Abstract
To produce a novel antihypertensive angiotensin I-converting enzyme (ACE) inhibitor from yeast, a yeast isolate, designated G-14 showing the highest ACE inhibitory activity was obtained and identified as Malassezia pachydermatis based on morphological, biochemical and cultural characteristics. The maximal extracellular ACE inhibitor production was obtained from M. pachydermatis G-14 when the strain was cultured in YEPD medium containing 0.5% yeast extract, 3.0% peptone and 2.0% glucose at 30℃ for 24 h and the final ACE inhibitory activity was 48.9% under the above condition.
Keywords: Angiotensin I-converting enzyme inhibitor, Antihypertensive, Malassezia pachydermatis
Angiotensin I-converting enzyme (ACE, dipeptidyl carboxypeptidase I, kininase II, EC. 3.4.15.1) is a multifunctional, zinc-containing enzyme located in different tissues which plays a key physiological role in the control of blood pressure by virtue of rennin-angiotensin system (Fujita, 2000; Ondetti, 1982; Rencland, 1994). ACE converts the inactive decapeptide, angiotensin I, to the potent vasopressor octapeptide, angiotensin II, and inactivates bradykinin (Ukeda, 1991).
Several ACE inhibitors show antihypertensive effects and may also have beneficial effects on glucose and lipid metabolism (Choi, 2001; Oshima, 1979; Pal, 1995; Pollare, 1989), decreasing insulin requirements in diabetes and increasing exercise tolerance (Pollare, 1989; Gohlke, 1994; Tomiyama, 1994).
Since the original discovery of ACE inhibitors in snake venom (Elisseeva, 1971), captopril (d-3-mercapto-2-methylpranory-l-proline), enalapril and lisinopril, effective oral inhibitors, have been developed and are currently used as clinical antihypertensive drugs (Ondetti, 1977). However, even though synthetic ACE inhibitors including captopril are remarkably effective as antihypertensive drugs, they have certain side effects such as cough, allergies, taste disturbance, skin rashes, etc. Therefore, research and development on safer, innovative, and economical ACE inhibitors is necessary for the prevention and remedy of hypertension.
Many research groups have screened for novel ACE inhibitors from natural products and microbial sources (Demain, 1989) including Doratomyces putredinis, Nocardia orientalis, Streptomycetes, Actinomycetes, Actinomadura spp. Food-derived ACE inhibitory peptides have been isolated from food or enzymatic digestion of food proteins (Fujita, 2000) including gelatin (Ondetti, 1982), casein (Morigiwa, 1986), fish (Matsumura, 1993; Sugiyama, 1991; Sun, 1997), fig tree latex (Maruyama, 1989) and α-zein (Miyoshi, 1991). Other ACE inhibitors were found from sake and its by-products (Saito, 1994), Korean traditional rice wines and liquors (Kim, 2002), cereals and legumes (Rhyu, 1996), and microbes such yeasts (Kim, 2003) and Basidiomycetes (Lee, 2003).
In this paper, we described the isolation and characterization of a novel yeast that produced potent extracellular ACE inhibitor.
Materials and Methods
Yeast strains, enzyme and reagents
Several Meju yeasts (Lee, 1997), industrial yeasts and other yeasts were obtained from the Lab. of Biotechnology, Paichai University, Korea Culture Center of Microorganism (KCCM) and Korea Collection for Types Cultures (KCTC).
The angiotensin I-converting enzyme (ACE) used in this experiment was extracted from rabbit lung acetone powder (Sigma Chemical Co., St. Louise, MO., USA) and its activity was determined using Hippuric acid-Histidine-Leucine (Hip-His-Leu) (Sigma Chemical Co.) as substrate. One unit was defined as the amount catalyzing the formation of 1 µM hippuric acid from Hip-His-Leu in 1 minute at 37℃ under standard assay condition (Ukeda, 1991). Unless otherwise specified, all chemicals and solvents were analytical grade. Pepsin, trypsin, trifluoroacetic acid and acetonitril were purchased from Sigma Chemical Co.
Screening and identification of the extracellular ACE inhibitor-producing strain
Yeasts from several sources were inoculated in the YEPD medium (0.5% yeast extract, 3.0% peptone and 2.0% glucose) and cultured at 30℃ for 72 h. After centrifugation at 15,000×g for 15 min, ACE inhibitory activities of the supernatants were determined. On the other side, the cells were suspended in the phosphate buffer (pH 7.0), washed, resuspended in phosphate buffer (pH 7.0), and then disrupted by glass bead. After centrifugation at 15,000×g (for 10 min at 4℃), ACE inhibitory activities of the cell free extract were determined.
Morphological, biochemical and cultural characteristics of the selected yeasts were investigated according to Taxonomy and Methods for the Identification of Microorganisms and Yeasts (Hasegawa, 1984) and The Yeast (Kreger-van, 1984).
Assay for ACE inhibitory activity
The activity of ACE inhibition was assayed by a modification of the method of Cushman and Cheung's (Ariyosh, 1993). A mixture containing a 100 mM sodium borate buffer (pH 8.3), 300 mM NaCl, 3 unit of ACE and an appropriate amount of the inhibitor solution was preincubated for 10 min at 37℃. The reaction was initiated by adding 50 µl of Hip-His-Leu at a final concentration of 5 mM, and terminated after 30 min of incubation by adding 250 µl of 1.0 N HCl. The hippuric acid liberated was extracted with 1 ml of ethyl acetate, and 0.8 ml of the extract was evaporated. The residue was then dissolved in 1 ml of sodium borate buffer. The absorbance at 228 nm was measured to estimate the ACE inhibitory activity (Choi, 2001). The concentration of ACE inhibitor required to inhibit 50% of the ACE activity under the above assay conditions was defined as IC50.
Results
Screening and identification of the extracellular ACE inhibitor-producing yeast
To select an extracellular ACE inhibitor-producing yeast, culture broth of 350 kinds of yeasts were tested for ACE inhibitory activities. Among them, strain G-14 showed the greatest ACE inhibitory activity. Therefore, G-14 was selected as a yeast for production of extracellular ACE inhibitor.
Morphological, biochemical and cultural characteristics of the G-14 strain are summarized in Table 1, 2 and Fig. 1. The selected G-14 strain was a spherical shaped yeast that did not form an ascospore. The strain formed a pseudomycelium and had no urease activity and also can not assimilate nitrate. A white color was shown in the TTC colorization test and the G+C content was 52.5 mol%. Although the strain assimilated almost of the sugars except soluble starch and xylose, it could not ferment all sugars. From these characteristics, the strain, was identified as Malassezia pachydermatis (Kreger-van, 1984).
Table 1.
Microbiological characteristics of the strain G-14
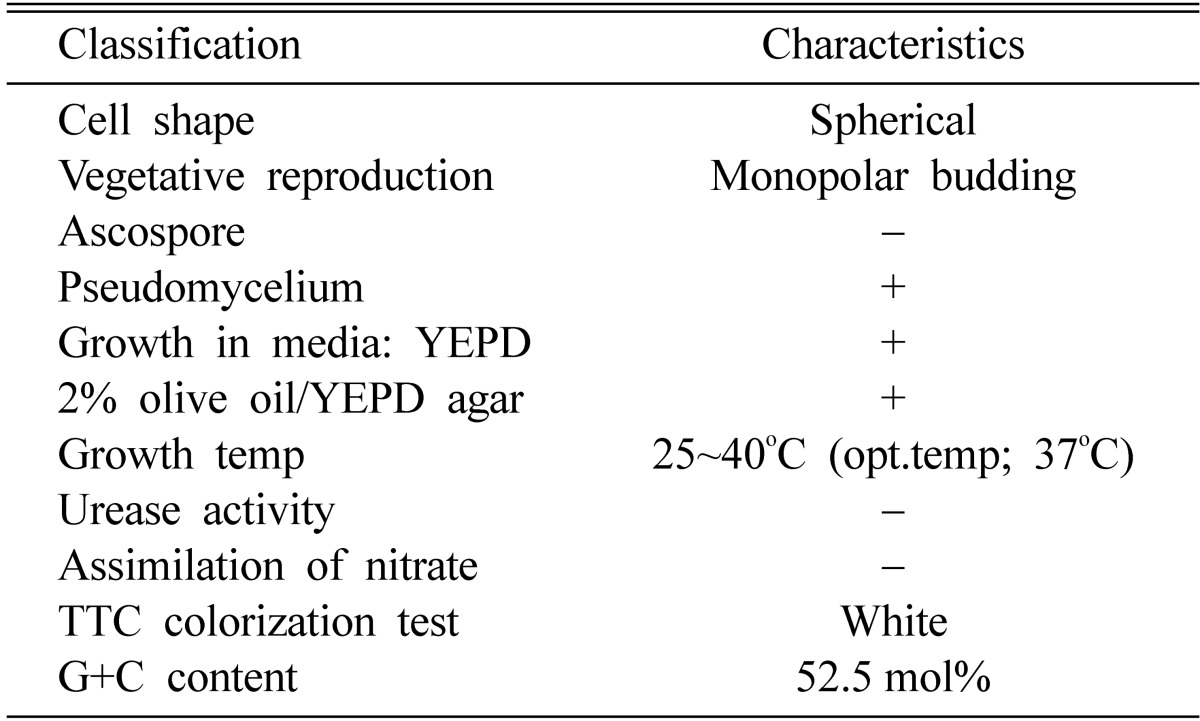
Table 2.
Assimilation and fermentability of carbon sources by the strain G-14
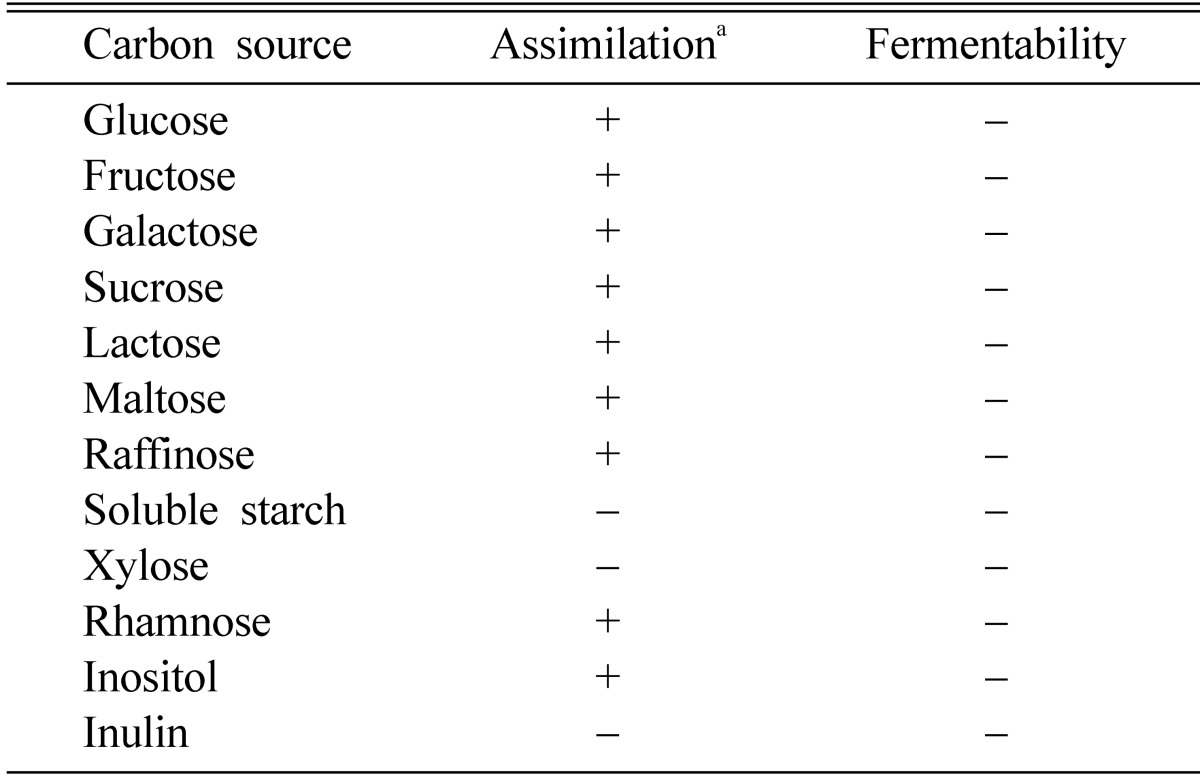
a+; assimilatable or fermentable, -; not assimilatable or fermentable.
Fig. 1.
Scanning electron micrograph of the strain G-14.
Furthermore, the supernatants of M. pachydermatis G-14 culture broth were digested with pepsin and trypsin under each optimal reaction condition (Rhyu, 1996) and then ACE inhibitory activity of the hydrolysates was determined. Original ACE inhibitory activity did not decreased and scarcely maintained even after digestion by pepsin (42.5%) and trypsin (43.8%) (data not shown).
Optimal conditions for the extracellular ACE inhibitor production
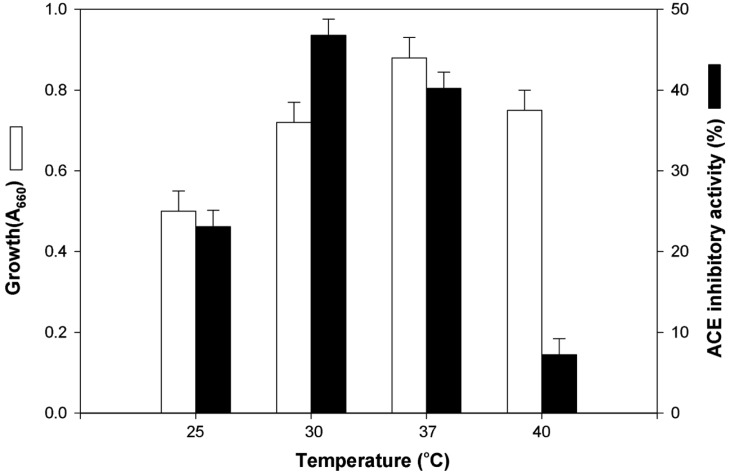
The effects of nitrogen sources on the production of the ACE inhibitor were investigated in basal medium containing 2.0% glucose. As shown in Table 3, M. pachydermatis G-14 did not grow with inorganic nitrogen sources and ACE inhibitor was not produced. The maximum production of the ACE inhibitor was obtained in YPED medium and casamino acid was a good nitrogen source for the production of ACE inhibitor. Furthermore, optimum concentration of yeast extract and peptone for the production of ACE inhibitor was 0.5% and 3.0%, respectively and its ACE inhibitory activity was 46.8% (Table 4). The effects of culture temperature on the production of the ACE inhibitor were examined in various temperature (25~40℃). For its growth M. pachydermatis preferred 37℃, whereas optimal temperature for the ACE inhibitor production was 30℃ (Fig. 2). Therefore, we decided to culture the strain at 30℃ for mass production of ACE inhibitor.
Table 3.
Effects of nitrogen sources on the production of the extracellular ACE inhibitor from M. pachydermatis G-14
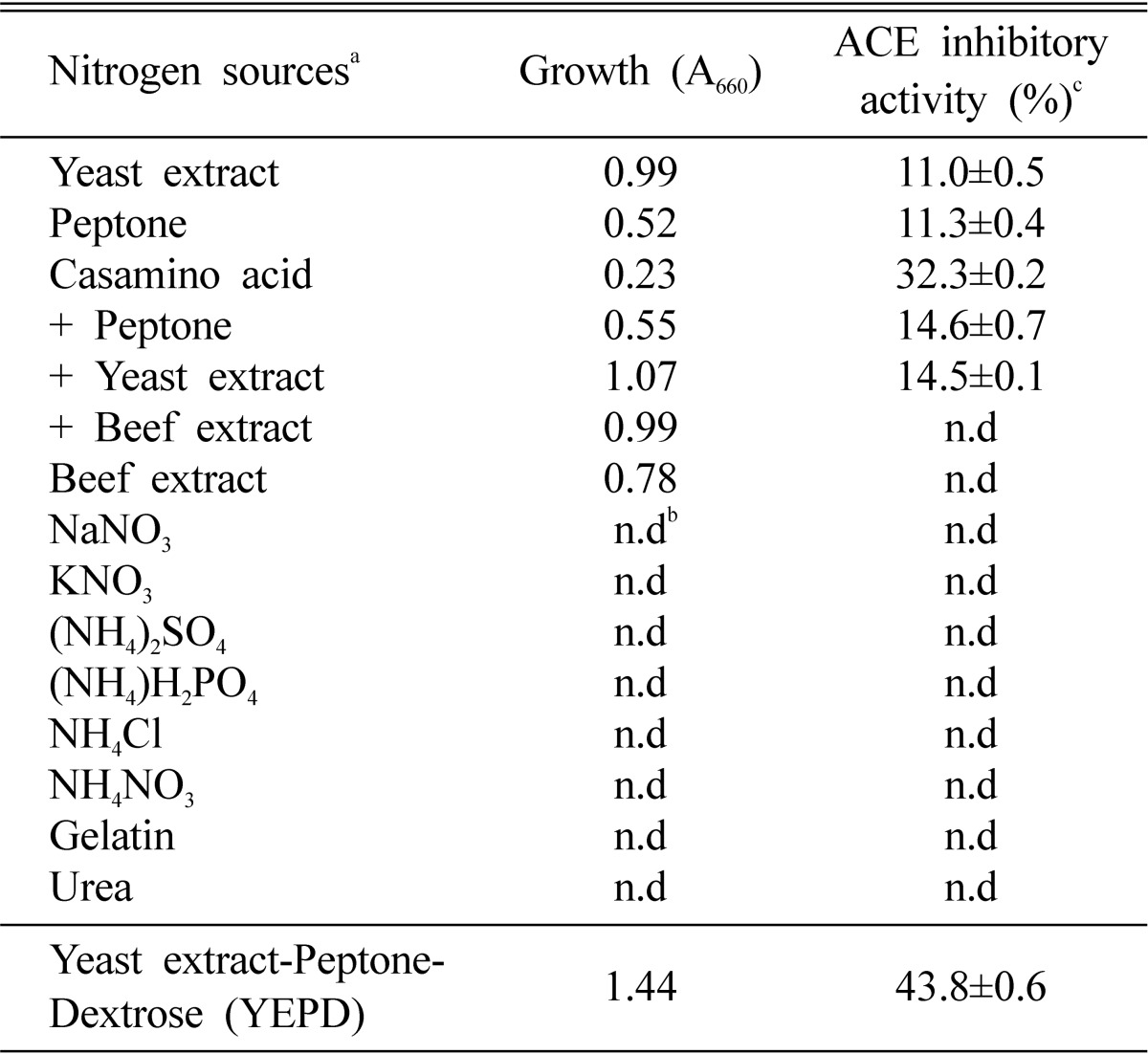
aEach nitrogen source was added into in the basal medium containing 2% glucose and cultured Malassezia pachydermatis at 30℃ for 2 days.
bn.d; not determined or below 10% of activity.
cValues are means±S.D. of three determinants.
Table 4.
Effects of yeast extract and peptone of YEPD medium on the production of the extracellular ACE inhibitor from M. pachydermatis G-14
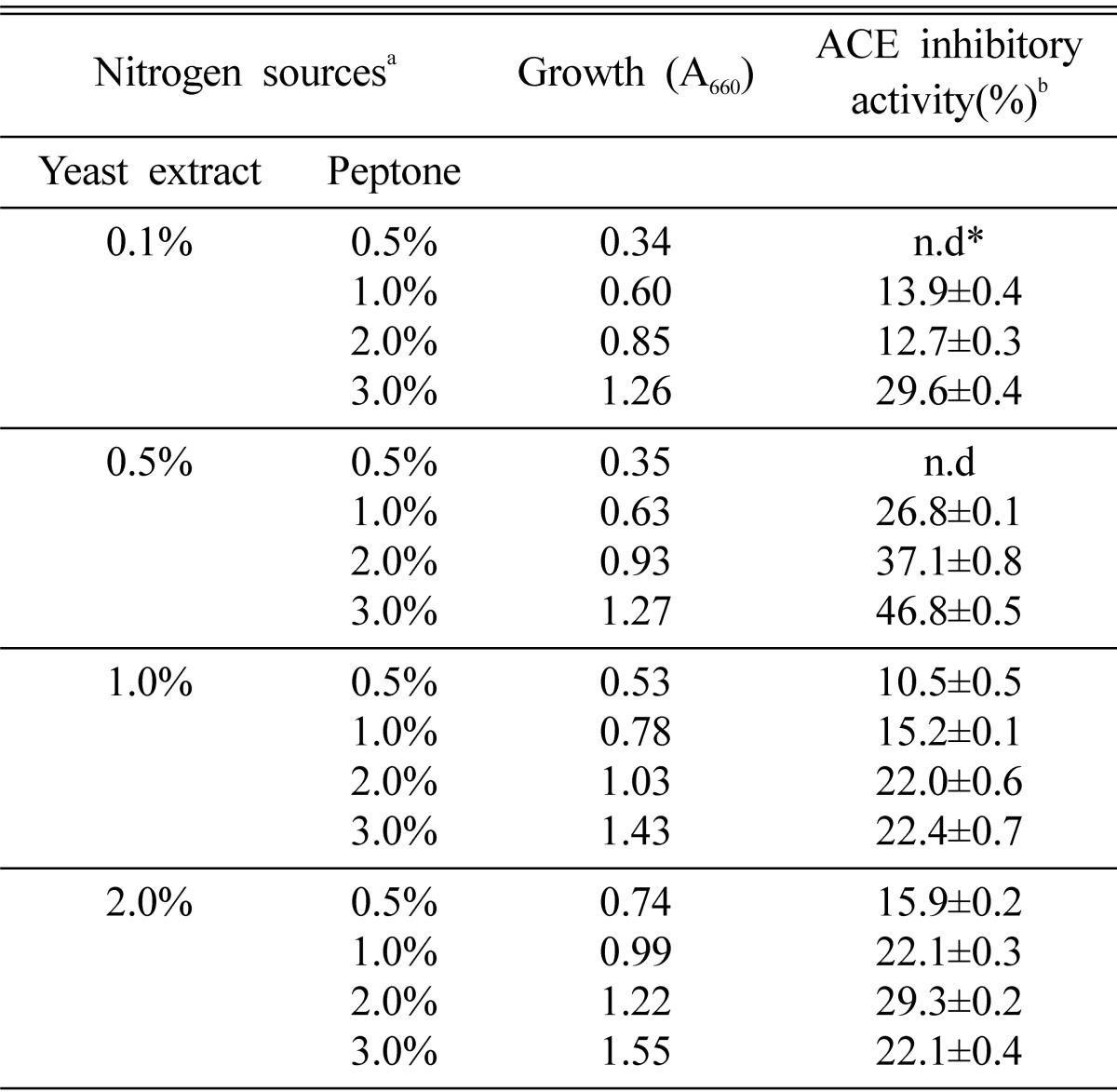
aM. pachydermatis was cultured at 30℃ for 2 days in YEPD medium containing 2.0% glucose and indicated concentration of yeast extracts and peptone, respectively.
bValues are means±S.D. of three determinants.
Fig. 2.
Effect of temperature on the production of the extracellular ACE inhibitor from M. pachydermatis G-14.
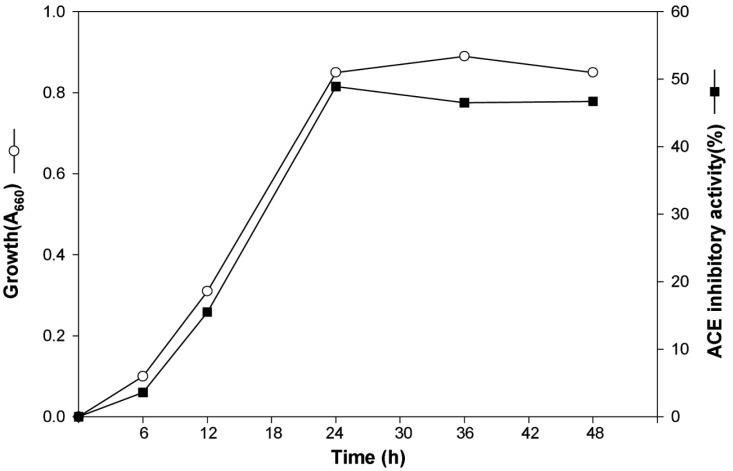
Time course of the ACE inhibitor production was determined in flask culture under the optimal culture conditions. As shown in Fig. 3, ACE inhibitor production increased with cell growth, and the maximum cell growth and ACE inhibitor production were obtained at 24 h of cultivation and its ACE inhibitory activity was 48.9%.
Fig. 3.
Effect of culture time on the production of the extracellular ACE inhibitor from M. pachydermatis G-14.
Considering the above results, the optimum culture condition for production of the ACE inhibitor was that medium was composed of 0.5% yeast extract, 3.0% peptone and 2.0% glucose, and culture temperature and time were 30℃ and 24 h, respectively.
Discussion
There are two kinds of system in the regulation of blood pressure, that is, renin-angiotensin system and kallikrein-kinin system. Among them, angiotensin I-converting enzyme (ACE, dipeptidyl carboxy peptidase I, E.C 3.4.15.1) is the key enzyme in the renin-angiotensin system, which catalyzes production of active hypertensive hormone angiotensin II (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe) from inactive pro-hormone angiotensin I (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu). This conversion action endows ACE with a very important role in regulating blood pressure through the direct action of angiotensin II on blood vessels, sympathetic nerves and adrenal glands (Folkow, 1961).
There are known some ways to remedy or prevent hypertension such as utilization of the ACE inhibitor or receptor blocker of angiotensin II. However, ACE inhibitors have long been used effectively because they inhibited both ACEs in renin-angiotensin system and kallikrein-kinin system, and their pharmaceutical effects are good and have less side-effect than chemically synthesized, antihypertensive drug, captopril. Since the ACE inhibitor was discovered for the first time from snake venom (Ferreira, 1970), many antihypertensive ACE inhibitors have been isolated and characterized from various natural sources such as foods, enzymatic hydrolysates of proteins, alcohol beverages and its by-products, cereals and legumes, etc (Fujita, 2000; Ondetti, 1982; Morigiwa, 1986; Matsumura, 1993; Sugiyama, 1991; Sun, 1997; Maruyama, 1989; Miyoshi, 1991; Saito, 1994).
Many research groups have been screened for novel ACE inhibitors of microbial origin such as Doratomyces putredinis, Nocardia orientalis (Ando, 1987), Virgaria nigra (Ando, 1988), Actinomycetes (Kido, 1983), baker's yeast (Kohama, 1990) and Basidiomycetes (Lee, 2003). WF-10129, obtained from Doratomyces putredinis, is an ACE inhibitor resembling to the potent synthetic ACE inhibitor, enalapril, and is a substituted N-carboxymethyl dipeptide. WF-10129 inhibited ACE in a dose-dependent manner and its IC50 was 14 nM. Kohama et al. (1990) isolated 3 kinds of ACE inhibitory peptides from baker's yeast glyceraldehyde-3-phosphate dehydrogenase. Among them, YG-1(Gly-His-Lys-Ile-Ala-Thr-Phe-Gln-Glu-Arg, IC50 : 0.4 µM) was the most potent yeast ACE inhibitor. Morigiwa et al. (1986) isolated strong antihypertensive triterpene compounds such as ganoderal A, ganoderols A, and B, and ganoderic acids K and S from 70% methanol extract of Ganoderma lucidum. Recently, Lee et al. (2003) isolated an ACE inhibitory peptide from Tricholoma gigantum.
Even though a number of microbial ACE inhibitors were reported (Kim, 2003), almost of them were intracellular ACE inhibitors. However, M. pachydermatis G-14 was the first strain in yeast as a producer of extracellular ACE inhibitor.
That optimum growth temperature of M. pachydermatis (37℃) was higher than that of general yeast (25~30℃) and ACE inhibitory activity was also shown high at 37℃ of cultivation, suggestsing that ACE inhibitor from M. pachydermatis may be thermostable.
Many natural ACE inhibitors and synthetic ACE inhibitors such as captopril, enalapril and lisinopril were known as remarkably effective antihypertensive drug (Ondetti, 1977). Since, M. pachydermatis G-14 produces as extracellular ACE inhibitor, its use will be easy and economic for the inhibitor production. Furthermore, the ACE inhibitor has high resistance to some proteases attack without side effects. Therefore, ACE inhibitor from M. pachydermatis G-14 might be useful in the preparation of antihypertensive drug or foods. Further purification and characterization of the ACE inhibitor are underway.
Acknowledgment
This work was supported by the Korea Science and Engineering Foundation (KOSEF) through the Bio-Medicinal Resources Research Center at PaiChai University.
References
- 1.Ando T, Okada S, Uchida I, Hemmi K, Nishikawa M, Tsurumi Y, Fujie A, Yoshida K, Okuhara M. WF-10129, a novel angiotensin converting enzyme inhibitor produced by a fungus, Doratomyces putredinis. J Antibiot. 1987;40:468–475. doi: 10.7164/antibiotics.40.468. [DOI] [PubMed] [Google Scholar]
- 2.Ando T, Tsurumi Y, Ohata N, Uchida I, Yoshida K, Okuhara M. Vinigrol, a novel antihypertensive and platelet aggregation inhibitory agent produced by a fungus, Virgaria nigra. Taxonomy, fermentation, isolation, physico-chemical and biological properties. J Antibiot. 1988;41:25–30. doi: 10.7164/antibiotics.41.25. [DOI] [PubMed] [Google Scholar]
- 3.Ariyosh Y. Angiotensin converting enzyme inhibitors derived from food protein. Trends Food Sci Technol. 1993;4:139–144. [Google Scholar]
- 4.Choi HS, Cho HY, Yang HC, Ra KS, Suh HJ. Angiotensin I-converting enzyme inhibitor from Grifola frondosa. Food Res Inter. 2001;34:177–182. [Google Scholar]
- 5.Demain AL, Somkuti GA, Hunter-Cevera JC, Rossmoore HW. Novel microbial products for medicine and agriculture. Elsevier Science Pubishers: 1989. [Google Scholar]
- 6.Elisseeva YE, Orekhovich VN, Pavlikhina LN, Alexeenko LP. Carboxycathepsin--A key regulatory component of two physiological systems involved in regulation of blood pressure. Clin Chim Acta. 1971;31:413–419. doi: 10.1016/0009-8981(71)90412-8. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira SH, Bartelt DC, Greene LJ. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry. 1970;9:2583–2592. doi: 10.1021/bi00815a005. [DOI] [PubMed] [Google Scholar]
- 8.Folkow B, Johansson B, Mellander S. The comparative effects of angiotensin and noradrenaline on consecutive vascular sections. Acta Physiol Scand. 1961;53:99–104. doi: 10.1111/j.1748-1716.1961.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 9.Fujita H, Yokoyama K, Yoshikawa M. Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J Food Sci. 2000;65:564–569. [Google Scholar]
- 10.Gohlke P, Linz W, Schokens BA, Kuwer I, Bartenbach S, Schell A, Unger T. Angiotensin converting enzyme inhibition improves cardiac function. Hypertension. 1994;23:411–418. doi: 10.1161/01.hyp.23.4.411. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa T. Taxonomy and identification of Microorganism. Tokyo: Hakhoey Pub. Center; 1984. [Google Scholar]
- 12.Kido Y, Hamakado T, Yoshida T, Anno M, Motoki Y, Wakamiya T, Shiba T. Isolation and characterization of ancovenin, a new inhibitor of angiotensin I converting enzyme produced by Actinomycetes. J Antibiot. 1983;36:1295–1299. doi: 10.7164/antibiotics.36.1295. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH. Biotechnological characterization of a novel antihypertensive angiotensin I-converting enzyme inhibitor from yeast and its industrial application. Paichai Univ. Graduate school; 2003. Ph.D. dissertation. [Google Scholar]
- 14.Kim JH, Lee DH, Choi SY, Lee JS. Characterization of physiological functionalities in Korean traditional liquors. Korean J Food Sci Technol. 2002;34:118–122. [Google Scholar]
- 15.Kohama Y, Nagase Y, Oka H, Nakagama T, Teramoto T, Murayama N, Tsujibo H, Inamori Y, Mimura T. Production of angiotensin-converting enzyme inhibitors from baker's yeast glyceraldehyde-3-phosphare dehydrogenase. J Pharmacobio-Dyn. 1990;13:766–771. doi: 10.1248/bpb1978.13.766. [DOI] [PubMed] [Google Scholar]
- 16.Kreger-van F. The yeast, a taxonomic study. 3rd ed. Amsterdam: Elsevier Sci; 1984. [Google Scholar]
- 17.Lee DH, Kim JH, Cheong JC, Gong WS, Yoo YB, Park JS, Yoo CH, Lee JS. Screening of mushrooms having angiotensin I-converting enzyme inhibitor. Korean J Mycol. 2003;31:148–154. [Google Scholar]
- 18.Lee JS, Yi SH, Kwon SJ, Ahn C, Yoo JY. Isolation, identification and cultural condition of yeasts from traditional meju. Korean J Microbiol Biotechnol. 1997;25:435–441. [Google Scholar]
- 19.Maruyama S, Miyoshi S, Tanaka H. Angiotensin I converting enzyme inhibitor derived from Ficus carica. Agric Biol Chem. 1989;53:2763–2769. [Google Scholar]
- 20.Matsumura N, Fujii M, Takeda Y, Sugita K, Shimizu T. Angiotensin I-converting enzyme inhibitory peptides derived from bonito bowels autolysate. Biosci Biotech Biochem. 1993;57:695–697. doi: 10.1271/bbb.57.695. [DOI] [PubMed] [Google Scholar]
- 21.Miyoshi S, Ishikawa H, Kaneko T, Fukui F, Tanaka H. Structures and activity of angiotensin-converting enzyme inhibitors in an α-Zein hydrolysate. Agric Biol Chem. 1991;55:1313–1318. [PubMed] [Google Scholar]
- 22.Morigiwa A, Kitabatake A, Fujimoto Y, Ikekawa N. Angiotensin converting enzyme-inhibitory triterpenes from G. lucidum. Chem Pharm Bull. 1986;34:3025–3028. doi: 10.1248/cpb.34.3025. [DOI] [PubMed] [Google Scholar]
- 23.Ondetti MA, Rubin B, Cushman DW. Design of specific inhibitors of angiotensin converting enzyme: New class of orally active antihypertensive agents. Science. 1977;196:441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- 24.Ondetti MA, Rubin B, Cushman DW. Enzyme of the rennin-angiotensin system and their inhibitors. Ann Rev Biochem. 1982;51:283–308. doi: 10.1146/annurev.bi.51.070182.001435. [DOI] [PubMed] [Google Scholar]
- 25.Oshima G, Shimabukuro H, Nagasawa K. Peptide inhibitors of angiotensin I-converting enzyme in digestsof gelatin by bacterial collagenase. Biochim Biophys Acta. 1979;566:128–137. doi: 10.1016/0005-2744(79)90255-9. [DOI] [PubMed] [Google Scholar]
- 26.Pal LV, Janet SR, Laura EL, Ralph ES, Patrick EW. Angiotensin and bradykinin metabolism by peptidases identified in cultured human skeletal muscle myocytes and fibroblasts. Peptides. 1995;16:1367–1373. doi: 10.1016/0196-9781(95)02034-9. [DOI] [PubMed] [Google Scholar]
- 27.Pollare T, Lithell H, Berne C. A comparison of the effects of hydrochorothiazide and captopril on glucose and lipid metabolism in patients with hypertension. N Engl J Med. 1989;321:868–873. doi: 10.1056/NEJM198909283211305. [DOI] [PubMed] [Google Scholar]
- 28.Reneland R, Lithell H. Angiotensin converting enzyme in human skeletal muscle. A simple in vitro assay of activity in needle biopsy specimens. Scand J Clin Lab Invest. 1994;54:105–111. doi: 10.3109/00365519409086516. [DOI] [PubMed] [Google Scholar]
- 29.Rhyu MR, Nam YJ, Lee HY. Screening of angiotensin converting enzyme inhibitors in cereals and legumes. Food Biotechnol. 1996;5:334–337. [Google Scholar]
- 30.Saito Y, Wanezaki K, Kawato A, Imayasu S. Structure and activity of angiotensin I converting enzyme inhibitory peptides from sake and sake lees. Biosci Biotechnol Biochem. 1994;58:1767–1771. doi: 10.1271/bbb.58.1767. [DOI] [PubMed] [Google Scholar]
- 31.Sugiyama K, Takada K, Egawa M, Yamamoto I, Onzuka H, Oba K. Hypertensive effect of fish protein hydrolysate. Nippon Nogeikagaku Kaishi. 1991;65:35–41. [Google Scholar]
- 32.Sun HJ, Cho SJ, Whang JH, Lee H, Yang HC. Characterization of angiotensin converting enzyme inhibitor from squid hydrolysate. Food Biotechnol. 1997;6:122–128. [Google Scholar]
- 33.Tomiyama H, Kushiro T, Abeta H. Kinins contribute to the improvement of insulin sensitivity during treatment with angiotensin converting enzyme inhibitor. Hypertension. 1994;23:450–455. doi: 10.1161/01.hyp.23.4.450. [DOI] [PubMed] [Google Scholar]
- 34.Ukeda H, Matsuda H, Kuroda H, Osajima K, Matsufuji H, Osajima Y. Preparation and separation of angiotensin I converting enzyme inhibitory peptides. Nippon Nogekagaku Kaishi. 1991;65:1223–1228. [Google Scholar]