Arnon DI. The water culture method for growing plants without soil. Calif Agric Exp Stn Bull. 1938;347:36–39. [Google Scholar]
- 11.Hoffland E, Hakulinen J, Van Pelt JA. Comparison of systemic resistance induced by a virulent and nonpathogenic Pseudomonas species. Phytopathology. 1996;86:757–762. [Google Scholar]
- 12.Hofte M, Mergeay M, Verstraete W. Marking the rhizopseudomonas strain 7NSK2 with a Mu d (lac) element for ecological studies. Appl Environ Microbiol. 1990;56:1046–1052. doi: 10.1128/aem.56.4.1046-1052.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofte M. Classes of microbial siderophores. In: Barton LL, Hemming BC, editors. Iron chelation in plants and soil microorganisms. San Diego: Academic Press; 1993. pp. 3–6. [Google Scholar]
- 14.Kloepper JW. Plant growth promoting rhizobacteria as biological control agents. In: Matting B, editor. Soil microbial technologies. New York: Marcel Dekker; 1992. pp. 142–152. [Google Scholar]
- 15.Leeman M, van Pelt JA, den Ouden FM, Heinsbroek M, Bakker PAHM, Schippers B. Induction of systemic resistance against Fusarium wilt of radish by lipopolysaccharide of Pseudomonas fluorescens. Phytopathology. 1995;85:1021–1027. [Google Scholar]
- 16.Leeman M, den Ouden FM, van Pelt JA, Dirkx FPM, Steijl H, Bakker PAHM, Schippers B. Iron availability affects induction of systemic resistance to Fusarium wilt of radish by Pseudomonas fluorescens. Phytopathology. 1996;86:149–155. [Google Scholar]
- 17.Leong J. Siderophores: Their biochemistry and possible role in the biocontrol of plant pathogens. Ann Rev Phytopathol. 1986;24:187–209. [Google Scholar]
- 18.Liu L, Kloepper JW, Tuzum S. Induction of systemic resistance in cucumber against Fusarium wilt by plant growth promoting rhizobacteria: Duration of protection and effect of host resistance on protection and root colonization. Phytopathology. 1995;85:1064–1068. [Google Scholar]
- 19.Meena B, Ramamoorthy V, Marimuthu T, Velazhahan R. Pseudomonas fluorescens mediated systemic resistance against late leaf spot of groundnut. J Mycol Pl Pathol. 2000;30:151–158. [Google Scholar]
- 20.Meena B, Marimuthu T, Velazhahan R. Salicylic acid induced resistance in Groundnut against late leaf spot caused by Cercosporidium personatum. J Mycol Pl Pathol. 2001;31:139–145. [Google Scholar]
- 21.Meyer GD, Hofte M. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induced resistance to leaf infection by Botrytis cinerea on bean. Phytopathology. 1997;87:587–593. doi: 10.1094/PHYTO.1997.87.6.588. [DOI] [PubMed] [Google Scholar]
- 22.Pareek RP, Gour AC. Organic acids in the rhizosphere of Zea mays and Phaseolus aureus plants. Plant Soil. 1973;39:441–444. [Google Scholar]
- 23.Pieterse CMJ, Van Wees SCM, Hoffland E, Van Pelt JA. Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell. 1996;8:1225–1237. doi: 10.1105/tpc.8.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Press CM, Loper JE, Kloepper JW. Role of iron in rhizobacteria-mediated induced systemic resistance of cucumber. Phytopathology. 2001;91:593–598. doi: 10.1094/PHYTO.2001.91.6.593. [DOI] [PubMed] [Google Scholar]
- 25.Press CM, Wilson M, Tuzun S, Kloepper JW. Salicylic acid produced by Serratia marcescens 90-166 is not the primary determinant of induced systemic resistance in cucumber or tobacco. Mol Plant-Microbe Interact. 1997;10:761–768. [Google Scholar]
- 26.Raaijmakers JM, Bitter W, Punte HLM, Bakker PAHM, Weisbeek PJ, Schippers B. Siderophore-receptor PupA as a marker to monitor wild-type Pseudomonas putida WCS358 in natural environments. Appl Environ Microbiol. 1994;60:1184–1190. doi: 10.1128/aem.60.4.1184-1190.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saikia R, Singh T, Kumar R, Srivastava J, Srivastava AK, Singh K, Arora DK. Role of salicylic acid in systemic resistance induced by Pseudomonas fluorescens against Fusarium oxysporum f. sp. ciceri in chickpea. Microbiol Res. 2003;158:203–213. doi: 10.1078/0944-5013-00202. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui IA, Shaukat SS. Systemic resistance in tomato induced by biocontrol bacteria against the root-knot nematode, Meloidogyne javanica is independent of salicylic acid production. J Phytopathol. 2004;152:48–54. [Google Scholar]
- 30.Srivastava AK, Singh T, Jana TK, Arora DK. Induced resistance and charcoal rot in Ciceri arietinum (chickpea) by Pseudomonas fluorescens. Can J Bot. 2001;79:787–795. [Google Scholar]
- 31.van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Ann Rev Phytopathol. 1998;36:453–485. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 32.Visc P, Ciervo A, Sanfilippo V, Orsi N. Iron-regulated salicylate synthesis by Pseudomonas spp. J Gen Microbiol. 1993;139:1995–2001. doi: 10.1099/00221287-139-9-1995. [DOI] [PubMed] [Google Scholar]
- 33.Yalpani N, Silverman P, Wilson TMA, Kleier DA, Raskin I. Salicylic acid is a systemic signal and an inducer of pathogenesis - related proteins in virus-infected tobacco. Plant Cell. 1991;3:809–818. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeole RD, Dube HC. Increased plant growth and yield through seed bacterization. Indian Phytopathol. 1997;50:316. [Google Scholar]