Abstract
HIV-1 persists in a latent state in resting CD4+ T lymphocytes of infected adults despite prolonged highly active antiretroviral therapy (HAART). To determine whether a latent reservoir for HIV-1 exists in infected children, we performed a quantitative viral culture assay on highly purified resting CD4+ T cells from 21 children with perinatally acquired infection. Replication-competent HIV-1 was recovered from all 18 children from whom sufficient cells were obtained. The frequency of latently infected resting CD4+ T cells directly correlated with plasma virus levels, suggesting that in children with ongoing viral replication, most latently infected cells are in the labile preintegration state of latency. However, in each of 7 children who had suppression of viral replication to undetectable levels for 1–3 years on HAART, latent replication-competent HIV-1 persisted with little decay, owing to a stable reservoir of infected cells in the postintegration stage of latency. Drug-resistance mutations generated by previous nonsuppressive regimens persisted in this compartment despite more than 1 year of fully suppressive HAART, rendering untenable the idea of recycling drugs that were part of failed regimens. Thus the latent reservoir for HIV-1 in resting CD4+ T cells will be a major obstacle to HIV-1 eradication in children.
Introduction
The success of combination therapy for the treatment of HIV-1 infection has resulted in a focus on sources of persistent HIV-1 in treated individuals. HIV-1 exists in different forms in several compartments with different rates of turnover. HIV-1 is present as free virions in plasma or bound to follicular dendritic cells, as integrated provirus in productively infected activated CD4+ T cells and macrophages, and in latent forms in resting CD4+ T cells (1). In resting CD4+ T cells, the virus is found in 2 distinct latent forms: a labile, cytoplasmic form consisting of reverse-transcribed, unintegrated HIV-1 DNA (2) and a second and more stable integrated form (3). The labile, unintegrated form is found in recently infected resting CD4+ T cells and is thought to result from a block in viral replication in resting lymphocytes, possibly at the level of nuclear import of the preintegration complex (2, 4). Whereas some resting CD4+ T lymphocytes are permissive to entry by HIV-1, T-cell activation is necessary for the subsequent steps in the virus life cycle, including nuclear import and integration of HIV-1 into the host genome. The preintegration form of HIV-1 latency is relatively unstable, with an estimated half-life as short as 6 days (J. Blankson and R.F. Siliciano, unpublished data).
A second, more stable form of latency is seen in resting memory CD4+ T cells with integrated HIV-1 DNA. The precise mechanisms involved in the generation of the pool of latently infected resting memory CD4+ T lymphocytes with integrated provirus are unknown. One possibility is that these cells are generated when infected, activated CD4+ lymphoblasts revert to a resting state having survived the cytopathic effects of HIV-1 and the host cytolytic effector mechanisms (3). These cells would carry an integrated copy of the HIV-1 genome that is not fully expressed in cells in a resting state.
Studies of the kinetics of the free virus turnover in the plasma of HIV-1–infected adults and children who are treated with highly active antiretroviral therapy (HAART) have shown similar decay patterns. Within days of initiating HAART, a rapid decay in plasma HIV-1 RNA is observed. This rapid decay is attributed to inhibition of the generation of newly infected CD4+ lymphoblasts and the rapid turnover of both free virus in the plasma (t1/2 < 6 hours) and the productively infected CD4+ lymphoblasts that produce most of the plasma virus (t1/2 = 1 day) (5–9). A second, slower phase decay in plasma HIV-1 RNA becomes apparent several weeks after the initiation of HAART and most likely reflects the turnover of compartments with longer half-lives, such as infected macrophages (10, 11).
Whereas combinations of antiretroviral agents are effective at suppressing viral replication to below the detection limits of standard plasma HIV-1 RNA assays and PBMC culture assays, they are ineffective at eliminating latent forms of HIV-1. HIV-1 has been shown in recent studies in adults (12–14) to persist latently in replication-competent forms in resting CD4+ T lymphocytes despite prolonged suppressive treatment with HAART. The persistence of HIV-1 in this compartment suggests that these cells may contribute to a very slow third-phase decay that may reflect the longer half-life of memory CD4+ T lymphocytes (15, 16). Whereas the kinetics of the decay of free virus in the plasma of children treated with HAART have been shown to be similar to those observed in adults (8, 9), the existence of a latent cellular reservoir for HIV-1–infected children has not been established, and the kinetics and the evolution of latent HIV-1 in the resting CD4+ T lymphocytes in children treated with HAART have not been reported.
A priori, there are several reasons to believe that the dynamics of the latent reservoir might be different in children with perinatally acquired HIV-1 infection than in adults. In children who acquire HIV-1 from their mothers, infection is established in the setting of a developing T-cell repertoire, in the presence of high levels of viral replication, and in the presence of deficient cytotoxic T-cell activity (17–22), and it might even be expected that the frequency of resting CD4+ T cells with integrated HIV-1 would be higher in infected children. In adults, most of the latent virus in resting CD4+ T cells resides in cells with a memory phenotype (23). At birth, memory cells are absent. During the first decade of life, memory cells are generated and come to comprise about 50% of the total T-cell population by age 15 (24). Because in perinatally infected children the memory cell compartment is actively generated in the setting of ongoing viral replication, there was concern that a large reservoir of latently infected resting memory CD4+ T cells would be established. The present study was undertaken to establish the presence of a latent reservoir for HIV-1 in infected children, to address the hypothesis that the frequency of latently infected cells might be higher in children than in adults, and to determine the kinetics and evolution of latent HIV-1 in resting CD4+ T lymphocytes in children who were rendered aviremic by HAART.
Methods
Study population.
Children with perinatally acquired HIV-1 infection were eligible. Infection was diagnosed in children older than 18 months of age by an HIV-1 ELISA with Western blot confirmation. In children who were less than 18 months of age, infection was confirmed by the detection of plasma HIV-1 RNA. Informed consent was obtained from the parent or guardian for all study subjects, and assent was obtained from those children who were aware of their diagnosis.
HIV-1 plasma RNA.
Plasma HIV-1 RNA was measured by HIV-1 RNA PCR (Amplicor HIV-1 Monitor Test; Roche Diagnostic Systems, Branchburg, New Jersey, USA).
Culture assay for latently infected cells.
Resting CD4+ T cells were isolated as described previously (3, 12, 23), using mAb/magnetic bead depletion of monocytes, natural killer (NK) cells, B cells, CD8+ T cells, and activated CD4+ T cells, followed by sorting for small CD4+ HLA-DR–cells. The purity was determined by flow-cytometric analysis of the sorted cells for the expression of CD4 and the absence of HLA-DR. Purities were typically greater than 97%. For children in whom it became difficult to culture HIV-1 from 2 to 5 million purified resting CD4+ lymphocytes, the cell-sorting step was omitted and bead-depleted cells (purity ∼90%, data not shown) were assayed. Latently infected cells were detected by a previously described (5, 23) limiting dilution culture assay in which purified CD4+ HLA-DR–cells were activated with phytohemagglutinin and irradiated allogeneic PBMC from an HIV-1 seronegative donor in medium containing IL-2, then cocultured with CD8-depleted CD4+ lymphoblasts from an HIV-1 seronegative donor. Supernatants were collected on day 14 and analyzed for p24 antigen by ELISA (Coulter Electronics Ltd., Hialeah, Florida, USA).
Mathematical and statistical methods.
Virus culture data were analyzed by a maximum likelihood method (25). The frequencies were expressed as infectious units per million (IUPM) resting CD4+ cells. In cases where all determinations were negative, an upper bound on the infected cell frequency was estimated by making the conservative assumption that the next highest cell concentration would be positive. Correlations between IUPM and plasma RNA levels (plotted on a logarithmic scale) and percent of CD4 were made using Spearman rank correlation methods and the Mann-Whitney-Wilcoxon test.
Sequencing of the HIV-1 pol gene.
HIV-1 pol sequences were amplified by PCR from cell-associated DNA from cultures of CD4+ lymphoblasts infected with viruses isolated from latently infected cells. Genomic DNA was isolated using standard methods (Gentra Systems Inc., Minneapolis, Minnesota, USA). The pol gene was amplified using published primers with PRT O5 primer (nucleotides 2008 to 2031; 5′GCCCCTAGGAAAAAGGGCTGTTGG3) and OUT3′ (nucleotides 4263 to 4295; 5′CATTGCTCTCCAAT-TACTGTGATATTTCTCATG3′) or PRT15 (nucleotides 2057 to 2080; 5′ TGAAAGATTGT-ACTGAGAGACAGG3′) and IN3 (nucleotides 4212 to 4246; 5′TCTATTCCATCTAAAA-ATAGTACTTTCCTGATTCC3′). The positions of the primers are numbered according to the pol gene of the HXB2R isolate (http://hiv-web.lanl.gov/NUM-HXB2/HXB2.MAIN.html). The DNA samples were heated to 94°C for 3 minutes, then 30 amplification cycles were performed (94°C for 30 seconds, 57°C for 30 seconds, 68°C for 2 minutes and 15 seconds) followed by an incubation at 68°C for 5 minutes. Bands were gel purified using QIAquick PCR purification kit (QIAGEN, Valencia, California, USA) and cloned into a pCR-Blunt vector (Invitrogen Corp., Carlsbad, California, USA) or pCR-Blunt11-TOPO (Invitrogen). Plasmid DNA purified using the QIAGEN purified plasmid DNA kit was sequenced on a DNA sequencer (ABI prism model 377; Perkin Elmer Applied Biosystems, Foster City, California, USA) using the following sequencing primers for forward sequencing (the SP6 promoter, or Prot5′ CAACT-CCCTCTCAGAAGCAGGAGCCC, and RT5′AAATCCATACAATACTCCAGTATTTGC) and the following antisense primers for reverse sequencing (T7 promoter, or 3′Prot GCAAATGGAGTATTGTATGGATTT, and 3RT CTGTATGTCATTGACAGTCCA or TCTGTATGTCATTGACAGTCC). For the isolates in which the full-length pol gene could not be amplified, the reverse transcriptase (RT) and/or the protease regions of the pol gene were amplified separately using the published primers for the RT (26) and the protease (27) genes. Sequence validation was carried out by recommended methods (28). Sequencing was performed on viral isolates that represent distinct biological clones obtained with a limiting dilution culture technique. Basic local alignment search tool (BLAST) searches of the Genbank revealed that none of the sequences matched laboratory or patient isolates published previously. Phylogenic analysis by standard methods (29–31) revealed the expected clustering of sequences of distinct biological clones obtained from individual patients. Observed resistance mutations were consistent with treatment history of individual patients. These sequences have been submitted to Genbank (accession numbers AF242321–AF242346).
Results
Clinical features.
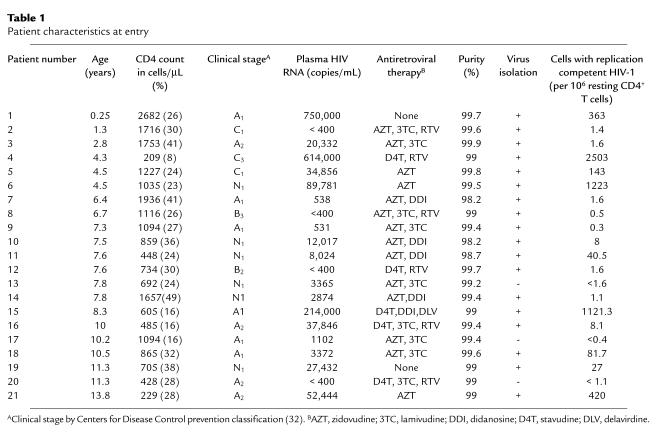
Table 1 summarizes the characteristics of the 21 children enrolled the initial cross-sectional study of the latent reservoir for HIV-1. The median age at entry was 7.6 years (range 0.25–13.8 years). The majority (85%) of children had mild to moderate HIV-1 disease as defined by the Centers for Disease Control (CDC) classification (33). For the entire cohort, the median RNA level at entry was 5,698 copies/mL (range < 400 to > 750,000), the median CD4+ cell count was 950 cells/mm3 (range 209–2682 cells/mm3), and the median CD4 percentage was 28 (range 8–49). Seventeen children (80%) had levels of plasma HIV-1 RNA of more than 400 copies/mL at entry, and 19 children were receiving antiretroviral therapy. Two children had received no antiretroviral therapy at entry. For the majority of children (70%), previous therapy consisted of 1–2 nucleoside RT inhibitors. Six children were receiving combination therapy with 2 nucleoside analogues and the protease inhibitor ritonavir.
Table 1.
Patient characteristics at entry
A subset of 9 children in the initial cohort achieved durable suppression of viral replication on HAART and were followed longitudinally to determine whether there was time-dependent decay in the latent reservoir. Included in this group were 6 children (patients 7, 10, 11, 14, 15, and 21) who were started on HAART and followed longitudinally. Before the initiation of HAART, the median age of the 6 children in this group was 7.7 years, the median CD4+ cell count was 732 cells/mm3, and the median viral load was 10,020 copies/mL. Three additional children (patients 2, 8, and 12), who at entry had been receiving HAART for 4, 16, and 8 months, respectively, and who had sustained suppression of viral replication to undetectable levels, were also followed longitudinally for 17–21 months.
Recovery of replication-competent HIV-1 from the resting CD4+ lymphocytes of infected children.
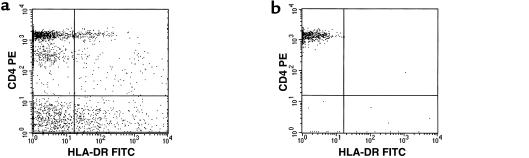
To establish the presence of a latent reservoir for HIV-1 in children with perinatally acquired HIV-1 infection, virus culture assays were carried out on highly purified populations of resting CD4+ lymphocytes (more than 98% pure; see Table 1 and Figure 1). In 18 of the 21 children, sufficient numbers of resting CD4+ lymphocytes (> 2.5 × 106) were obtained for culture. Replication-competent HIV-1 was recovered from the resting CD4+ T lymphocytes of all 18 children. Because HIV-1 does not replicate in resting CD4+ T cells, any virus recovered by activation of the resting cells can be considered to have come from a latent reservoir in resting CD4+ T cells. These results therefore establish the existence of this cellular reservoir in children.
Figure 1.
Representative flow cytometric analysis of CD4 and HLA-DR antigen expression on monocyte-depleted PBMCs before (a) and after (b) sequential magnetic bead depletion and cell sorting.
The frequency of latently infected resting CD4+ T lymphocytes and its correlation with plasma HIV-1 RNA levels and percentage of CD4+ lymphocytes.
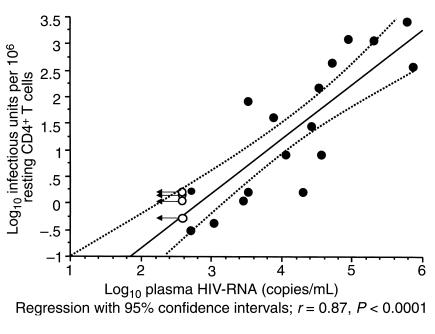
In children not yet on HAART, the frequencies of resting CD4+ T lymphocytes harboring latent, replication-competent HIV-1 ranged over 4 logs (0.3–2503 per 106 resting CD4+ cells; geometric mean frequency = 10/106 cells). The frequencies correlated directly with levels of plasma HIV-1 RNA (r = 0.86, P < 0.0001; Figure 2) and inversely with the percentage of CD4+ lymphocytes (r = –0.60, P < 0.005). The culture assay used to detect latent HIV-1 can detect cells in the preintegration state of latency and cells in the more stable postintegration state of latency. Previous studies in adults suggest that in patients who are viremic, most of the virus present in resting CD4+ T cells is in the preintegration state of latency (23, 33). The direct correlation between plasma HIV-1 RNA levels and the frequency of latently infected resting CD4+ cells shown in Figure 2 suggests that when the viral load drops to the limit of detection of the most sensitive assays (20 copies/mL), then the frequency of latently infected cells should be 0.03 IUPM or lower. However, as shown below, the frequency of latently infected cells remains above 0.1 IUPM even in children who have had long-term suppression of viral replication on HAART. This is due to the contribution of cells with the stable integrated form of HIV-1 DNA.
Figure 2.
Correlation between the frequency of latently infected resting CD4 T cells (IUPM) and the plasma HIV-1 RNA (copies/mL). Closed symbols represent children who had detectable levels of plasma HIV-1 RNA at study entry. Open symbols represent children who had undetectable (< 400 copies/mL) levels of plasma HIV-1 RNA at the time of study entry. Values are arbitrarily plotted at the upper bound of the plasma virus assay at 400 copies/mL.
Effect of HAART on the frequencies of latently infected resting CD4+ lymphocytes.
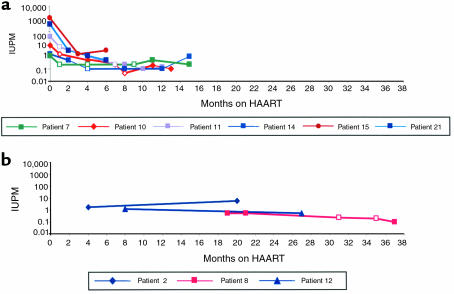
Longitudinal analysis of the effects of HAART on the size and turnover of the pool of latently infected resting CD4+ lymphocytes was carried out in 6 children (patients 7, 10, 11, 14, 15, and 21) who were tested before and after the initiation of HAART. A striking 2-phase decline in the frequency of latently infected resting CD4+ lymphocytes was observed (Figure 3a). Within 1 to 3 months of initiation of HAART, the frequency of latently infected resting CD4+ lymphocytes decreased by 1 log or more. The rapid initial decline in the proportion of resting cells with latent HIV-1 likely is due to the decay of the labile preintegration form of HIV-1 in recently infected, resting CD4+ T lymphocytes (23, 33; J. Blankson and R.F. Siliciano, unpublished data). Four of the 6 children (patients 7, 10, 11, 12, and 14) maintained suppression of viral replication (< 400 copies/mL) for 1 year or more on HAART. All 4 children had decreases in the CD8 percentages (range 6–12% decrease). In these 4 children, within 1 to 8 months of therapy with HAART it became difficult to culture HIV-1 at 1 or more time points using the standard input of 2.5 million purified resting CD4+ T cells. This result reflects that fact that the frequency of resting CD4+ T cells with a stable form of latent infection is low and close to the limits of detection of the assay. To establish that HIV-1 persisted in this compartment in the children who maintained suppression of viral replication, it was important to obtain higher input numbers of resting CD4+ T lymphocytes for culture analysis. Because of the limitation in the amount of blood that could be obtained from the children, increasing the input numbers of resting CD4+ T lymphocytes required the use of cells purified by magnetic bead depletion alone (90% pure). Replication-competent HIV-1 was recovered in all 4 children after 9–11 months of HAART using the higher input numbers of resting cells. It is unlikely that the virus detected was present in the low number of contaminating activated T cells because the frequency of infected activated CD4+ T cells is also extremely low (12, 23). In repeat analysis, after 12–15 months of HAART, replication-competent HIV-1 could be readily recovered from highly purified (> 98%) resting CD4+ T lymphocytes in each of the 4 children, providing definitive confirmation that replication-competent HIV-1 persisted in resting CD4+ T cells in these patients despite 12–15 months of HAART.
Figure 3.
Decay in the frequency of latently infected resting CD4+ T lymphocytes (in IUPM) versus time on HAART in months. (a) Decay of latently infected T cells in 6 children followed longitudinally before and after the initiation of HAART. (b) Decay of latently infected T cells in 3 children studied after the initiation of HAART. Closed symbols represent the time points at which replication-competent HIV-1 was recovered from the resting CD4+ T lymphocytes. Open symbols represent time points at which viral cultures on resting CD4+ T lymphocytes were negative. An upper bound on the frequency of latently infected resting CD4+T lymphocytes was determined as described.
HIV-1 persistence despite HAART and increases in CD4+ T lymphocytes in HIV-1–infected children.
Recent studies have shown that HIV-1 persists in the resting CD4+ T lymphocytes of infected adults treated with prolonged courses (up to 40 months) of suppressive antiretroviral therapy (12–14, 34). To determine whether HIV-1 also persists in a replication-competent form in children treated with HAART for more than 1 year, we measured, at 2 or more time points, the frequency of resting CD4+ lymphocytes harboring replication-competent virus in 3 children (patients 2, 8, and 12) who had suppression of viral replication to undetectable levels for 16–37 months (Figure 3b). Patient 2 started zidovudine, lamivudine, and ritonavir at 11 months of age after having received 6 weeks of zidovudine therapy for postnatal prophylaxis. On combination therapy, she has had continued suppression of viral replication to less than 400 copies/mL, and on 3 separate occasions when ultrasensitive assays were performed, plasma HIV-1 RNA levels were undetectable (< 20 copies/mL). At 15 months of age, the frequency of cells carrying replication-competent HIV-1 was 1.6 per million resting CD4+ T lymphocytes. At repeat evaluation, after 21 months of HAART, the frequency of latently infected resting CD4+ T lymphocytes was 5.3 per million resting CD4+ T cells. Thus, there was no decay in this reservoir despite prolonged suppression of viral replication. The percent of CD8+ cells in this patient has remained about 25% despite prolonged suppression of viral replication.
The other two children (patient 8 and patient 12) had marked increases (10-fold) in their CD4+ T lymphocyte counts while on protease-inhibitor therapy. Patient 8 was started on combination therapy with ritonavir 19 months before the first analysis. At the time therapy was initiated, his CD4+ cell count was 9 cells/mm3 and his plasma HIV-1 RNA was 207,554 copies/mL. Since the initiation of HAART, the patient has maintained undetectable levels of plasma HIV-1 RNA (less than 400 copies/mL) and has had levels of less than 20 copies/mL reported whenever ultrasensitive assays were done. He also experienced a dramatic increase in his CD4 cell count to 1,200 cells/mm3. Replication-competent virus was isolated from his resting CD4+ lymphocytes at 2 separate time points at 19 and 21 months of HAART despite increases in CD4 cells to normal levels in the presence of undetectable plasma HIV-1 RNA. However, in repeat studies at 31 and 35 months of HAART, culture of 5 million highly purified resting CD4+ T lymphocytes failed to yield HIV-1 replication-competent HIV-1. Subsequent analysis at 37 months using 14 million resting CD4+ T lymphocytes of 90% purity obtained by magnetic bead depletion alone yielded replication-competent HIV-1 at a frequency of 0.1 per million resting CD4+ T cells. These results demonstrate persistence of latent HIV-1 near the limit of detection, with possible slow decay. Whether the observed decrease actually represents a slow decay in the frequency of latently infected CD4+ lymphocytes will require further study over the course of the next several years. Patient 12 was started on HAART 8 months before entry into the study, at which time her CD4+ cell count was 79 cells/mm3 and her plasma HIV-1 RNA level was 214,760 copies/mL. While on HAART, the patient had an increase in her CD4 cell count to 734 cells/mm3, and her plasma HIV-1 RNA level remained undetectable. The percent of CD8+ T cells has remained constant. Virus was recovered from her resting CD4+ lymphocytes 8 months after the initiation of HAART at a frequency of 1.6 per million resting cells and again at 28 months after HAART at approximately the same frequency. Taken together, these results suggest that HIV-1 persists in the resting CD4+ lymphocytes of children treated with prolonged suppressive therapy with HAART and despite increases in CD4+ T cells to normal levels.
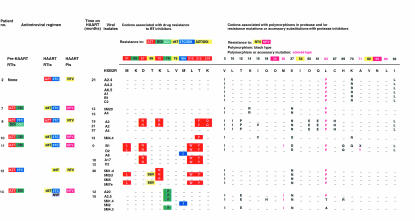
Analysis of drug-resistance mutations in viral isolates from latent reservoir.
The mechanisms involved in maintaining latent, replication-competent HIV-1 in resting CD4+ T lymphocytes at a stable frequency in treated adults are unclear. Possibilities include the persistence of cells in an inactive state for long periods of time or gradual renewal of the latent pool by low levels of ongoing viral replication (35–38). In the former case, the composition of the latent reservoir should not change on therapy and should reflect the virologic status before HAART. If the reservoir is maintained by ongoing viral replication, then the possible emergence of drug-resistant virus is a serious concern. We therefore analyzed viruses isolated from latently infected cells for the persistence or emergence of known drug-resistant mutations in the HIV-1 pol gene. Isolates from the 7 children who had suppression of viral replication for 1 year or more were sequenced. Figure 4 summarizes the relevant amino acid substitutions detected in the RT and protease region of the HIV-1 pol gene. None of the 7 children developed primary antiretroviral resistance substitutions at the amino acid positions 30, 54, 82, 88, or 90 that are reported to confer phenotypic resistance to the protease inhibitors used (nelfinavir or ritonavir). However, all 7 children had individual patient-specific sequence patterns at positions in the pol gene associated with natural polymorphisms recognized previously, including the L63P substitution that is a common polymorphism in protease inhibitor–naïve individuals and that is also an accessory substitution in some protease inhibitor–resistant viruses. One child (patient 2), whose primary antiretroviral regimen consisted of combination therapy with zidovudine, lamivudine, and ritonavir and who has had suppression of viral replication to less than 20 copies/mL, had no mutations associated with genotypic resistance to zidovudine or lamivudine. In contrast, 5 of the 6 children who were treated previously with incompletely suppressive regimens of zidovudine and didanosine and who had suppression of viral replication for 12–30 months on HAART had persistence of replication-competent HIV-1 harboring mutations conferring resistance to zidovudine and didanosine. These included mutations at positions 41, 67, 70, 210, 215, or 219 in the RT gene that confers high-level resistance to zidovudine and/or mutations at positions 74 or 184 that confer resistance to didanosine. In 2 children (patients 11 and 14) the M184I mutation was detected after 10 and 15 months of HAART, respectively. Because the M184I mutation is associated with drug resistance to both didanosine and lamivudine and both children received previous nonsuppressive therapy with didanosine, then were subsequently treated with a suppressive regimen that contained lamivudine, it is not possible to determine to which drug this mutation should be attributed. Interestingly, patient 12, who was treated previously with zidovudine and didanosine and subsequently received suppressive therapy with stavudine and ritonavir for 30 months, has had intermittent levels of plasma HIV-1 RNA between 30 to 50 copies over a 2-year period. In this child, none of the 4 HIV-1 isolates cultured after 30 months of suppressive therapy with ritonavir and stavudine had genotypic substitutions in the protease gene that confer resistance to ritonavir. However 2 of the 4 isolates contained amino acid insertions at position 69 [69 Ser- (Ser-Val)] reported to occur with treatment with zidovudine in combination with didanosine or zalcitabine and associated with high-level resistance to the nucleoside RT inhibitors (29, 39–41). The preservation of drug-resistance mutations selected by previous nonsuppressive therapy emphasizes the stability of the latent reservoir for HIV-1 in resting CD4+ T cells.
Figure 4.
HIV-1 RT and protease sequences from viral isolates cultured from the resting CD4+ T lymphocytes. Dashes represent amino acids that are unchanged from the reference sequence HXB2R. Blanks represent codons not determined. Numbers in the top row indicate RT codons associated with drug resistance and protease codons associated with drug resistance, common polymorphisms, or polymorphism and accessory substitutions to protease inhibitors. Mutations associated with resistance to antiretroviral drugs are shown in blocks of the appropriate color. Positions in protease at which polymorphisms occur are shown in black type. Known polymorphisms that are also seen as accessory substitutions contributing to resistance to ritonavir (RTV) or nelfinavir (NFV) are shown in colored type.
A particularly dramatic illustration of the stability of the latent reservoir is provided by the case of the child who has had the longest duration (37 months) of suppression of viral replication with HAART (patient 8). This child was treated with nonsuppressive antiretroviral regimens consisting of zidovudine, lamivudine, didanosine, and zalcitabine for 3.5 years before initiation of a suppressive HAART regimen consisting of zidovudine, lamivudine, and ritonavir. Failure of the earlier regimens was documented by the presence of high-level viremia at the time of initiation of HAART (207,554 copies/mL). Virus isolated from the latent reservoir at 19 and 21 months showed multiple mutations conferring resistance to zidovudine. However, wild-type virus was recovered from his resting CD4+ T cells after 37 months of HAART. Thus, in this patient who had clearly developed zidovudine-resistant virus and who is still on zidovudine, both zidovudine-sensitive virus and zidovudine-resistant virus can be found in the latent reservoir. The zidovudine-sensitive viruses may have entered the reservoir 6 years earlier, before the initiation of zidovudine therapy, whereas the zidovudine-resistant virus may have entered the reservoir 3–6 years earlier during the period of nonsuppressive therapy. Thus, this study demonstrates that both wild-type virus and drug-resistance virus selected by previous nonsuppressive therapy persist for long periods of time in latently infected resting CD4+ T cells of children on HAART whereas new drug-resistance mutations clearly attributable to the suppressive regimens do not appear.
Discussion
The purpose of this study was to demonstrate the existence and determine the size and turnover rate of the latent reservoir of resting CD4+ T lymphocytes in HIV-1–infected children. Before initiation of HAART, replication-competent HIV-1 was readily recovered from highly purified populations of resting CD4+ T lymphocytes of all children from whom sufficient cells were obtained for analysis. Thus, the reservoir of latently infected resting CD4+ T cells is present in HIV-1–infected children. This reservoir has a biphasic decay pattern after the initiation of HAART, with a rapid initial drop in the first 3 months of therapy followed by a second phase in which there is minimal decay. It is important to point out that the method currently used to detect the replication-competent forms of HIV-1 persisting in resting CD4+ T lymphocytes does not differentiate between the labile, cytoplasmic form and the stable, integrated form of persistent HIV-1 infection. The direct correlation observed between the frequency of resting CD4+ T lymphocytes harboring replication-competent HIV-1 and plasma HIV-1 RNA levels in children with plasma HIV-1 RNA more than 400 copies/mL suggests that many of the infected resting CD4+ T lymphocytes detected may be recently infected cells with the labile, unintegrated form of HIV-1. Because in vitro T-cell activation methods are used to recover the replication-competent forms of HIV-1 present in the resting CD4+ lymphocytes, it is uncertain what proportion of the resting cells harboring this form of HIV-1 would progress to productive HIV-1 infection in vivo. Nevertheless, this pool of latently infected resting CD4+ lymphocytes constitutes an inducible reservoir for productive HIV-1 infection in the setting of a developing T-cell repertoire and for the generation of the more stable postintegration form for HIV-1 existing in the resting memory CD4+ lymphocyte compartment. The rapid initial decay observed in the frequency of latent HIV-1 in resting CD4+ T lymphocytes early in the course of HAART that parallels the decay in plasma HIV-1 RNA probably reflects the decay of the preintegration form of HIV-1 latency in recently infected resting CD4+ T cells. In children with levels of HIV-1 RNA greater than 400 copies/mL, this form may be the predominant form of HIV-1 latency.
Previous studies in adults have demonstrated the presence of integrated HIV-1 DNA in resting CD4+ T lymphocytes (3, 23, 34). In the children who had successful suppression of viral replication to less than 400 copies/mL on HAART, the predominant form of HIV-1 latency in resting CD4+ is most likely this stable integrated form present in resting memory CD4+ T lymphocytes. Extrapolation of the observed relationship between viral load and the frequency of latently infected resting CD4+ T cells in children with plasma HIV-1 RNA greater than 400 copies/mL (Figure 2) suggests that at plasma HIV-1 RNA levels of less than 20 copies/mL, the contribution of the labile unintegrated form of HIV-1 latency should be no more than 0.1 IUPM resting CD4+ lymphocytes. The fact that in most children on HAART who have undetectable plasma virus the frequency of latently infected cells is in the range of 0.1–5 IUPM suggests that a stable form of latent infection is persisting. This is confirmed by our longitudinal studies, which demonstrate very little decay in this stable latent reservoir after the first 3 months of therapy, during which time the preintegration form of latency is undergoing decay.
In comparing the latent reservoir for HIV-1 in children and adults, the following conclusions can be drawn. First, the fraction of latently infected cells is similar. The mean frequency of latently infected resting CD4+ T lymphocytes in children treated with HAART was similar to that measured in treated adults (mean 0.4 vs. 0.8 per million resting CD4+ lymphocytes, respectively). Thus although children are establishing a pool of memory cells, the fraction of these cells that carry replication-competent HIV-1 does not appear to be significantly higher than in adults. Second, the frequency of resting CD4+ T cells harboring latent HIV-1 shows a biphasic decline following the initiation of therapy in both children (as shown here) and in adults (J. Blankson and R.F. Siliciano, unpublished results). After the rapid initial decay in the first 3 months of treatment, little subsequent decay is detected. Thus, as observed in adults, HIV-1 persists in a latent form in resting CD4+ T cells in children despite 1–3 years of HAART. The precise mechanisms involved in the generation of this latent reservoir are unknown; the occasional reversion of HIV-1–infected, activated T lymphocytes to a resting memory state with integrated HIV-1 is one plausible mechanism. Although the number of children who have had long-term suppression of HIV-1 replication on HAART is too low to permit a precise calculation of the decay rate of this latent pool, the data presented here strongly suggest that the extremely slow decay observed in adults (t1/2 = 44 months, ref. 34) will also apply to children with perinatally acquired HIV-1 infection.
The persistence of HIV-1 within the resting CD4+ lymphocyte compartment despite prolonged treatment with HAART and despite significant increases in CD4+ cells is consistent with the function of memory T lymphocytes to provide immunologic memory for prolonged periods. Analysis of the viral isolates cultured from this compartment in a group of children who were pretreated with nonsuppressive antiretroviral regimens showed the persistence, for up to 2 years, of drug-resistant substitutions selected by previous nonsuppressive antiretroviral therapies. The persistence of multiply drug-resistant, latent, replication-competent HIV-1 in resting CD4+ lymphocytes for up to 2 years of HAART suggests that the reuse of drugs used in previous nonsuppressive regimens may not be possible. However, we did not detect new substitutions clearly attributable to drugs in the HAART regimen. No new resistance substitutions to the protease inhibitors were observed even in isolates from the 2 children who had increases in plasma HIV-1 RNA to 230 and 438 copies/mL at the time the culture assay was performed and in the 1 child who had plasma HIV-1 RNA in the range of 30 to 50 copies/mL at several time points. Additional longitudinal studies are needed to determine the kinetics and evolution of latent HIV-1 in this cellular reservoir in children treated for longer periods and to determine the timing and impact of early treatment with HAART on the development of this cellular reservoir in children with perinatally acquired HIV-1 infection. However, the data presented here indicate that the latent reservoir for HIV-1 is established in infected children and will likely represent a major barrier to virus eradication that will have to be considered in all therapeutic strategies for the treatment of pediatric HIV-1 infection.
Acknowledgments
We wish to thank Susan Marvin and Mary Joyner for help in the recruitment of study participants; Tom Hall (North Carolina State University) for providing the sequence analysis program BioEdit; and the Pediatric AIDS Clinical Trials Group for providing the funding and structure that allowed the rigorous follow-up of the children on HAART. This work was supported by a Child Health Research Center award (P30HD27799) and a Doris Duke Charitable Foundation award to D. Persaud and a National Institutes of Health grant (AI-43222) to R.F. Siliciano.
References
- 1.Finzi D, Siliciano RF. Viral dynamics in HIV-1 infection. Cell. 1998;93:665–671. doi: 10.1016/s0092-8674(00)81427-0. [DOI] [PubMed] [Google Scholar]
- 2.Zack JA, et al. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, et al. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 4.Bukrinsky MI, et al. Active nuclear import of human immunodeficiency virus type 1 pre-integration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho DD, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 6.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 7.Wei X, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 8.Melvin AJ, et al. HIV-1 dynamics in children. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:468–473. doi: 10.1097/00042560-199904150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Luzuriaga K, et al. Dynamics of human immunodeficiency virus type 1 replication in vertically infected infants. J Virol. 1999;73:362–367. doi: 10.1128/jvi.73.1.362-367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perelson AS, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 11.Cavert W, et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 12.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 13.Chun TW, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 15.McLean AR, Michie CA. In vivo estimates of division and death rates of human T lymphocytes. Proc Natl Acad Sci USA. 1995;92:3707–3711. doi: 10.1073/pnas.92.9.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 17.Mofenson LM, et al. The relationship between serum human immunodeficiency virus type 1 (HIV-1) RNA level, CD4 lymphocyte percent, and long-term mortality risk in HIV-1-infected children. National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. J Infect Dis. 1997;175:1029–1038. doi: 10.1086/516441. [DOI] [PubMed] [Google Scholar]
- 18.Palumbo PE, et al. Viral measurement by polymerase chain reaction-based assays in human immunodeficiency virus-infected infants. J Pediatr. 1995;126:592–595. doi: 10.1016/s0022-3476(95)70357-8. [DOI] [PubMed] [Google Scholar]
- 19.Shearer WT, et al. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. N Engl J Med. 1997;336:1337–1342. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- 20.Pikora CA, Sullivan JL, Panicali D, Luzuriaga K. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J Exp Med. 1997;185:1153–1161. doi: 10.1084/jem.185.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luzuriaga K, Koup RA, Pikora CA, Brettler DB, Sullivan JL. Deficient human immunodeficiency virus type 1-specific cytotoxic T cell responses in vertically infected children. J Pediatr. 1991;119:230–236. doi: 10.1016/s0022-3476(05)80732-2. [DOI] [PubMed] [Google Scholar]
- 22.Luzuriaga K, et al. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J Immunol. 1995;154:433–443. [PubMed] [Google Scholar]
- 23.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 24.Hayward AR, Lee J, Beverley PCL. Ontogeny of expression of UCHL1 antigen on TcR1+ (CD4/8) and TcRd+ cells. Eur J Immunol. 1989;18:1653–1661. doi: 10.1002/eji.1830190430. [DOI] [PubMed] [Google Scholar]
- 25.Myers LE, McQuay LJ, Hollinger FB. Dilution assay statistics. J Clin Microbiol. 1994;32:732–739. doi: 10.1128/jcm.32.3.732-739.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richman DD, Guatelli JC, Grimes J, Tsiatis A, Gingeras T. Detection of mutations associated with zidovudine resistance in human immunodeficiency virus by use of polymerase chain reaction. J Infect Dis. 1991;164:1075–1081. doi: 10.1093/infdis/164.6.1075. [DOI] [PubMed] [Google Scholar]
- 27.Hertogs K, et al. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob Agents Chemother. 1998;42:269–276. doi: 10.1128/aac.42.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Learn GH, Jr, et al. Maintaining the integrity of human immunodeficiency virus sequence databases. J Virol. 1996;70:5720–5730. doi: 10.1128/jvi.70.8.5720-5730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond, J., Calef, C., Larder, B., Schinazi, R., and Mellors, J.W. 1998. Mutations in retroviral genes associated with drug resistance. In Human retroviruses and AIDS 1998: a compilation and analysis of nucleic acid and amino acid sequences. B.T. Korber et al., editors. Los Alamos National Laboratory. Los Alamos, NM. pp. III-36–III-79.
- 30.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1996;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 31.Felsenstein, J. 1999. PHYLIP (Phylogeny Inference Package). Version 3.572c. Department of Genetics, University of Washington, Seattle, WA.
- 32.Revised classification system for human immunodeficiency virus in children less than 13 years of age. MMWR Morb Mort Wkly Rep. 1994;43(RR-12):1–10. [Google Scholar]
- 33.Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 35.Furtado MR, et al. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med. 1999;340:1614–1622. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 36.Grossman Z, et al. Ongoing HIV dissemination during HAART. Nat Med. 1999;5:1099–1104. doi: 10.1038/13410. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 38.Dornadula G, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 39.Larder BA, et al. A family of insertion mutations between codons 67 and 70 of human immunodeficiency virus type 1 reverse transcriptase confer multinucleoside analog resistance. Antimicrob Agents Chemother. 1999;43:1961–1967. doi: 10.1128/aac.43.8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross L, Johnson M, Graham N, Shaefer M, St. Clair M. The reverse transcriptase codon 69 insertion is observed in nucleoside reverse transcriptase inhibitor-experienced HIV-1-infected individuals, including those without prior or concurrent zidovudine therapy. J Hum Virol. 1999;2:290–295. [PubMed] [Google Scholar]
- 41.Winters MA, et al. A 6-basepair insert in the reverse transcriptase gene of human immunodeficiency virus type 1 confers resistance to multiple nucleoside inhibitors. J Clin Invest. 1998;102:1769–1775. doi: 10.1172/JCI4948. [DOI] [PMC free article] [PubMed] [Google Scholar]