Abstract
By observing the real-time behavior of focal liver lesions at three vascular phases (arterial, portal-venous, and late), contrast-enhanced ultrasound (CEUS) has been successfully applied to differentiate benign from malignant hepatic nodules. In recent years, numerous studies highlighted the usefulness of CEUS also for other applications such as abdominal trauma, renal, pancreatic, thyroid, and inflammatory bowel diseases, supporting its role even in differentiating benign from malignant splenic nodules. Therefore, the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) recently updated the guidelines for the use of ultrasound contrast agents in clinical practice, pointing out the indication to characterize splenic parenchymal inhomogeneity or suspected lesions found on conventional ultrasound (BUS). We describe the case of a patient with a history of colon cancer and finding, at BUS and CEUS, of hypoechoic lesions with a highly suggestive pattern for metastases, subsequently histologically proved to be splenic localizations of a benign and multisystemic granulomatous disease such as sarcoidosis. We therefore reviewed the current literature focusing on the role of CEUS in differentiating benign from malignant splenic lesions, emphasizing on the lack of data and numerical shortage of sarcoidosis derived-lesions in the available studies. We conclude that sarcoidosis remains a diagnosis of exclusion and new studies are needed before defining precise indications of CEUS in these patients.
Keywords: Sarcoidosis, CEUS, Spleen, Guidelines
Riassunto
L’ecografia con mezzo di contrasto (CEUS) è una metodica diagnostica minimamente invasiva ed ampiamente utilizzata, negli ultimi decenni, per differenziare le lesioni epatiche benigne dalle maligne, valutandone l’aspetto contrastografico nelle tre fasi vascolari (arteriosa, portale-venosa e tardiva). Negli ultimi anni, numerosi studi hanno messo in evidenza ulteriori indicazioni della CEUS, tra cui il trauma addominale, le lesioni focali renali, pancreatiche, tiroidee e le malattie infiammatorie intestinali, supportandone un ruolo anche nella differenziazione tra lesioni spleniche benigne e maligne. Pertanto, la Federazione Europea delle Società di Ultrasonologia in Medicina e Biologia (EFSUMB), ha recentemente aggiornato le linee guida per l’utilizzo dell’ecografia con mezzo di contrasto nella pratica clinica, sottolineando, tra le varie indicazioni, quella di caratterizzare le lesioni spleniche o disomogeneità parenchimali sospette, riscontrate incidentalmente all’esame ecografico convenzionale. Nel seguente articolo descriviamo il caso di una paziente con anamnesi per carcinoma del colon e riscontro, all’esame ecografico convenzionale, di lesioni ipoecogene di non univoca interpetazione e caratterizzate, alla CEUS, da un pattern contrastografico altamente suggestivo per metastasi. Tali lesioni si sono poi rivelate essere, all’esame istologico, localizzazioni di una malattia benigna, multisistemica e granulomatosa quale la sarcoidosi. Abbiamo pertanto effettuato una revisione approfondita della letteratura focalizzando sul ruolo della CEUS nel differenziare le lesioni spleniche benigne dalle maligne, enfatizzando sulla carenza di studi riguardanti la caratterizzazione, attraverso la CEUS, delle lesioni derivate dalla sarcoidosi. Tale malattia rimane, pertanto, una diagnosi di esclusione e sono necessari ulteriori studi prima di definire precise indicazioni della CEUS nei pazienti affetti.
Introduction
Sarcoidosis is a multisystemic granulomatous disease, which may affect any organ or tissue and cause significant morbidity and mortality. The area with the highest incidence is Northern Europe, with approximately 5–40 cases per 100,000, although an unexpectedly high prevalence of sarcoidosis (upper to 48 of 100,000) has been recently found in a US Metropolitan population. Females are more affected than males with a F:M ratio 2:1 and two peaks of incidence, between 25–40 and 45–65 years [1–3]. Lungs and intrathoracic lymph nodes are the most frequently involved sites, though other organs may often be affected, such as heart, liver, kidneys, central and peripheral nervous system, gastrointestinal tract, skin and peripheral lymph nodes [4, 5]. Rarely (5–10 %), sarcoidosis may cause splenic enlargement, usually neither giving symptoms nor blood cell count reduction [1–6]. Several studies have shown that contrast-enhanced sonography (CEUS) may support the diagnosis of focal splenic lesions by differentiating benign from malignant [7, 8]. We describe the case of a patient with a history of colon cancer and finding, at conventional baseline ultrasound (BUS) and CEUS, of hypoechoic lesions highly suggestive for metastases, subsequently proved to be splenic localizations of systemic sarcoidosis.
Case report
A 45-year-old woman came to our observation for worsening fatigue, weight loss (10 kg in the last 6 months) left ocular pain, and headache. She did not report changes in bowel habits. She reported a history of colon cancer undergone to a previous surgical resection and adjuvant chemotherapy, and of polycystic ovary syndrome and migraine. The physical examination was normal. A mild laterocervical lymphadenopathy was found. The complete blood counts documented the presence of mild thrombocytopenia and lymphopenia, with reduction of CD4 and CD4/CD8 ratio.
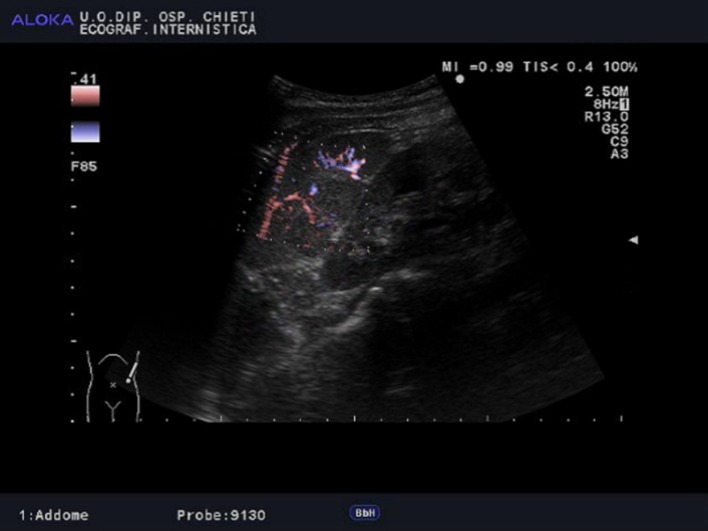
Neoplastic markers [carcinoembryonic antigen (CEA), membrane glycoproteins such as Ca 19-9 and Ca 125, and alpha fetoprotein (AFP)] were in the normal range. Serological tests for viruses [e.g. Cytomegalovirus (CMV) and Epstein Barr virus (EBV)] and parasites were negative. The quantiferon test was normal. Baseline gray-scale sonography revealed the presence of enlarged spleen (area = 60 cm2) and almost completely subverted by the presence of multiple and roundish hypoechoic lesions (Fig. 1). Color Doppler sonogram showed absence of vascularity inside the lesions (Fig. 2). The largest of these measured 17 × 22 mm and was localized near the lower splenic pole. BUS did not show the presence of hepatic lesions. According to the recent update of the European Federation of Ultrasound in Medicine and Biology (EFSUMB) guidelines concerning the non-hepatic applications of CEUS, which recommend this method to characterize splenic parenchymal inhomogeneity or suspected lesions found on conventional US (Recommendation Level:B;1b) [9], we performed CEUS with sulfur hexafluoride to characterize the suspected nodules. After an initial, rapid, and transiently inhomogeneous enhancement phase [similar to the “zebra” pattern seen on dynamic contrast-enhanced computer tomography (CECT) or Magnetic Resonance (CEMR)], the healthy parenchyma became homogeneous, showing a strong and persistent late phase enhancement. The lesions showed a slight and more peripherally located enhancement in the arterial phase (Fig. 3) followed by a rapid and marked wash-out of the injected contrast in the parenchymal and late phases (Fig. 4). According to the EFSUMB guidelines, and in light of the patient’s history for colon cancer, we considered the described pattern as highly suggestive for malignancy (metastases). Usually, however, colon cancer spreads to the liver, and this preferential localization has been attributed to the abundant hepatic circulation (approximately 25 % of the cardiac output), to the sinusoids ability to trap tumor cells and to the favorable biochemical environment. Metastases involve the spleen in a small percentage of cases (less than 1 %), and are usually associated with a rise in CEA [10], unlike our case. Computer Tomography (CT) did not add further information, confirming the poor enhancement with a hypodense appearance of the splenic lesions. Chest X-ray, esophagogastroduodenoscopy, and colonoscopy were negative. The patient also performed Positron emission tomography (PET)–CT, which documented the presence of tracer uptake by spleen and peri-bronchial, under-clavicular and mediastinal lymph nodes, and a magnetic resonance imaging (MRI) of the brain which showed an increased size of the pituitary gland. A biopsy was therefore necessary and revealed the presence of granulomatous and no necrotizing lesions, excluding the presence of malignant cellularity. The collected data were consistent with a diagnosis of systemic sarcoidosis. Angiotensin Converting Enzyme (ACE) levels, although nonspecific, were greatly increased. After carefully excluding other granulomatous diseases, we started a treatment with medium doses of corticosteroids. After 6 months of well-conducted therapy, the patients became asymptomatic and the lesions gradually reduced, as confirmed by the follow-up performed with BUS and CEUS.
Fig. 1.
Enlarged and subverted spleen by the presence of multiple and roundish hypoechoic lesions at baseline gray-scale sonography
Fig. 2.
Color Doppler sonogram showed absence of vascularity inside the lesions
Fig. 3.
CEUS, arterial phase: slight and more peripherally located enhancement of the splenic lesions derived from sarcoidosis
Fig. 4.
CEUS, late phase: rapid and marked wash-out of the injected contrast resulting in a hypoechoic appearance of the splenic lesions derived from sarcoidosis
Discussion
In recent years, numerous studies have documented the usefulness of CEUS other than for the characterization of focal liver lesions, also for applications such as abdominal trauma, renal, pancreatic, thyroid, and inflammatory bowel diseases. Thus, Piscaglia et al. recently published an update of the EFSUMB guidelines, focusing on non-hepatic applications of CEUS [9]. The spleen, acting primarily as a blood filter, shows a peculiar behavior after contrast injection. Its peculiar vascularization, mainly represented by splenic capillaries and venous sinuses, leads to a rapid and transiently inhomogeneous enhancement pattern, similar to the “zebra” behavior seen on dynamic CECT or CEMRI. After a minute, the parenchyma becomes homogeneous, showing a strong and persistent (longer than 5 min) late phase enhancement [9, 11].
In the last years, the characterization of focal splenic lesions has been debated in several studies, primarily aimed at distinguishing benign from malignant characteristic patterns. Most of them compared the results of CEUS with those from non-invasive examinations such as CT and MRI or from biopsy, and showed interesting results. Von Herbay et al. investigated 35 patients with as many focal splenic lesions detected by BUS, examining them for 3 min using low-mechanical index ultrasonography with contrast-specific software. Of these lesions, 14 were malignant (6 metastases, 6 non-Hodgkin lymphomas, and 2 Hodgkin lymphomas) while 21 were benign (6 ischemic lesions, 5 echogenic cysts, 4 abscesses, 3 hemangiomas, 1 splenoma, 1 hematoma and 1 Hemophagocytic syndrome). Malignant lesions were identified by a contrast enhancement in the early phase followed by a rapid wash-out and demarcation of the lesions, without contrast enhancement in the parenchymal phase. The authors identified two typical benign patterns, characterized by the absence or presence of contrast enhancement in both early and parenchymal phase, respectively. Nevertheless, no sarcoid lesions were included in the study [12]. Görg et al. found that, among patients with diagnosis of infarction (n = 9), lymphoma (n = 7), metastasis (n = 6), haemangioma/splenoma (n = 6), injury (n = 4), accessory spleen (n = 3), cyst (n = 2), and unknown cause (n = 9), CEUS was highly necessary in 13 cases (28 %), such as 6 hemangiomas/splenomas, 5 infarctions and 2 ruptures, while was helpful in 9 (20 %; 4 infarctions, 3 accessory spleens and 2 ruptures) and of little value in 24 (52 %; 9 unknown, 7 lymphomas, 6 metastases and 2 cysts). However, no cases of sarcoid granulomas were included in their work [7]. Stang et al. have instead documented that arterial hyperenhancement or isoenhancement seemed to be an independent predictor of benign vascular neoplasms such as hemangiomas and hamartomas; however, they excluded patients with a diagnosis of sarcoidosis, infarction, abscess, and diffuse micronodular infiltration by lymphoma [13]. In a previous study, which included 147 different splenic lesions (68 benign and 79 malignant) from 147 patients, the same authors showed that benign lesions predominantly appeared non- or isoenhancing relative to parenchyma of the spleen, whereas malignant lesions appeared predominantly and progressively hypoenhancing. The lesions derived from sarcoidosis were numerically undersized (only 4 cases) and appeared as progressively hypoenhancing up to 3 cm in diameter [14]. Through a retrospective analysis of 279 focal splenic lesions, other authors also found that metastases are characterized, in most cases, by a reduced contrast enhancement in both arterial and parenchymal phases, improving the visualization of these in about 40 % of cases, in comparison to standard B-mode US [15]. Furthermore, another work found that CEUS seemed to be as effective as CT to characterize non-traumatic focal lesions of the spleen, showing specificity comparable to CT (77.2 %) to diagnose 7 splenic infarcts, 5 hemangiomas, 3 lacerations, 2 benign cysts, 1 abscess, 1 granuloma, 1 lymphoma and 2 lesions of unknown etiology. CEUS was inconclusive for the only case of sarcoid granuloma included in the study; the nodules were hypoechoic during all the phases [16]. Recently, Yu et al. documented that 25 of 56 benign lesions (23 hemangiomas, 14 cysts, 8 infarctions, 4 splenic ruptures, 3 lesions derived from tuberculosis, 2 abscesses, 1 lymphangioma and 1 pseudoaneurysm) showed no enhancement, while among 31 benign lesions with enhancement, 27 (87.1 %) showed iso- or hyperenhancement in the arterial phase and 22 (71.0 %) lesions iso- or hyperenhancement in the parenchymal phase. Of 19 malignant lesions, 9 [5 of 10 metastases (50.0 %) and 4 of 7 lymphomas (57.1 %)] showed hypoenhancement in the arterial phase and 18 (94.7 %) in the parenchymal phase. Sensitivity, specificity, and accuracy to diagnose focal splenic lesions were 91.1, 95.0, and 92.0 % for CEUS and 75.0, 84.2, and 77.3 % for the BUS, respectively. Neither this study included splenic lesions resulting from sarcoidosis [8].
As shown in Table 1, CEUS may be useful in the diagnosis of certain benign splenic lesions, by identifying suggestive behaviors such as hyper-enhancement in both arterial and parenchymal phases. Sometimes, however, benign and malignant patterns may overlap resulting in problems of differential diagnosis [9]. In our experience, splenic localizations of sarcoidosis showed a slight and most peripherally located enhancement in arterial phase and rapid wash out in parenchymal phase, generating problems of differential diagnosis with metastases, also considering the patient’s history for colon cancer. The pattern was indeed more suggestive for malignant lesions, as shown by Von Herbay et al. [12], and made the biopsy necessary; this behavior was consistent with that observed for sarcoidosis by Stang et al., which reported slightly less enhancement of the lesions in the arterial phase, when compared with adjacent splenic tissue, and a progressively hypoenhancement in the parenchymal phase [14]. Pérez-Grueso et al. and Chiavaroli et al. have instead documented a complete absence of enhancement of the lesions both in the arterial and parenchymal phases [16, 17].
Table 1.
Lack of data and numerical shortage of lesions derived from sarcoidosis in the available studies
| Authors/publication year | Nr. and nature of splenic lesions | Arterial phase | Parenchymal phase | Nr of lesions derived from sarcoidosis |
|---|---|---|---|---|
| Von Herbay et al. 2009 [12] | 21 Benign | Hyper-enhancement | Hyper-enhancement | 0 |
| Hypo-enhancement | Hypo-enhancement | |||
| 14 Malignant | Hyper-enhancement | Hypo-enhancement | ||
| Stang et al. 2009 [14] | 68 Benign | Iso-enhancement | Iso-enhancement | 4 |
| Hypo-enhancement (sarcoidosis) | Hypo-enhancement (sarcoidosis) | |||
| No enhancement | No enhancement | |||
| 79 Malignant | Hypo-enhancement | Hypo-enhancement | ||
| Neesse et al. 2010 [15] | 0 Benign | – | – | 0 |
| 12 Malignant | Hypo-enhancement | Hypo-enhancement | ||
| Stang et al. 2011 [13] | 58 Benign | Hyper-enhancement | Iso- or hypo-enhancement | 0 |
| 78 Malignant | Hypo-enhancement | Hypo-enhancement | ||
| Chiavaroli et al. 2011 [16] | 19 Benign | Hyper-enhancement | Hyper-enhancement | 1 |
| Hypo-enhancement (e.g. sarcoidosis) |
Hypo-enhancement (e.g. sarcoidosis) |
|||
| 1 Malignant | Hypo-enhancement | Hypo-enhancement | ||
| Yu et al. 2012 [8] | 56 Benign | Iso- or hyper-enhancement | Iso- or hyper-enhancement | 0 |
| No enhancement | No enhancement | |||
| 19 Malignant | Hypo-enhancement | Hypo-enhancement |
In conclusion, even if CEUS can provide some valuable clues to differentiate certain splenic abnormalities [18], seems to have a limited role in sarcoidosis, which actually remains a diagnosis of exclusion that can be achieved by detecting non-caseating granulomas at biopsy of affected organs or tissues, demonstrating clinical and radiological compatible data and excluding known agents capable to form granulomas [1, 2]. Splenic lesions derived from sarcoidosis do not actually show a specific pattern on CEUS that can indeed overlap with a malignant one, as shown in our case report and in previously published studies. The main limit of the available data are the lack of ad-hoc studies in patients with splenic sarcoidosis and the scant representation of sarcoidosis among the published reports, as shown in the Table 1. New studies are needed before defining precise indications of CEUS in these patients.
Conflict of interest
None.
References
- 1.Tana C, Tana M, Mezzetti A, Schiavone C. Sarcoidosis: old certainties and new perspectives. Ital J Med. 2012;6(3):186–194. doi: 10.1016/j.itjm.2012.05.002. [DOI] [Google Scholar]
- 2.Iannuzzi MC, Fontana JR (2011) Sarcoidosis: clinical presentation, immunopathogenesis, and therapeutics. JAMA 305(4):391–399 [DOI] [PubMed]
- 3.Erdal BS, Clymer BD, Yildiz VO, Julian MW, Crouser ED (2012) Unexpectedly high prevalence of sarcoidosis in a representative U.S. Metropolitan population. Respir Med 106(6):893–899 (Epub 2012 Mar 13) [DOI] [PubMed]
- 4.Nunes H, Soler P, Valeyre D. Pulmonary sarcoidosis. Allergy. 2005;60(5):565–582. doi: 10.1111/j.1398-9995.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- 5.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 6.Moller DR. Rare manifestations of sarcoidosis. Eur Respir Monogr. 2005;10:233–250. doi: 10.1183/1025448x.00032015. [DOI] [Google Scholar]
- 7.Görg C, Bert T. Contrast enhanced sonography of focal splenic lesions with a second-generation contrast agent. Ultraschall Med. 2005;26:470–477. doi: 10.1055/s-2005-858904. [DOI] [PubMed] [Google Scholar]
- 8.Yu X, Yu J, Liang P, Liu F (2012) Real-time contrast-enhanced ultrasound in diagnosing of focal spleen lesions. Eur J Radiol 81(3):430–436 (Epub 2011 Jan 26) [DOI] [PubMed]
- 9.Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M et al (2012) The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 33(1):33–59 (epub 2011 Aug 26) [DOI] [PubMed]
- 10.Abi Saad GS, Hussein M, El-Saghir NS, Termos S, Sharara AI, Shamseddine A (2011) Isolated splenic metastasis from colorectal cancer. Int J Clin Oncol 16(4):306–313 (epub 2011 Jan 22) [DOI] [PubMed]
- 11.Lim AK, Patel N, Eckersley RJ, Taylor-Robinson SD, Cosgrove DO, Blomley MJ. Evidence for spleen-specific uptake of a microbubble contrast agent: a quantitative study in healthy volunteers. Radiology. 2004;231:785–788. doi: 10.1148/radiol.2313030544. [DOI] [PubMed] [Google Scholar]
- 12.von Herbay A, Barreiros AP, Ignee A, Westendorff J, Gregor M, Galle PR, et al. Contrast-enhanced ultrasonography with SonoVue: differentiation between benign and malignant lesions of the spleen. J Ultrasound Med. 2009;28(4):421–434. doi: 10.7863/jum.2009.28.4.421. [DOI] [PubMed] [Google Scholar]
- 13.Stang A, Keles H, Hentschke S, von Seydewitz CU, Dahlke J, Habermann C et al (2011) Incidentally detected splenic lesions in ultrasound: does contrast-enhanced ultrasonography improve the differentiation of benign hemangioma/hamartoma from malignant lesions? Ultraschall Med 32(6):582–592 (Epub 2011 Dec 9) [DOI] [PubMed]
- 14.Stang A, Keles H, Hentschke S, von Seydewitz CU, Dahlke J, Malzfeldt E, et al. Differentiation of benign from malignant focal splenic lesions using sulfur hexafluoride-filled microbubble contrast-enhanced pulse-inversion sonography. AJR Am J Roentgenol. 2009;193(3):709–721. doi: 10.2214/AJR.07.3988. [DOI] [PubMed] [Google Scholar]
- 15.Neesse A, Huth J, Kunsch S, Michl P, Bert T, Tebbe JJ et al (2010) Contrast-enhanced ultrasound pattern of splenic metastases—a retrospective study in 32 patients. Ultraschall Med 31(3):264–269 (Epub 2009 Nov 6) [DOI] [PubMed]
- 16.Chiavaroli R, Grima P, Tundo P. Characterization of nontraumatic focal splenic lesions using contrast-enhanced sonography. J Clin Ultrasound. 2011;39(6):310–315. doi: 10.1002/jcu.20831. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Grueso MJ, Repiso A, Gómez R, Gonzalez C, de Artaza T, Valle J, et al. Splenic focal lesions as manifestation of sarcoidosis: characterization with contrast-enhanced sonography. J Clin Ultrasound. 2007;35(7):405–408. doi: 10.1002/jcu.20322. [DOI] [PubMed] [Google Scholar]
- 18.Catalano O, Sandomenico F, Matarazzo I, Siani A. Contrast-enhanced sonography of the spleen. AJR Am J Roentgenol. 2005;184(4):1150–1156. doi: 10.2214/ajr.184.4.01841150. [DOI] [PubMed] [Google Scholar]