Abstract
Three fungi newly intercepted from importing plants were identified in 2004. They were Ascochyta chrysanthemi on Lactuca sativa from China, A. spinaciicola on Spinacia oleracea from Denmark, and Leptosphaerulina australis on Brassica oleracea var. capitata from China. The characters of these fungi were described and illustrated.
Keywords: Ascochyta, Identification, Leptosphaerulina, Plant quarantine
In making plant quarantine decisions it is essential that the fungi present in subject plant materials should be identified accurately, not only to exclude non-indigenous, pathogenic fungi, but also to allow the entry of material infected by fungi that are already present in a country and therefore not of plant quarantine concern (Palm, 1999).
Importing plant and plant products to Korea are inspected at the port by National Plant Quarantine Service (NPQS) inspectors. When a fungus is found during the inspection, the inspectors responsible for identifying fungi make a determination. If the fungus cannot be identified at the port, the specimen is forwarded to the consulting team of NPQS for pest identification. In 2004, the authors identified three fungal specimens newly intercepted from importing plants as the consulting team members of NPQS.
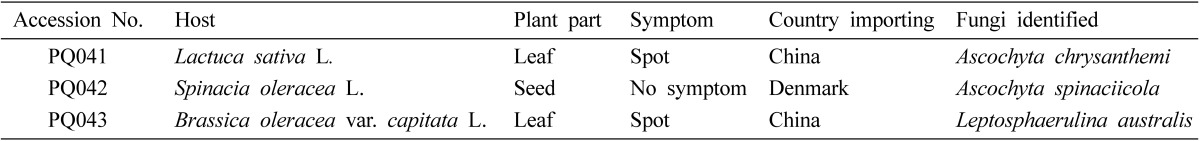
These specimens were made from Lactuca sativa (lettuce) sample importing from China, Spinacia oleracea (spinach) from Denmark, and Brassica oleracea var. capitata (Chinese cabbage) from China, respectively (Table 1). Leaf samples were incubated in a moist chamber for three to five days. Seed sample was incubated for seven days by the blotter method (Neergaard, 1977). After sporulation, morphological characters were observed for the identification.
Table 1.
List of plant specimens and fungi identified in this study
On the basis of the morphology, three fungi intercepted from lettuce, spinach and Chinese cabbage were identified as Ascochyta chrysanthemi F. Stevens, A. spinaciicola Melnik, and Leptosphaerulina australis McAlp., respectively. Identification was done by referring to Mel'nik (2000) and Punithalingam (1980) for the Ascochyta species, and to Graham and Luttrell (1961) for Leptosphaerulina australis. These three fungi were characterized as follows:
Ascochyta chrysanthemi F. Stevens
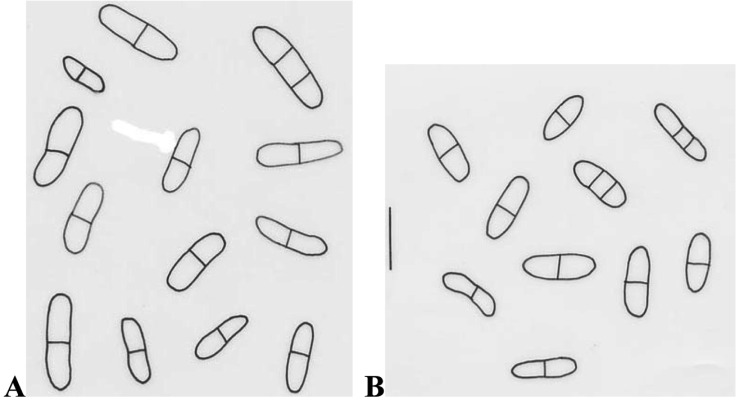
Conidiomata pycnidial, epiphyllous, scattered on lesions of leaves, immersed, punctiform with papillate ostiole. Conidia cylindrical or oblong-ellipsoidal, straight or sometimes slightly curved, both ends rounded, sometimes narrowed to one end, not or only slightly constricted at the septa, hyaline, 10~22.5 × 3~6.3 µm (Fig. 1A).
Fig. 1.
Conidia of Ascochyta chrysanthemi (A) and A. spinaciicola (B). Bar = 15 µm.
This fungus was detected from the leaves of lettuce showing spot symptom. Punithalingam (1980) recorded lettuce as a host by inoculation. However, principal hosts of this fungus are Chrysanthemum cinerariifolium and C. morifolium (Punithalingam, 1980). A chrysanthemum is one of the most important plants in floriculture in Korea. Once introduced and established in an area, this fungus is both difficult and costly to eradicate, and control of the disease remains as additional production expense. This fungus was recorded in Africa, Australasia & Oceania, Europe, North America and Asia (Punithalingam, 1980), however this fungus is not reported in Korea according to the Korean Society of Plant Pathology (2004). Its teleomorph is Didymella ligulicola (syn. Mycosphaerella ligulicola), designated as a plant quarantine pathogen in Korea.
Ascochyta spinaciicola Melnik
Conidiomata pycnidial, scattered, immersed, 110~170 µm in diam. Conidia cylindrical or oblong-ellipsoidal, both ends rounded, straight or sometimes slightly curved, not constricted or rarely slightly constricted at the septa, hyaline, 10~17.5 × 3.8~5 µm (Fig. 1B).
Mel'nik (2000) recorded Asia (George) as a distribution area of the fungus. Interestingly this fungus was intercepted from the spinach from Denmark. Mel'nik (2000) observed this fungus on leaves of spinach. In this work, it was detected from seeds of spinach. So far, information on this fungus is not known well.
Leptosphaerulina australis McAlp
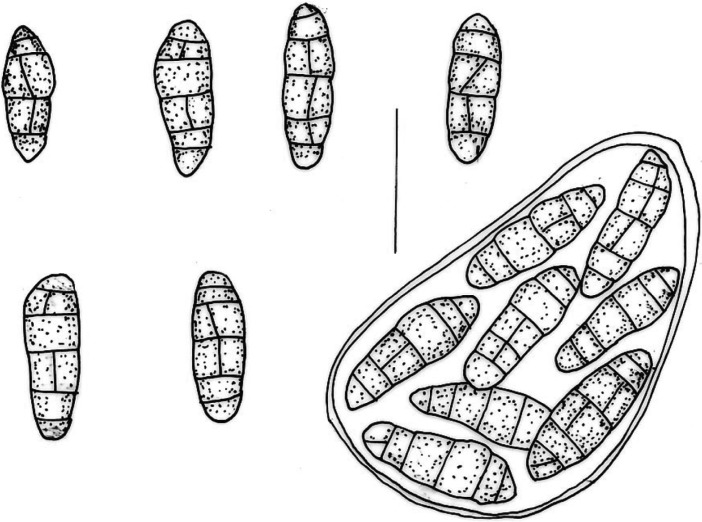
Ascomata pseudothecial, uniloculate, immersed. Asci bitunicate, thick-walled, 8-spored, 75~95 × 45~58 µm. Ascospores ellipsoid to oblong with gelatinous sheath, 3~5 transverse septa, 1~3 longitudinal septa, hyaline to brown, 26~38 × 9~13 µm (Fig. 2).
Fig. 2.
Ascospores and ascus of Leptosphaerulina australis. Bar = 30 µm.
According to Graham and Luttrell (1961), Leptosphaerulina trifolii, L. briosiana and L. arachidicola were pathogenic, but L. australis, L. argentinensis and L. americana were saprobic. These six species of Leptosphaerulina are recognized on forage legumes in the U.S.A. However, Irwin and Davis (1985) reported that L. argentinensis was pathogenic to Stylosanthes guianensis. In the present work, the fungus was detected from the spot lesions of Chinese cabbages. Pathogenicity of the fungus is also needed to study.
On the other hand, Booth and Pirozynski (1967), and Irwin and Davis (1985) regarded L. australis as a taxonomic synonym of L. trifolii. L. trifolii is plurivorus but especially on economic plants from the following families: Cruciferae, Euphorbiaceae, Gramineae, Leguminosae and Solanaceae. It causes leaf spot or pepper spot on account of leaves and petioles of diverse plants, particularly forage legumes (Booth and Pirozynski, 1967).
All three fungi intercepted are not reported in Korea according to the Korean Society of Plant Pathology (2004), and may have risks to domestic plants. It is necessary to inspect and identify thoroughly the presence of these fungi during plant quarantine procedures.
References
- 1.Baker KF, Dimock AW, Davis LH. Cause and prevention of the rapid spread of the Ascochyta disease of chrysanthemum. Phytopathology. 1961;51:101. [Google Scholar]
- 2.Booth C, Pirozynski KA. Leptosphaerulina trifolii. Commonw. Mycol. Inst. Descriptions of Pathogenic Fungi and Bacteria No. 146. 1967. [Google Scholar]
- 3.Farr DF, Bills GF, Chamuris GP, Rossman AY. Fungi on Plants and Plant Products in the United States. St. Paul: APS Press; 1989. p. 1255. [Google Scholar]
- 4.Graham JH, Luttrell ES. Species of Leptoshpaerulina on forage plants. Phytopathology. 1961;51:680–693. [Google Scholar]
- 5.Irwin JA, Davis RD. Taxonomy of some Leptosphaerulina spp. on legumes in eastern Australia. Aust J Bot. 1985;33:233–237. [Google Scholar]
- 6.Mel'nik VA. Key to the Fungi of the Genus Ascochyta Lib. (Coelomycetes) Berlin: BBA; 2000. p. 192. [Google Scholar]
- 7.Neergaard P. Seed Pathology. London and Basingstoke: The Macmillan Press Ltd.; 1977. p. 839. [Google Scholar]
- 8.Palm ME. Mycology and world trade: a view from the front line. Mycologia. 1999;91:1–12. [Google Scholar]
- 9.Punithalingam E. Didymella chrysanthemi. Commonw. Mycol. Inst. Descriptions of Pathogenic Fungi and Bacteria No. 662. 1980. [Google Scholar]
- 10.The Korean Society of Plant Pathology. List of Plant Diseases in Korea. 2004. p. 779. [Google Scholar]