Abstract
Pot greenhouse experiments were carried out to attempt to increase the salinity tolerance of one of the most popular legume of the world; cowpea; by using dual inoculation of an Am fungus Glomus clarum and a nitrogen-fixer Azospirillum brasilense. The effect of these beneficial microbes, as single- or dual inoculation-treatments, was assessed in sterilized loamy sand soil at five NaCl levels (0.0~7.2 ds/m) in irrigating water. The results of this study revealed that percentage of mycorrhizal infection, plant height, dry weight, nodule number, protein content, nitrogenase and phosphatase activities, as well as nutrient elements N, P, K, Ca, Mg were significantly decreased by increasing salinity level in non-mycorrhized plants in absence of NFB. Plants inoculated with NFB showed higher nodule numbers, protein content, nitrogen concentration and nitrogenase activities than those of non-inoculated at all salinity levels. Mycorrhized plants exhibited better improvement in all measurements than that of non-mycorrhized ones at all salinity levels, especially, in the presence of NFB. The concentration of Na+ was significantly accumulated in cowpea plants by rising salinity except in shoots of mycorrhizal plants which had K+/Na+ ratios higher than other treatments. This study indicated that dual inoculation with Am fungi and N-fixer Azospirillum can support both needs for N and P, excess of NaCl and will be useful in terms of soil recovery in saline area.
Keywords: Glomus, Growth, Fixation, Legumes, Nitrogen, Nodule, Nutrients, Symbiosis
About one-third of the land area of the world comprises arid and semiarid climates. Arid desert soils were previously considered economically unimportant; however, during the past three decades, the economic and agricultural utilization of arid lands has emerged as a critical element in maintaining and improving the world's food supply (Zahran, 1999).The progressive salinization of land is considered as the major environmental factor limiting plant growth and productivity of the arid and semiarid regions. Increases in the salinity of soils or water supplies used for irrigation result in decreased productivity of most crop plants and lead to marked changes in the growth pattern of plants (Cordovilla et al., 1994). Soil infertility in arid zones is often due to the presence of large quantities of salt, and the introduction of plants capable of surviving under these conditions (salt-tolerant plants) is worth investigating (Delgado et al., 1994; Muscolo et al., 2003). Thus, there is a need to develop highly salt-tolerant crops to recycle agricultural drainage waters, which are literally rivers of contaminated water that are generated in aridzone irrigation districts (Glenn et al., 1999).
Salt tolerance in plants is a complex phenomenon that involves morphological and developmental changes as well as physiological and biochemical processes. Salinity decreases plant growth and yield, depending upon the plant species, salinity levels, and ionic composition of the salts. Arbuscular mycorrhizal fungi (Am fungi) widely exist in salt-affected soils (Juniper and Abbott, 1993; Bohrer et al., 2003). Many studies have demonstrated that inoculation with Am fungi improves growth of plants under a variety of salinity stress conditions (Ruiz-Lozano et al., 1996; Al-Karaki et al., 2001; Tain et al., 2004). To some extent, these fungi have been considered as bio-ameliorators of saline soils (Singh et al., 1997; Rao, 1998; Burke et al., 2003). Therefore, knowledge of the relationship between plants and the fungi is important for successful utilization of Am fungi under particular conditions.
Many species of bacteria such as Azospirillum, Rhizobia and Frankia adapt to saline conditions by the intracellular accumulation of low-molecular-weight organic solutes called osmolytes (Csonka and Hanson, 1991; Fischer et al., 2003). The accumulation of osmolytes is thought to counteract the dehydration effect of low water activity in the medium but not to interfere with macromolecular structure or function (Smith et al., 1994). Bacteria of the genus Azospirillum are free-living nitrogen-fixing rhizobacteria found in close association with legumes roots, where they exert beneficial effects on plant growth. Yield increases have been attributed to mechanisms such as nitrogen fixation, phytohormone production, and nitrate reduction (Okon and Vanderleyden, 1997).
The contribution of Am fungi and of associative or obligate nitrogen-fixing microsymbionts (Azospirillum- and Rhizobium bacteria) to soil fertility, productivity and crop yield has been well-documented (Biro et al., 2000). The organisms are, therefore, used to evaluate the functioning of ecosystems (Giller and Cadish, 1995), especially under nutrient unbalanced conditions. Well-known effects of AMF include improved uptake of water, and phosphorus or other macro- and microelements in non-optimal situations (Lesueur et al., 2001; Zandavalli et al., 2004). Rhizosphereñmycorhizosphere systems can therefore be tailored to help plants to establish and survive in nutrient-deficient, or degraded habitats or during periods of stress (Sanchez-Diaz et al., 199; Biro et al., 2000).
Although there are many researches on using Am fungi and N-Fixing bacteria to increase salinity tolerance in legumes plants, the available literatures do not document using dual inoculation of Am fungus Glomus clarum and a nitrogen-fixer Azospirillum in increasing salinity tolerance of these plants. Thus this article is considered a good attempt to increase the salinity tolerance of one of the most popular legume of the world, cowpea (Vigna sinensis), by using dual inoculation of the Am-fungi and nitrogen -fixing bacteria under salinity stress conditions.
Materials and Methods
A loamy sand soil contains 10.2 g/kg organic matter, 2.7 g/kg total nitrogen, 0.46 g/kg total phosphorus, 0.23 g/kg total potassium, 1.2 dSm-1 of EC and a pH of 7.8. The experiment was conducted in a greenhouse at a temperature range of 25~35℃. Vigna sinensis L. (cowpea) was used as a model plant. The soil was sieved (<2 mm), sterilized by autoclaving for 4h and placed into 30 cm diameter plastic pots at 2 kg per pot.
Provided from Botany Dept. Fac. of Sci., Mansura Univ. Egypt, Glomus clarum, previously isolated from saline soil, was selected as an efficient isolate used for improving plant growth under salinity stress. The mycorrhizal inoculum consisted of spores, mycelium and root segments of G. clarum isolates propagated with onion roots for four months. Each pot was inoculated with 20 g inoculum for the mycorrhizal treatments, or 20 g sterilized inoculum with 20 ml filtrates free from mycorrhizal propagules from the inoculum for the non-mycorrhizal treatments. Local strain of Azospirillum brasilense was isolated from rhizospheric soil (identified by A.Al-Humiany assistant professor of bacteriology Taief teacher's college Saudi Arabia) and was grown on modified nitrogen-deficient semi solid malate medium (Dobereiner, 1978) at 30℃ for 48 h. The suspension obtained (1 × 108 cells/ml) was used for seeds inoculation at the rate of 10 ml per pot. The same dose was added to each pot after 10 days of sowing.
The experiment was a 5 × 4 × 5 complete factorial which was comprised of 5 salinity levels and four inoculation treatments with 5 replicates for each treatment. The pots were arranged in a randomized block. It was carried out with the following treatments: non-inoculation control and inoculation with G. clarum and A. brasilense as a single and paired inoculum, each at five salt levels (5 NaCl concentrations) of 1.8, 3.6, 5.4, and 7.2 dS/m. Each NaCl concentration, dissolved in nutrient solution containing N at 100 mg l-1, P at 50 mg l-1 and K at 50 mg l-1, was added to the soil at a rate of 100 ml every week. 5 seeds were sown into each pot and were thinned to two seedlings per pot after emergence. Tap water was supplied daily and the pots were weighted every week to adjust water content. Fertilization rates of all pots were 60 kg fed-1 (NH4NO3 33.5%) and 48 kg K2O fed-1 (k2SO4 48%).
After 65 days from planting, inoculated and un-inoculated representative plants (5 plants/replicate) were carefully uprooted. The roots were dipped in water to remove soil particles, and washed with distilled water. Plant height and root nodule number were estimated. Total dry weight of plant, root and shoot dry weight were determined after drying at 70℃ for 48hr.The oven dried plants were grounded and mineralized by sulfuric-perchloric acids (Piper, 1950). Content of total nitrogen (Jackson et al., 1973) and phosphorus (Jackson, 1967) were determined. Potassium and sodium were measured by a flame photometer, while calcium and magnesium were estimated by atomic absorption (Allen et al., 1984). Protein content was measured according to Bradford 1976.
Nitrogenase activity of plant was measured by using acetylene reduction assay (Hardy et al., 1973). Values of nitrogenase activity were recorded as n moles C2H4 gm plant -1 h-1. Soluble acid and alkaline phosphatases were determined according to (Gianinazzi-Pearson and Gianinazzi, 1976).
The roots were cleared and stained by using the methods by Philips and Hayman (1970) and the percentage of mycorrhizal colonization was estimated by the methods of Trouvelot and Gianinazzi (1986).
Statistical analysis of the results was subjected to ANOVA (Significance was set at *P 0.05 and **P < 0, 01. Means were compared using the least significant difference (LSD) procedure (Steel and Torrie, 1960).
Results
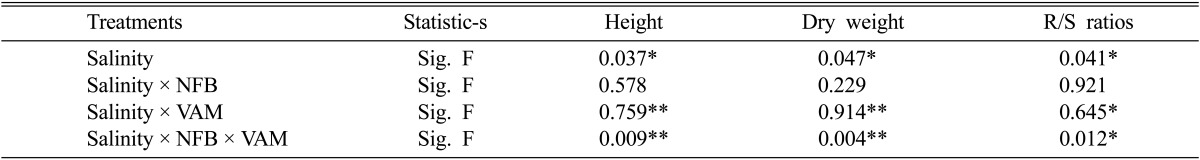
The results in Table 1 showed the effect of single and dual inoculations of Am fungi and nitrogen fixing bacteria (NFB) on height, dry weight and root: shoot dry weight ratios of cowpea plants under various levels of salinity. With increasing salinity level the height and dry weight of plants were significantly decreased especially in control plants (without inoculation). Inoculation of cowpea plants with NFB significantly and insignificantly reduced the suppression effect of salinity on plant height at high salinity levels (5.4 and 7.2 dSm-1) and at lower levels (1.8 and 3.6 dSm-1) respectively. On the other hand, inoculation of plants with Am fungi significantly increases salinity tolerance of cowpea plants at all salinity levels used in this study. The plant height increased from 59 and 21 cm in non-mycorrhized to 99 and 57 cm in mycorrhized plants at 1.8 dSm-1 and 7.2 dSm-1 salinity levels respectively. Dual inoculation of Am fungi and NFB to cowpea plants significantly reduces the harmful effects of salinity on the height at all salinity levels used, where the maximum height of plants was obtained in plants inoculated with both VAM and NFB at corresponding levels of salinity.
Table 1a.
Effect of salinity levels on plant height, dry weight and root shoot ratios of mycorrhizal, and non-mycorrhizal plants with and without nitrogen-fixing bacteria
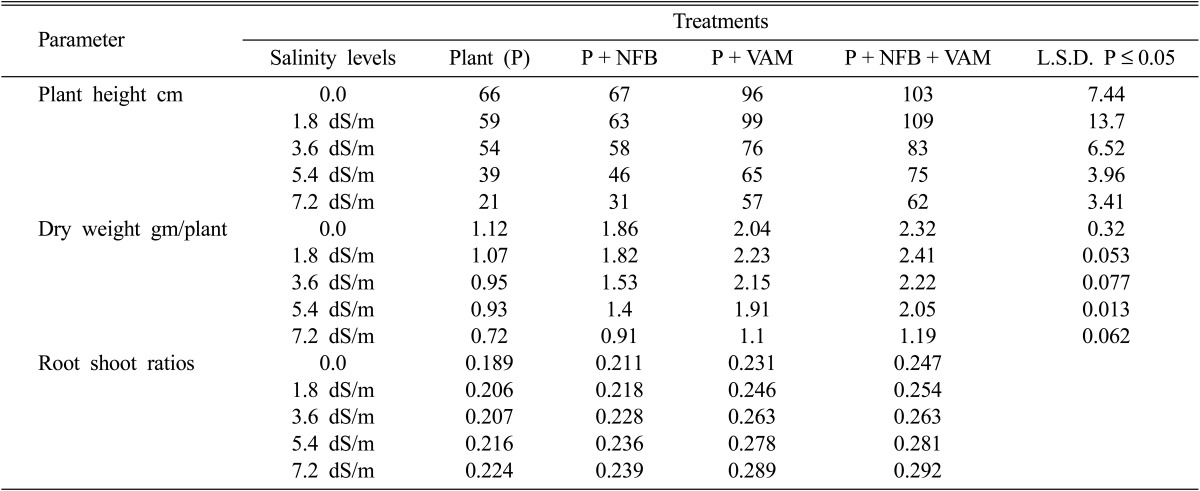
NFB = Nitrogen-fixing bacteria VAM = Vesicular arbuscular mycorrhiza.
It was also revealed that the effect of single and dual inoculation of NFB and Am fungi on dry weight of cowpea plants under salinity conditions follow similar trends of these inoculants on the height of cowpea plants under the same salinity conditions. The highest values of dry height were recorded for mycorrhizal plants, co-inoculated with NFB at all corresponding salinity levels. On the other hand, increasing levels of salinity caused an increase of root shoot dry weight ratios in all treatments in this study. The highest ratio was obtained in Am plants inoculated with NFB at all corresponding levels of salinity as shown in Table 1.
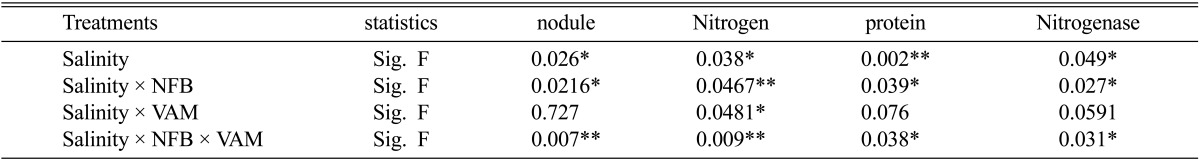
The results of Table 2 showed that the number of nodules was slightly decreased at low levels of salinity (1.8 and 3.6 dSm-1) and greatly decreased at high salinity levels (5.4 and 7.2 dSm-1) in cowpea plants without any inoculants. The presence of microbial inoculants of NFB and Am fungi caused significant increases in nodule numbers of inoculated plants at each corresponding levels of salinity in this study. The maximum counts of nodules were recorded in plants treated with NFB and Am fungi at all salinity levels especially at 1.8 dSm-1 salinity in each treatment.
Table 2a.
Effect of salinity levels on number of nodules, nitrogen content protein content and nitrogenase activity of of mycorrhizal,and non-mycorrhizal plants with and without nitrogen-fixing bacteria
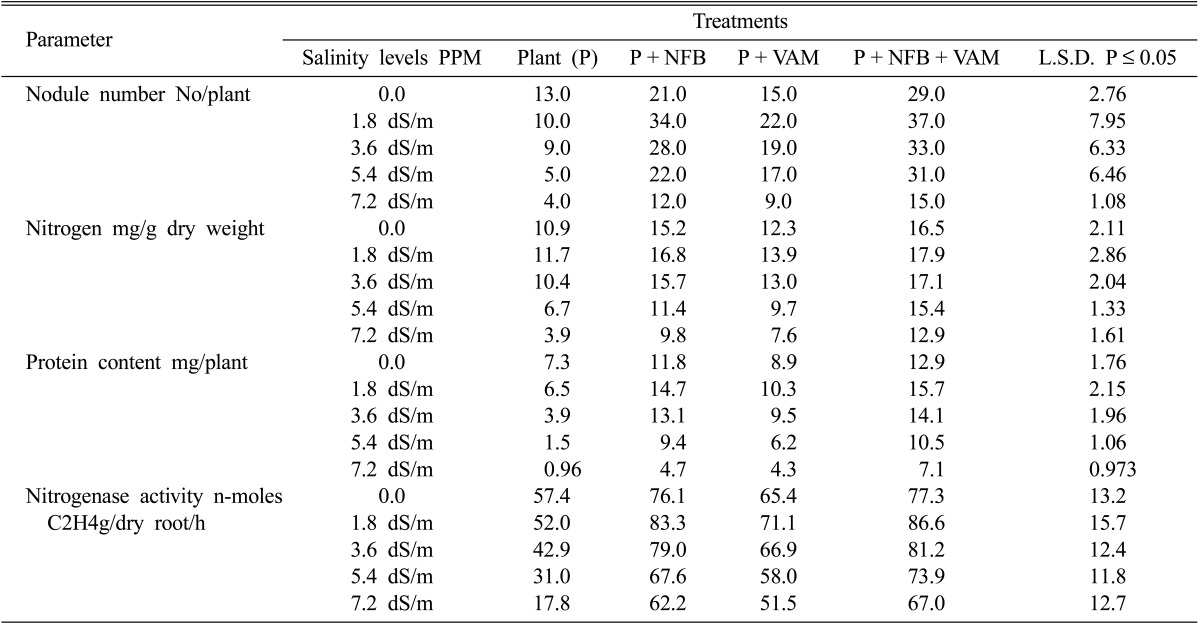
NFB = Nitrogen-fixing bacteria VAM = Vesicular arbuscular mycorrhiza
The nitrogen content of uninoculated cowpea plants was significantly influenced by increasing salinity levels whereas it was decreased from 11.7 mg dry weight-1 at 1.8 dSm-1 salinity to 3.9 mg dry weight-1 at 7.2 dSm-1 salinity. Inoculation of cowpea plants with NFB either singly or paired with Am fungi significantly increased nitrogen content compared to control plant at all salinity levels. Moreover, plants inoculated with Am fungi showed insignificant and significant increase in nitrogen content compared with non-mycorrhizal plants either in the presence or in the absence of NFB at low (1.8 and 3.6 dSm-1) and high (5.4 and 7.2 dSm-1) salinity levels respectively.
On the other hand, protein content of uninoculated cowpea plants was greatly suppressed with increasing salinity levels, whereas it was reduced from 6.5 mg/plant at 1.8 dSm-1 salinity to 0.96 mg plant-1 at 7.2 dSm-1 salinity (Table 2). Inoculating cowpea plants with Am fungi significantly increased their protein content, and the highest content was obtained at 1.8 dSm-1 salinity. In addition, the results of the table also showed that inoculation of NFB caused significant increase in protein content of cowpea plants compared with uninoculated plants at each level of salinity. Moreover, inoculation of NFB and Am fungi significantly increased protein content of cowpea plants compared with other treatments at all salinity levels with highest count at 1.8 dSm-1 salinity.
Nitrogenase activities were significantly inhibited with increasing salinity in all treatments in this study. Close examination of Table 2 revealed that the presence of bio-inoculants either as single or paired reduces the inhibitory effects of salinity on nitrogenase activities of cowpea plants. A-mycorrhizal infection of cowpea plants showed higher nitrogenase activities compared with non-infected plants at all salinity levels. Inoculation of cowpea plants with NFB significantly increased nitrogenase activities specially in presence of Am fungi, and the high activities were obtained at all salinity levels used (Table 2).
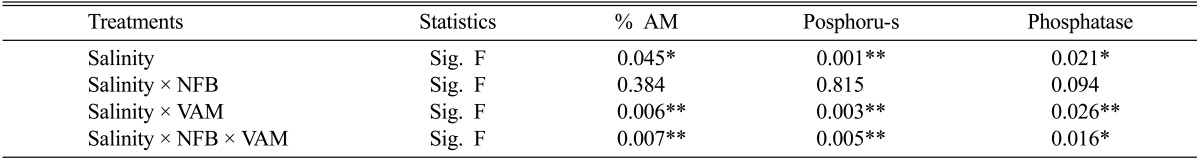
The results of Table 3 showed that the intensity of mycorrhizal infection of cowpea plants was not affected by relatively low salinity levels (1.8 and 3.6 dSm-1), meanwhile, it showed slightly increase compared with that under non-saline condition. On the other hand, these intensities were reduced at higher levels of salinity (5.4 and 7.2 dSm-1) from 69% and 74% at 1.8 ds/m salinity to 36% and 41% at 7.2 ds/m in the absence and presence of NFB respectively.
Table 3a.
Effect of salinity levels on % mycorrhizal infection, Phosphorus content and phoshatases activities of mycorrhizal, and non-mycorrhizal plants with and without nitrogen-fixing bacteria
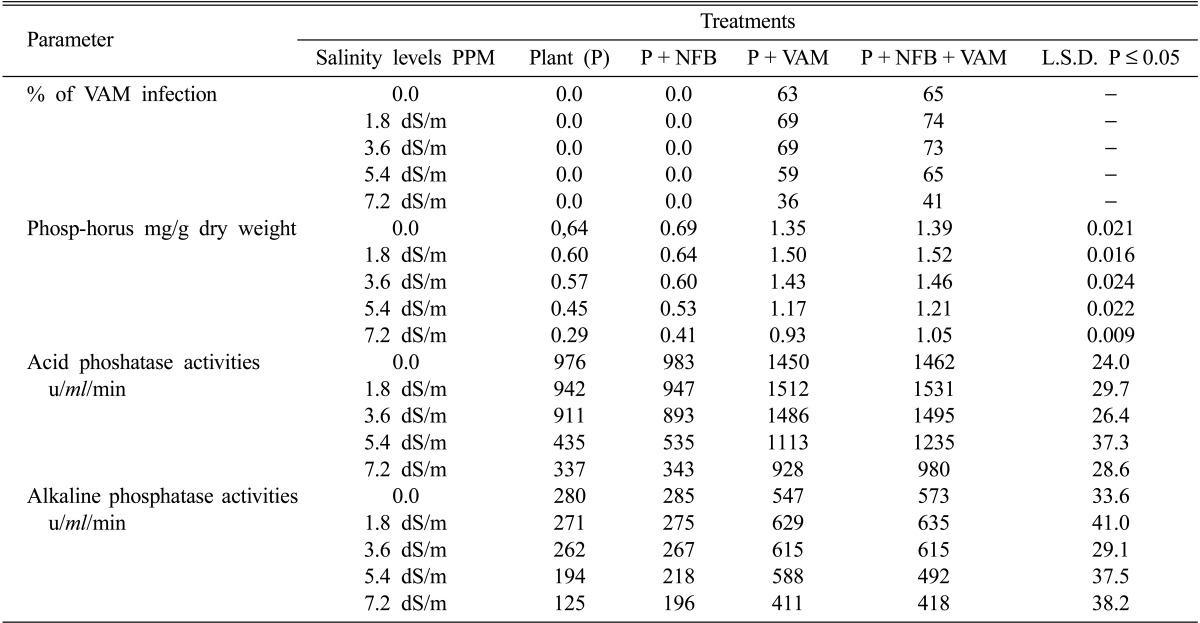
NFB = Nitrogen-fixing bacteria VAM = Vesicular arbuscular mycorrhiza
With increasing salinity, the changes in P concentrations in mycorrhizal and non-Am plants were significantly different. The P concentration of non-Am plants declined at relatively lower salinity levels (1.8 and 3.6 dSm-1), particularly in the absence of NFB, while the opposite trend was found for mycorrhizal plants (Table 3). On the other hand, higher levels of salinity (5.4 and 7.2 dSm-1) caused significant reduction in P concentration of mycorrhized and non-mycorrhized plants. Compared with non-mycorrhizal plants, plants inoculated with Am fungi had higher P concentrations at all NaCl levels, while the inoculation of NFB to Am plants increased P concentrations in these plants than those of non-inoculated Am plants at all salinity levels.
The results of Table 3 also indicated inhibitory effects of high salinity levels (5.4 and 7.2 dSm-1) on phosphatases activities (acid and alkaline phosphatases). It was clearly shown in all treatments especially in non-inoculated cowpea plants. On the other hand, Am infected cowpea plants results in significant increases in acid and alkaline phosphatases activity irrespective to the presence and absence of NFB at low salinity levels (1.8 and 3.6 dSm-1). NFB inoculated to cowpea plants caused slight insignificant increase in acid and alkaline phosphatases compared with control plants. However, the highest acid and alkaline phosphatase activities were recorded in cowpea plant inoculated with both NFB and Am fungi at all salinity levels.
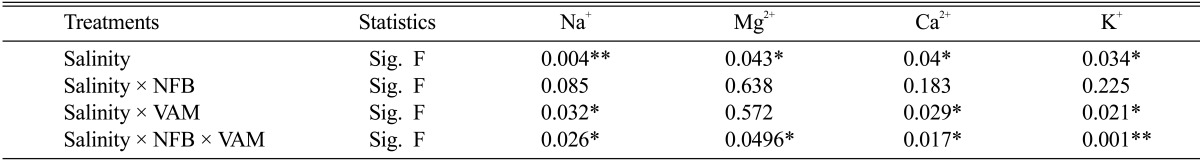
The results of Table 4 showed that Sodium ions (Na+) were greatly accumulated in root and shoot of non-inoculated cowpea plants by rising salinity levels in irrigating water. The inoculation of cowpea plants with NFB also increase Na+ in infected plants with rising salinity levels but there is no significant differerance between the inoculated and non-inoculated cowpea plants. On the other hand, inoculation of cowpea plants with Am fungi in the presence or absence of NFB caused more accumulation of Na+ in root system at all salinity levels used. Unlike the root system, shoots of mycorrhizal plants had insignificant increases in Na+ with increasing salinity levels and there is no significant difference between Na+ ions concentrations at 1.8 and 3.6 dS/m salinity.
Table 4a.
Effect of salinity levels on sodium (Na), magnesium (Mg), calcium (Ca) and potassium (K) contents of mycorrhizal, and non-mycorrhizal plants with and without nitrogen fixing bacteria
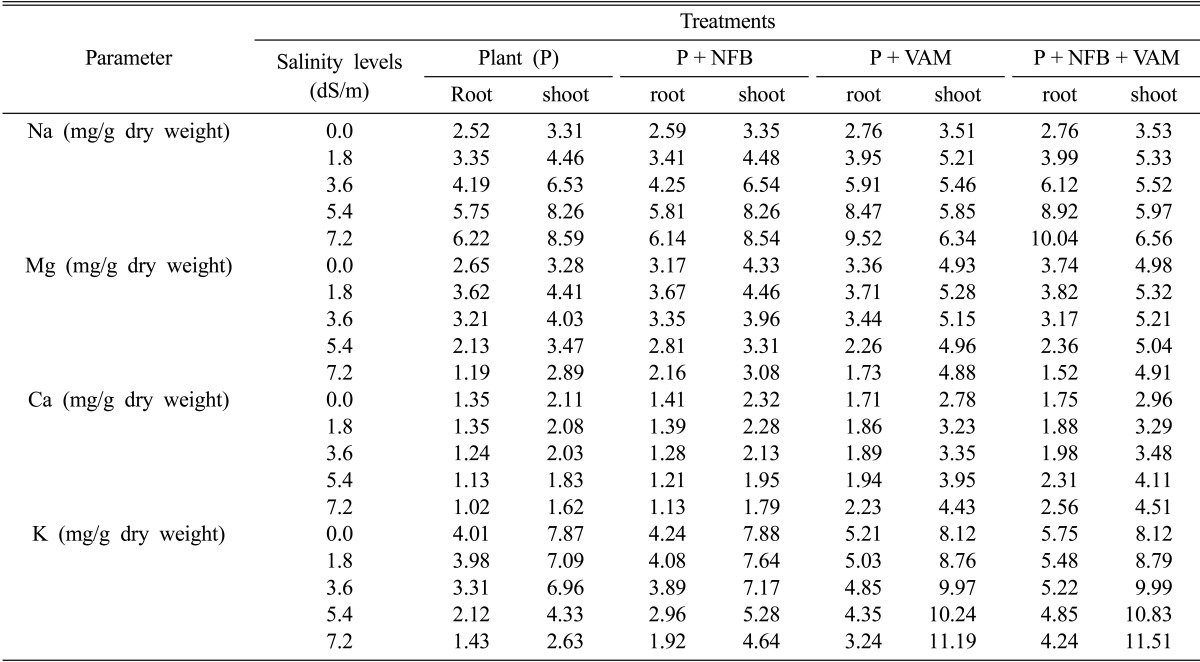
NFB = Nitrogen-fixing bacteria VAM = Vesicular arbuscular mycorrhiza
Magnesium concentration (Mg+2) of cowpea plants was decreased at higher salinity levels (5.4 and 7.2 dSm-1) (Table 4). On the contrary, Mg+2 content of cowpea plants was slightly increased at lower salinity levels and the highest Mg+2 contents of roots and shoots were recorded at 1.8 dSm-1 salinity for all treatments. Inoculated of cowpea plants with NFB did not show significant differences in Mg+2 content compared to non-inoculated one in Am and non-Am plants. On the other hand, Am fungi infected cowpea plants decreased Mg+2 contents of roots from 3.71 mg and 3.82 mg at 1.8 dSm-1 salinity to 1.73 mg and 1.52 mg at 7.2 dSm-1 salinity in the absence and presence of NFB respectively. Nevertheless, Am fungi improved Mg+2 contents of shoot of infected plants compared to non-Am plants at all salinity levels. Meanwhile this improvement, Mg+2 contents of mycorrhized plant shoots kept near that of these plants in non-saline condition.
The results in Table 4 also showed that non-mycorrhizal plants either in the absence or presence of NFB reduced calcium ions (Ca+2) concentrations in roots and shoots by increasing salinity in irrigating water. However, inoculation of Am fungi singly or in paired with NFB to cowpea plants caused relative slight increase of Ca+2 content, particularly in the presence of NFB, in roots and shoots of plants as a result of increasing salinity in irrigating water.
Potassium ions (K+) concentration in non-mycorrhizal plants either in the presence or absence NFB was significantly reduced with increasing salinity in irrigating water and still plant-infected with NFB had higher K+ content in root and shoot at all salinity levels. Am cowpea plants showed higher K+ content at all salinity levels compared with non-mycorrhizal one, especially in the presence of NFB. However, close examination of the results in (Table 4) reveals that while roots of Am plants had a slight decrease in K+ content by increasing salinity levels, shoots of these plants showed increasing K+ concentrations at corresponding salinity levels. Moreover, K+/Na+ ratios of mycorrhizal plant shoots, particularly in the presence of NFB were increased with rising salinity.
Discussion
Associative and symbiotic nitrogen ñfixing bacteria and Am fungi are common beneficial microbes of leguminous-plants. It is frequently suggested that Am may improve P nutrition, enhance N uptake, or improve disease resistance in their host plants. Other microbes, e.g., N-fixing bacteria or P-solubilising bacteria, may synergistically interact with Am fungi and thereby benefit plant development and growth. The mycorrhizal symbiosis becomes even more important in sustainable agricultural systems where nutrient inputs are low. The principal objective of this work was to attempt using dual inoculation of Am fungi and N-fixing bacteria to induce salinity tolerance of cowpea plants grown under the salinity conditions. This study indicates that dual inoculation with G. clarum and A. brasilense can increase the plant height, dry weight and root shoot ratio of cowpea plants more than single inoculation with Am fungi or NFB as well as control at all salinity levels. In this connection, Biro et al., 2000; Tian et al., 2002 proved that VA-mycorrhizal fungi and N-fixing bacteria can play an important role in the establishment of plants in soils with low nutrient levels.
It was clear in Table 2 that relatively low salt levels (1.8 dsm-1) reduced the nodule number to about 79% of non-salt treatment. High salt levels (5.4 and 7.2 dSm-1) depressed the nodule number of cowpea plants to about 38% and 30% respectively, compared to the control treatment. As expected plants inoculated with NFB showed better nodule number at all salinity levels and these numbers were increased in the presence of dual inoculants of Am fungi and NFB. The synergistic effects of dual inoculation Am fungi and NFB on nodule formation and activity were previously proved for different legumes plants under non-saline conditions (Lesueure et al., 2001; Saini et al., 2004; Rabie and Al-humiany, 2004). In spite of nitrogen and protein contents as well as nitrogenase activity of non-inoculated cowpea were negatively affected with relatively low salinity levels, single and dual inoculated plants showed positive effects at these levels. This may directly correlated with the presence of these inoculants. These results are in agreement with those reported by El-Mokadem et al., 1991; Zou et al., 1995; Rabie, 2005). Although nitrogen and protein contents as well as nitrogenase activity were significantly reduced in all treatments at higher salinity levels, inoculated plants were still higher than those of non-inoculated at corresponding salinity levels (Table 2). The depressive effect of salt stress on N2 fixation by legumes is directly related to the saltinduced decline in dry weight and N content in the shoot (Cordovilla et al., 1995; Veatch et al., 2004). The salt-induced distortions in nodule structure could also be reasons for the decline in the N2 fixation rate by legumes subject to salt stress (Zahran and Abu-Gharbia, 1995). Reduction in photosynthetic activity might also affect N2 fixation by legumes under salt stress (Georgiev and Atkias, 1993).
Careful examination of the data in Table 2 showed that bacterial-Am fungal-legume tripartite symbiosis showed better nitrogen fixation (nodule number, nitrogen and protein contents as well as nitrogenase activities) than that of bacterial-legume symbiosis. Yet, there are no significant differences between them at all salinity levels. Therefore, the data suggested that enhanced nitrogen fixation can be attributed to NFB and not to Am fungi. These results were consistent with Minerdi et al., 2001 who demonstrated the presence of genes for N fixation in endosymbiotic Burkholderia bacteria in AM-mycorrhizal hyphae and suggested that there may be a potential for improving N supply to mycorrhizal plants through fixation of atmospheric N. In fact, it is easy to understand that the nodules can fix atmospheric nitrogen, but its efficiency is mostly determined by the phosphorous nutrient condition of the host plant since appropriate phosphorous nutrient support is indispensable for the process of nitrogen fixation. Mycorrhizal infection could contribute to proper phosphorous uptake in Azospirillum and ensure the activity of the nitrogen fixation enzyme. In this connection, Jha et al., 1993; Valdes and Sannchez-Francia, 1996; Johansson et al., 2004 showed that dual inoculation with mycorrhizae and NFB can support both the needs for N and P and increase the growth of host plant.
In this investigation, the mycorrhizal colonization of cowpea plants was significantly increased at relatively low salinity levels (Table 3). On the contrary, at higher salinity levels mycorrhizal colonization was significantly reduced although mycorrhizal plants inoculated with NFB still had higher mycorrhizal colonization. Previous research had shown that salinity may reduce mycorrhizal colonization by inhibiting the germination of spores (Hirrel, 1981), inhibiting growth of hyphae in soil and hyphal spreading after initial infection had occurred (McMillen et al., 1998), and reducing the number of arbuscules (Pfeiffer and Bloss, 1988). On the other hand Mycorrhizosphere bacteria may affect AM fungi and their plant hosts through a variety of mechanisms including (1) effects on the receptivity of the root; (2) effects on the root-fungus recognition; (3) effects on the fungal growth; (4) modification of the chemistry of the rhizospheric soil; and (5) effects on the germination of the fungal propagules. In this context, Nitrogen fixation further promotes mycorrhizal development (Puppi and Hoflich, 1994). Some mycorrhizosphere bacteria may be able to promote mycorrhizal establishment through improved spore germination, but so far there is no direct demonstrations of this in the field. The colonization enhancement may also be mutual between associated microorganisms. This has been reported following dual inoculation of Pseudomonas sp. and Glomus sp., which additionally increased the growth of the host plant in an additive manner (Johansson et al., 2004).
Saline stress induces P deficiency by reducing P uptake or translocation (Munns, 1993). The present study indicated that Am fungi increased P uptake and phosphatases activities at all salinity levels (Table 3) and saline stress in plants was thereby alleviated. This is consistent with previous findings explaining that the main mechanism for enhancing salinity tolerance in mycorrhizal plant was by improving of P nutrition (Copeman et al., 1996; Al-Karaki et al., 2001). In some cases, however, saline tolerance of mycorrhizal plants appears to be independent on plant P concentration (Ruiz-Lozano et al., 1996; Fing et al., 2002). Another interesting result in Table 3 evinces that the presence of NFB increases P concentration and acid and alkaline phosphatases activities in mycorrhizal plants at all salinity levels. It has been reported that NFB clearly has the potential to influence Am fungi. In this respect, Johansson et al., 2004 reported that bacteria associated with the VA-mycorrhiza can assist in mobilizing nutrients from soil. Abundant examples of these are available from bacterial-Am fungal-legume tripartite symbiotic relationships, where diazotrophic bacteria provide fixed N not only for the plant, but also for the fungus. Moreover, nodulation of legumes by N-fixing bacteria and establishment of Am often occur simultaneously and synergistically.
It has been widely accepted that plants are stressed in three ways by salinity (1) low water potential of the root medium leads to water deficits in crop plants, (2) toxic effect of ions, mainly Na and Cl and (3) nutrient imbalance caused by depression in uptake and/or shoot transport (Marschner, 1995; Adiku et al., 2001). In this study, as expected, Na+ concentration was significantly increased with increasing salinity levels in mycorrhized and non-mycorrhized plants irrespective with absence and presence of NFB. Surprisingly, while mycorrhizal plants accumulated more Na+ in their roots with increasing salinity, shoots of these plants had lower Na+ content and showed limited accumulation with increasing salinity (Table 4). These results are in agreement with previous work of Rabie (2004) and suggested that Am fungi protect leaf metabolism from Na+ toxicity.
The results in table 4 clearly showed reduction of nutrient elements Mg+, Ca+ and K+ concentrations in non-mycorrhizal plants with increasing NaCl levels which was proved previously by Bayuelo-Jimenez et al., 2003 and Netondo et al., 2004. The nutrient imbalance caused by salinity may be explained according to Khan et al. (2000) who reported that nutrient deficiencies can occur in plants when high concentration of Na+ reduces amounts of available Mg+, Ca+ and K+ or Na+ displaces membrane-bound Ca+, and Na+ may have a direct toxic effect, such as it interferes with the function of K+ as cofactor in various metabolic reactions. On the other hand, it has been reported that Am fungi would enhance nutrient uptake by infected plant under salinity conditions (Roa and Tak, 2002; Yano-Melo et al., 2003; Zandavalli et al., 2004). The results of this study are in consistent with these reports where mycorrhizal cowpea plants contained higher nutrient concentrations, particularly, in the presence of NFB, than that of non-mycorrhizal one at all salinity levels.
The most important result in Table 4 evinces that salinity levels K+ concentration in shoots of mycorrhizal plants increased while decreased in roots of these plants. In addition, K+/Na+ ratio in mycorrhizal shoot plants, especially in presence of NFB, was increased by rising of salinity levels and still much higher than that of non-mycorrhizal one at all salinity levels. These results emphasize that Am fungi improve salinity tolerance of cowpea plants, particularly in the presence of NFB. In this context, the higher ratios of K+/Na+ was proved to be one of the key determinants of plant salt tolerance by the work of Maathuis and Amatmann, 1999; Naidoo and Naidoo, 2001; Tomar et al., 2003, they proved higher K+/Na+ ratio in salinity tolerance plants under salinity conditions.
Bacterial-Am fungal-legume tripartite symbiosis clearly increased the tolerance of cowpea to salt stress and therefore constituted an alternative method to reduce cowpea plant stress caused by soil salinization. Studies of tripartite symbioses are still in their infancy; more research is also needed to come close to better understanding of the interactions between Am fungi and other NFB for the development of sustainable management of soil salinity.
Table 1b.
One way ANOVA for height, dry weight and root/shoot ratios of cowpea plants inoculated with single and dual NFB and VA fungi under salinity stress
* = significant differences (P < 0.05) ** = significant differences (P < 0.01).
Table 2b.
One way ANOVA for nodule, nitrogen, protein and nitrogenase of cowpea plants inoculated with single and dual NFB and VA fungi under salinity stress
* = significant differences (P < 0.05) ** = significant differences (P < 0.01)
Table 3b.
One way ANOVA for % of VA infection phosphorus and phosphatases of cowpea plants inoculated with single and dual NFB and VA fungi under salinity stress
* = significant differences (P < 0.05) ** = significant differences (P < 0.01).
Table 4b.
One way ANOVA for nodule, nitrogen, protein and nitrogenase of cowpea plants inoculated with single and dual NFB and VA fungi under salinity stress
* = significant differences (P < 0.05) ** = significant differences (P < 0.01).
References
- 1.Adiku G, Renger M, Wessolek G, Facklam M, Hech-Bucholtz C. Stimulation of dry matter production and seed yield of common beans under varying soil water and salinity conditions. Agric Water Manag. 2001;47:55–68. [Google Scholar]
- 2.Al-Karaki GN, Hammad R, Rusan M. Response of two tomato cultivars differing in salt tolerance to inoculation with mycorrhizal fungi under salt stress. Mycorrhiza. 2001;11:41–47. [Google Scholar]
- 3.Allen SF, Grimshaw HF, Rowl AB. Chemical analysis. In: Moore PD, Chapman SB, editors. Methods in plant ecology. Blackwell Oxford; 1984. pp. 185–344. [Google Scholar]
- 4.Bayuelo-Jimenez J, Debouck DG, Lynch JP. Growth, gas exchange, water relations and ion composition of Phaseolus species grown under saline conditions. Field Crop Res. 2003;80:207–222. [Google Scholar]
- 5.Biró B, Köves-Péchy K, Vörös I, Takács T, Eggenberger P, Strasser RJ. Interrelations between Azospirillum and Rhizobium nitrogen-fixers and arbuscular mycorrhizal fungi in the rhizosphere of alfalfa in sterile, AMF-free or normal soil conditions. App Soil Ecol. 2000;15:159–168. [Google Scholar]
- 6.Bohrer G, Kagan-Zur V, Roth-Bejerano N, Ward D, Beck G, Bonifacio E. Effects of different Kalahari-desert VA mycorrhizal communities on mineral acquisition and depletion from the soil by host plants. J Arid Environ. 2003;55:193–208. [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing theprincipal of protein-dye binding. Annal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Burke DJ, Hamerlynck EP, Hahn D. Interactions between the salt marsh grass Spartina patens, arbuscular mycorrhizal fungi and sediment bacteria during the growing season. Soil Biol Biochem. 2003;35:501–511. [Google Scholar]
- 9.Copeman RH, Martin CA, Stutz JC. Tomato growth in response to salinity and mycorrhizal fungi from saline or nonsaline soil. Hort Sci. 1996;31:341–344. [Google Scholar]
- 10.Cordovilla MP, Ligero F, Lluch C. The effect of salinity on N fixation and assimilation in Vicia faba. J Exp Bot. 1994;45:1483–1488. [Google Scholar]
- 11.Cordovilla MP, Ocana A, Ligero F, Lluch C. Salinity effects on growth analysis and nutrient composition in four grain legumes-Rhizobium symbiosis. J Plant Nutr. 1995;18:1595–1609. [Google Scholar]
- 12.Csonka LN, Hanson AD. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Plant Physiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- 13.Delgado MJ, Ligero F, Lluch C. Effects of salt stress on growth and nitrogen fixation by pea, faba-bean, common bean and soybean plants. Soil Biol Biochem. 1994;26:371–376. [Google Scholar]
- 14.Dobereiner J. Influence of environmental factor on the occurrence of S. lipoferum in soil and roots In: Environmental role of N2 ñfixing blue green algae and symbiotic bacteria. Ecol Bull (Stockholm) 1978;26:343–352. [Google Scholar]
- 15.El-Mokadem MT, Helemish FA, Abdel-Wahab SM, Abou-El-Nour MM. Salt response of clover and alfalfa inoculated with salt tolerant strains of Rhizobium. Ain Shams Sci Bull. 1991;28B:441–468. [Google Scholar]
- 16.Feng G, Zhang FS, Li XL, Tian CY, Tang C, Rengel Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza. 2002;12:185–190. doi: 10.1007/s00572-002-0170-0. [DOI] [PubMed] [Google Scholar]
- 17.Fischer S, Miguel MJ, Mori GB. Effect of root exudates on the exopolysaccharide composition and the lipopolysaccharide profile of Azospirillum brasilense Cd under saline stress. FEMS Microbiol Lett. 2003;219:53–62. doi: 10.1016/S0378-1097(02)01194-1. [DOI] [PubMed] [Google Scholar]
- 18.Georgiev GI, Atkias CA. Effects of salinity on N2 fixation, nitrogen metabolism and export and diffusive conductance of cowpea root nodules. Symbiosis. 1993;15:239–255. [Google Scholar]
- 19.Gianinazzi-Pearson V, Gianinazzi S. Enzymatic studies on the metabolism on Vesicular arbuscular mycorrhiza, Effect of mycorrhiza formation and phosphorus nutrition on soluble phosphatase activities in onion roots. Physiol Veg. 1976;14:833–841. [Google Scholar]
- 20.Giller KE, Cadish G. Future benefits from biological nitrogen fixation: an approach to agriculture. Plant Soil. 1995;174:255–277. [Google Scholar]
- 21.Glenn EP, Brown JJ, Blumwald E. Salt tolerance and crop potential of halophytes. Crit Rev Plant Sci. 1999;18:227–255. [Google Scholar]
- 22.Hardy R, Bums R, Holsten R. Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol Biochem. 1973;5:47–81. [Google Scholar]
- 23.Hirrel MC. The effect of sodium and chloride salts on the germination of Gigaspora margaria. Mycology. 1981;43:610–617. [Google Scholar]
- 24.Jackson ML. Soil chemical Analysis. New Delhi, India: Prence Hall of India Ltd; 1967. [Google Scholar]
- 25.Jackson NF, Miller RH, Forkiln RE. Soil chemical analysis. New Delhi: Prentic-Hall of India Private & Ltd; 1973. 2nd Indian Rep. [Google Scholar]
- 26.Jha DK, Sharma GD, Mishra RR. Mineral nutrition in the tripartite interaction between Frankia, Glomus and Alnus at different soil phosphorus regimes. New Phytol. 1993;123:307–311. [Google Scholar]
- 27.Johansson JF, Paul LR, Finlay RD. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol Ecol. 2004;48:1–13. doi: 10.1016/j.femsec.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Juniper S, Abbott L. Vesicular-arbuscular mycorrhizas and soil salinity. Mycorrhiza. 1993;44:45–57. doi: 10.1007/s00572-006-0046-9. [DOI] [PubMed] [Google Scholar]
- 29.Khan M, Unger I. Effect of salinity on growth water relation and ion accumulation of subtropical perennial Halophyte, Atriplex graffithii Var. stocksii. Annals of Bot. 2000;85:225–232. [Google Scholar]
- 30.Lesueur D, Ingleby K, Odee D, Chamberlain J., Wilson J, Manga TT, Sarrailh JM, Pottinger A. Improvement of forage production in Calliandra calothyrsus: methodology for the identification of an effective inoculum containing Rhizobium strains and arbuscular mycorrhizal isolates. J Biotech. 2001;91:269–282. doi: 10.1016/s0168-1656(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 31.Maayhuis FJM, Amtmann A. K+ Nutrition and Na+ toxicity: The basic of cellular K+/Na+ ratios. Annals Botany. 2004;84:123–133. [Google Scholar]
- 32.Marschner H. Mineral nutrition of higher plants. New York: Academic press; 1995. Saline soil; pp. 567–680. [Google Scholar]
- 33.McMillen BG, Juniper S, Abbott LK. Inhibition of hyphal growth of a vesicularñarbuscular mycorrhizal fungus in soil containing sodium chloride limits the spread of infection from spores. Soil Biol Biochem. 1998;30:1639–1646. [Google Scholar]
- 34.Minerdi D, Fani R, Gallo R, Boarino A, Bonfante P, Munns R. Physiological processes limiting plant growth in saline soils some dogmas and hypotheses. Plant Cell Environ. 1993;16:1524. [Google Scholar]
- 35.Minerdi D, Fani R, Gallo R, Boarino A, Bonfante P. Nitrogen Fixation genes in an endosymbiotic Burkholderia strain. Appl Environ Microbiol. 2000;67:725–732. doi: 10.1128/AEM.67.2.725-732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muscolo A, Panuccio MR, Sidari M. Effects of salinity on growth, carbohydrate metabolism and nutritive properties of KiKuyu grass (Pennisetum clandestinum Hochst) Plant Science. 2003;164:1103–1110. [Google Scholar]
- 37.Naidoo G, Naidoo Y. Effect of salinity and nitrogen on growth, ion relations and proline accumulation in Triglochin bulbosa. Wetland Ecol Managem. 2001;9:491–497. [Google Scholar]
- 38.Netondo GW, Onyango JC, Beck E. Sorghum and salinity. Crop Science. 2004;44:797–805. [Google Scholar]
- 39.Okon Y, Vanderleyden J. Root-associated Azospirillum species can stimulate plants. ASM News. 1997;63:366–370. [Google Scholar]
- 40.Pfeiffer CM, Bloss HE. Growth and nutrition ofguayule (Parthenium argentatum) in a saline soil as influenced by vesicularñarbuscular mycorrhiza andphosphorus fertilization. New Phytol. 1988;108:315–321. doi: 10.1111/j.1469-8137.1988.tb04168.x. [DOI] [PubMed] [Google Scholar]
- 41.Phillips J, Hayman D. Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55:158–161. [Google Scholar]
- 42.Piper CS. Soil and plant analysis. New York: Inter. Sci. Publ.; 1950. [Google Scholar]
- 43.Puppi G, Azcon R, Hoflich G. Management of positive interactions of arbuscular mycorrhizal fungi with essential groups of soil microorganisms. In: Gianinazzi S, Schouepp H, editors. Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems. 1994. pp. 201–215. [Google Scholar]
- 44.Rabie GH, Al-Humiany A. Role of VA-mycorrhiza on the growth of cowpea plant and their associative effect with N2-fixing and P-solubilizing bacteria as biofertilizers in calcareous soil. Food Agric Environ. 2004;2:185–191. [Google Scholar]
- 45.Rabie GH. Influence of VA-mycorrhizal fungi and kinetin on the response of mungbean plants to irrigation with seawater. Mycorrhiza. 2005 doi: 10.1007/s00572-004-0345-y. (in press) [DOI] [PubMed] [Google Scholar]
- 46.Rao DLN. Microbial Interactions in Agriculture and Forestry. vol. 1. Enfield, USA: Science Publishers; 1998. Biological amelioration of salt-affected soils; pp. 21–238. [Google Scholar]
- 47.Rao AV, Tak R. Growth of different tree species and their nutrition uptake in limestone mine spoil as influenced by arbuscular mycorrhizal (AM) fungi in India arid zone. J Arid Environ. 2002;51:113–119. [Google Scholar]
- 48.Ruiz-Lozano JM, Azcon R, Gomez M. Alleviation of salt stress by arbuscular-mycorrhizal Glomus species in Lactuca sativa plants. Physiol Plant. 1996;98:767–772. [Google Scholar]
- 49.Saini VK, Bhandari SC, Tarafdar JC. Comparison of crop yield, soil microbial C, N and P, N-fixation, nodulation and mycorrhizal infection in inoculated and noninoculated sorghum and chickpea crops. Field Crops Res. 2004;89:39–47. [Google Scholar]
- 50.Singh RP, Choudhary A, Gulati A, Dahiya HC, Jaiwal PK, Sengar RS. Response of plants to salinity in interaction with other a biotic and factors. In: Jaiwal PK, Singh RP, Gulati A, editors. Strategies for Improving Salt tolerance in Higher Plants. Enfield, USA: Science Publishers; 1997. pp. 25–39. [Google Scholar]
- 51.Steel RGD, Torrie IH. Principals and procedures of statistics. New York: McGraw Hill; 1960. [Google Scholar]
- 52.Tian C, He X, Zhong Y, Chen J. Effects of VA mycorrhizae and Frankia dual inoculation on growth and nitrogen fixation of Hippophae tibetana. Forest Ecol Managem. 2002;170:307–312. [Google Scholar]
- 53.Tain CY, Feng G, Li XL, Zhang FS. Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. Appl Soil Ecol. 2004;26:143–148. [Google Scholar]
- 54.Tomar OS, Minhas PS, Sharma VK, Gupt RJK. Response of nine forage grasses to saline irrigation and its schedules in a semi-arid climate of north-west India. J Arid Environ. 2003;55:533–544. [Google Scholar]
- 55.Trouvelot A, Kough J, Gianinazzi-Pearson V. Netical aspects of mycorrhizae. Paris: Institut National de la Recherche Agronomique. Press; 1986. Measure des taux de mycorhization VA d'UN system radiculaire. Recherche de methode d'estimation ayant une signification fonctionnelle; pp. 217–221. [Google Scholar]
- 56.Valdes M, Sanchez-Francia D. Response of Alnus and Casuarina to endomycorrhizal inoculation. Rev Mexicana Microbiol. 1996;12:65–67. [Google Scholar]
- 57.Veatch ME, Smith SE, Vandemark F. Shoot biomass productions of Medicago truncatula Exposed to NaCl. Crop Sci. 2004;44:1008–1013. [Google Scholar]
- 58.Yano-Melo AM, Saggin OJ, Maia LC. Tolerance of mycorrhized banana (Musa sp. cv. Pacovan) plantlets to saline stress. Agric Ecosyst Environ. 2003;95:343–348. [Google Scholar]
- 59.Zahran HH, Abu-Gharbia MA. Development and structure of bacterial root-nodules of two Egyptian cultivars of Vicia faba L. under salt and water stresses. Bull Fac Sci Assiut Univ. 1995;24:1–10. [Google Scholar]
- 60.Zahran HH. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Molecular Biol Rev. 1999;63:968–989. doi: 10.1128/mmbr.63.4.968-989.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zandavalli RB, Dillenburg LR, Paulo VD. Growth responses of Araucaria angustifolia (Araucariaceae) to inoculation with the mycorrhizal fungus Glomus clarum. Appl Soil Ecol. 2004;25(3):245–255. [Google Scholar]
- 62.Zou N, Dort PJ, Marcar NE. Interaction of salinity and rhizobial strains on growth and N2 fixation by Acacia ampliceps. Soil Biol Biochem. 1995;27:409–413. [Google Scholar]


