Abstract
Ultrasound (US) imaging of the spleen was considered of little use in the past and was performed only to distinguish between cystic and solid lesions. However, in the last decade due to experience acquired and the introduction of second-generation contrast agents, this technique has been re-evaluated as contrast-enhanced US (CEUS) allows detection and characterization of most focal lesions of the spleen with a high sensitivity and a good specificity. Gray-scale US presents a low specificity in splenic infarctions with a high rate of false negative cases, whereas specificity reaches 100 %, if the examination is performed using US contrast agents. Gray-scale US can provide a correct diagnosis in simple cysts, whereas CEUS is useful when cystic lymphangioma is suspected. In the study of splenic lesions, the most important problem is to differentiate between angioma, hamartoma, lymphoma, and metastasis. CEUS reaches a good specificity in the differentiation of benign from malignant splenic lesions, as hypo-enhancement in the parenchymal phase is predictive of malignancy in 87 % of cases. In conclusion, Gray-scale US and particularly CEUS are at present widely indicated in the study of focal splenic lesions.
Keywords: Focal splenic lesion, Splenic angioma/hamartoma, Splenic cysts
Riassunto
L’ecografia splenica è stata considerata nel passato poco utile ed indicata solo nella diagnosi differenziale tra lesioni cistiche e solide. Nell’ultimo decennio grazie alla maggior esperienza ed all’utilizzo dei mezzi di contrasto ecografici di II generazione (CEUS), questa metodica è stata rivalutata, in quanto consente di evidenziare e caratterizzare con elevata sensibilità e buona specificità la maggior parte delle lesioni focali della milza. Negli infarti splenici l’ecografia B-Mode ha una bassa specificità con elevata percentuale di falsi negativi, mentre questa risulta il 100 %, quanto l’esame è eseguito con i mezzi di contrasto ecografici. Nelle cisti semplici l’esame ecografico è sufficiente per porre una corretta diagnosi, mentre la CEUS può risultare utile nel sospetto di linfoangioma cistico. Il problema più importante a livello splenico è quello di definire con la maggior accuratezza possibile la diagnosi differenziale tra Angioma/Amartoma e Linfoma/Metastasi. La CEUS presenta nella lesioni spleniche una buona specificità nel differenziare una lesione benigna da una maligna, in quanto la presenza di ipo-ehnancement in fase parenchimale è predittiva nell’87 % dei casi di malignità. In conclusione l’ecografia B-Mode ed ancor più la CEUS trova al momento attuale ampie indicazioni nelle lesioni focali spleniche.
Introduction
In terms of structure and function, the spleen consists of two organs combined in one: the white pulp which plays an important role in the immune system as it produces lymphocytes, plasma cells and antibodies, and the red pulp which filters the blood removing antigens, microorganisms, and defective or worn-out red blood cells.
In the first stages of life, the red pulp also plays an important role in hematopoiesis and in the accumulation and storing of blood. The weight of the spleen varies from 50 to 300 g with a median weight of 150 g; the length does not exceed 13 cm in men and 12 cm in women, but in adults the size decreases with aging.
At US imaging, the normal spleen appears half-moon shaped with a homogeneous and uniform echostructure and the echogenicity is significantly higher than that of the liver. The spleen is rarely the primary site of a malignant disease; solid lesions of the spleen are rarely compared to other organs (liver, kidneys, pancreas, etc.) and the ratio of benign versus malignant focal splenic lesions is 1:3. Benign splenic lesions are often solitary, malignant lesions (lymphoma, metastasis) are more frequently multiple and fast growing, and solitary metastases are very rare.
Cysts are the most common benign disease of the spleen; congenital cysts account for 64.7 %, post-traumatic cysts 11.6 %, and dermoid cysts 5.8 % [1]. World population incidence of focal splenic lesions ranges from 0.103 to 0.20 %; of these lesions true cysts account for 21.35 %, angiomas 14.56 %, calcification and infarctions 9.7 %, pseudocysts, also referred to as false cysts, 8.73 %, lymphomas and abscesses 7.76 %, and metastases 4.85 % [2, 3]. Unlike liver lesions, focal lesions of the spleen have so far received little attention and they have often been considered to be poorly specific findings.
The ability of US to depict focal or multiple splenic lesions depends on several factors such as the size of the spleen and the focal lesions, the location and echotexture of these pathologies as well as US findings involving the adjacent organs. The patient’s clinical history and symptoms can definitely help the clinicians to formulate a correct differential diagnosis.
In the early 1980s, it was said that US examination of the spleen only served to differentiate a solid lesion from a cystic lesion because focal benign and malignant splenic lesions could present the same US pattern, malignant lesions of the same histology could present different US patterns, cystic lesions could have a different etiology and it is was not always possible to differentiate intrasplenic from extrasplenic lesions [4].
Also in the 1990s, it was not considered possible to formulate a diagnosis on the basis of US imaging only, although in these years some epidemiologic US characteristics of splenic diseases were defined showing that benign lesions accounted for 57–60 % that malignant lesions were hypo-echoic in 97 % of cases and that 83.33 % of these were lymphomas. These studies also showed that metastases often have an echoic halo or a target pattern, that high-grade non-Hodgkin lymphomas are often solitary lesions exceeding 3 cm in diameter, whereas low-grade non-Hodgkin lymphomas and Hodgkin’s disease are multiple lesions less than 3 cm in diameter or diffuse, with echostructure almost always hypo-echoic and very rarely hyperechoic [5].
Around the year 2000, the ability to study focal splenic pathologies was further improved and it was reported that US imaging could identify 88 % of splenic diseases detected at computed tomography (CT), but US was unable to detect iso-echoic subdiaphragmatic and subcapsular lesions and particularly 50 % of infarctions, 33.3 % of metastases, and 16.6 % of lymphomas.
The presence of solitary focal splenic lesions, anechoic masses or nodules with increased echogenicity due to gas or calcification were interpreted as benign lesions with a positive predictive value (PPV) of 85, 100, or 100 %, respectively, whereas complex or echoic, diffuse or multiple lesions, target lesions or associated with abdominal lesions were suspected of malignancy with a PPV of 70, 70, 100, 100 %, respectively. Recent studies have shown that the incidence of focal splenic lesions reaches 10 % with a benign to malignant ratio of 1.6, confirming that high-grade non-Hodgkin lymphomas are often large hypo-echoic lesions, whereas low-grade non-Hodgkin lymphomas and Hodgkin’s disease cause multiple nodular or diffuse involvement. Diagnostic accuracy of US reached 87.2 %, although it was demonstrated that different splenic diseases may present a similar echostructure (Table 1).
Table 1.
Echostructure of focal splenic lesions
| Hypo-echoic lesion | Hyperechoic lesion |
|---|---|
| Cyst | Hemorrhagic cyst |
| Metastasis | Metastasis |
| Abscess | Abscess |
| Acute hematoma | Subacute hematoma |
| Angioma | Angioma |
| Infarction | Subacute chronic infarction |
| Lymphoma | Calcification |
| Lymphangioma |
Currently the indications for US imaging of the spleen are the following: measurement of splenic dimensions, evaluation of changes in splenic volume in patients with hematologic diseases, diagnosis of focal lesions (cyst, tumor, infarction, abscess, calcification), evaluation of splenic vessels, portal hypertension and US-guided interventional procedures involving the spleen [1, 6–8].
The splenic vascularization is quite particular. The splenic artery divides into trabecular arteries before subdividing into small arterioles which become central arterioles surrounded by lymphoid follicles. After the lymphatic sheath, the white pulp, the arterioles divide into the penicillar arteries which give origin to the precapillary arterioles (sheathed capillaries) and they open into the venous sinuses which are lined with endothelial cells. The venous sinuses allow the blood to percolate the red pulp and continue in the veins of the pulp reaching the trabeculae. It is possible that this type of vascularization facilitates the study of the spleen using second-generation US contrast agents (CEUS). After only 6–12 s, there is a contrast enhancement of the arterial vessels which ramify from the splenic hilum into the splenic tissue. This phase is followed by parenchymal enhancement, which is initially inhomogeneous, the so-called “zebra pattern”, but after 50–60 s enhancement becomes intense and homogeneous [9]. The arterial phase is completed in 30–60 s and the spleen remains intensely echoic and homogeneous for up to 8–9 min. Then contrast agent is gradually eliminated and the organ slowly returns to the initial echostructure. In this period, hyperechoic spots may appear, this is probably due to the persistence of contrast agent within the venous sinuses, which are characterized by a slower circulation [10].
Second-generation contrast agents produce a spleen-specific enhancement which lasts longer than the vascular phase, probably due to contrast agent uptake in the parenchymal cells. Another hypothesis is that this specific uptake of contrast agent is due to the persistence of microbubbles in the splenic sinusoids. These findings are probably useful for detecting and characterizing focal lesions of the spleen [11, 12].
Contrast-enhanced US is, thus, useful in the study of splenic focal lesions as this method allows identification of the ectopic splenic tissue (splenosis and accessory spleen), characterization of splenic infarctions, necrotic lesions and abscesses, splenic lesions caused by trauma and cystic lesions (congenital cyst, parasitic cyst, secondary cyst, dermoid cyst, angioma and cystic lymphangioma).
Contrast-enhanced US also allows the formulation of a differential diagnosis between benign and malignant lesions (angioma and hamartoma versus lymphoma metastasis).
Splenic infarction
The incidence of splenic infarction is 9.7 %; infarction is caused by occlusion of the splenic artery or vein thrombosis of the splenic sinusoids. The whole spleen may be involved in splenic vein thrombosis causing massive splenomegaly.
Infarction usually occurs in patients with sickle cell anemia, hematological malignancies and immunological disorders, thrombophilia and emboli due to endocarditis, but more recently it has been reported as a complication of chemoembolization for hepatocellular carcinoma. Splenic infarction normally causes pain of varying intensity in the left upper quadrant and low-grade fever.
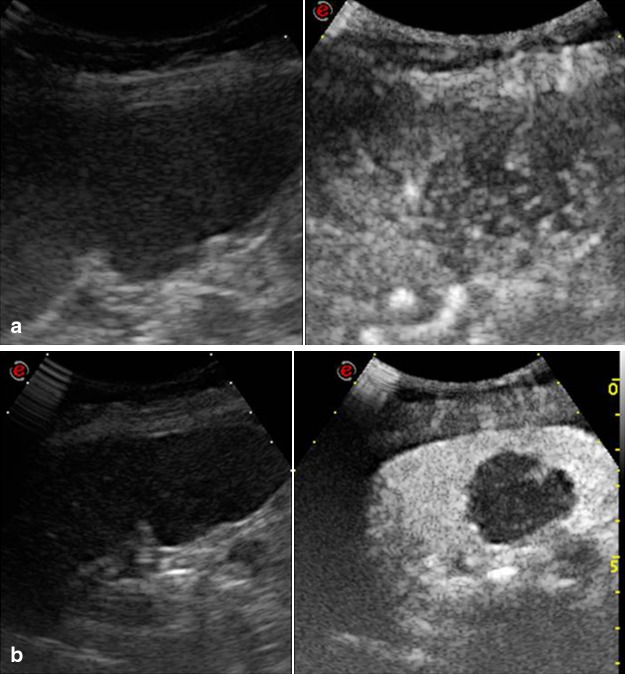
B-mode US is of little use in the acute phase of splenic infarction due to a high incidence of false negative results (US does not reveal acute infarction in 50 % of cases). However, US imaging sometimes depicts ill-defined hypo-echoic triangular-shaped lesions (Fig. 1a, b). In the healing phase, fibrosis causes the US image to become echoic showing a roughly triangular shape with the base facing the splenic capsule. If the infarction is large, color Doppler may show an area with no blood supply, but CT is the diagnostic method which has the highest sensitivity and specificity in these cases. In case of suspected splenic infarction, the use of second-generation US contrast agent permits identification of areas with/without enhancement in the arterial and venous phases in 100 % of cases showing a triangular, sometimes roundish or irregular shape with the base facing the splenic capsule (Fig. 2a, b) [2, 10].
Fig. 1.
a, b Left coronal scan. Hypo-echoic lesion; triangular shape, subcapsular base and apex pointing toward the hilum, secondary to recent splenic infarction
Fig. 2.
a, b Left subcostal oblique scan; a inhomogeneous splenomegaly; b CEUS depicts the lesions as hypo-anechoic avascular masses due to multiple infarctions
Splenic cysts
Splenic cysts are the most common benign lesions, even though they occur in a percentage lower than that of the liver, kidney and ovary. They can be divided into:
Primary (true) cysts (21.35 %) which have epithelium, endothelium or membrane lining:
congenital: epithelial, mesothelial, dermoid
parasitic: Echinococcus granulosus or Taenia solium
neoplastic (lymphangioma and cystic angioma)
Secondary (false) cysts which have no cellular lining:
Simple or epithelial cysts (25 % of true cysts) may be solitary, multiple or linked to a polycystic disease and they do not pose diagnostic problems as they appear at US imaging as anechoic lesions with well-defined regular contours, with no evident cyst wall or alteration of the surrounding tissue. Splenic cysts are always intra-parenchymal, as opposed to liver and kidney cysts that may be exophytic (Fig. 3).
Fig. 3.
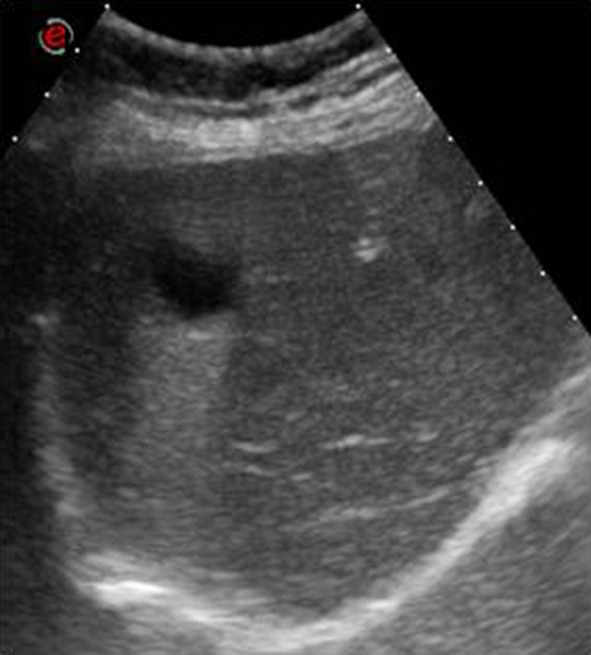
Left coronal scan. Anechoic splenic lesion with regular and well-defined margins: a simple cyst
Secondary (false) cysts are usually caused by a traumatic injury, infarction or infectious processes such as mononucleosis, tuberculosis, malaria, and/or abscess. These pseudocysts usually contain fluid and necrotic debris.
Hemorrhage and infection can change the echotexture also of simple cysts, as these conditions determine the presence of fine echoes, septa and echoic changes. This aspect is also typical of secondary cysts, which may also present irregular contours and sometimes calcifications. CEUS is not indicated in congenital cysts, especially if they are not associated with complications.
Epidermoid cysts of the spleen are most common in young people and in women, and they may be of familial origin. These cysts are usually asymptomatic, but can cause pain if they are large or complicated by hemorrhage and/or rupture. They are caused by a traumatic injury of the capsule followed by growth within the mesothelium and subsequent metaplastic changes.
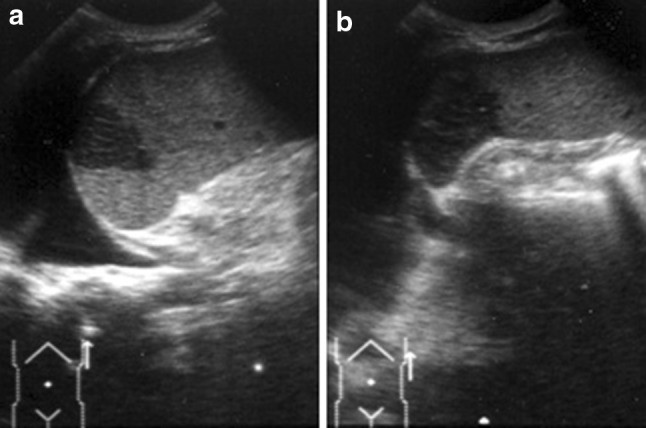
Dermoid cysts are lined by squamous epithelium which is positive for keratin and negative for factor VIII [13, 14]. Gray-scale US shows rounded hypo-anechoic lesions ranging from 5 to 300 mm in diameter with a capsule often characterized by peripheral trabeculation and finely echoic contents due to the presence of cholesterol crystals and/or lamellae of keratin; this image changes when the patient is lying down (snow globe effect) (Fig. 4). Color Doppler is useful for differentiating epidermoid cysts from cyst-like lesions such as lymphoma and cystic neoplasm. In this splenic disease, CEUS shows anechoic lesions in all phases, but in the arterial phase there may be enhancement of the wall if it is vascularized.
Fig. 4.
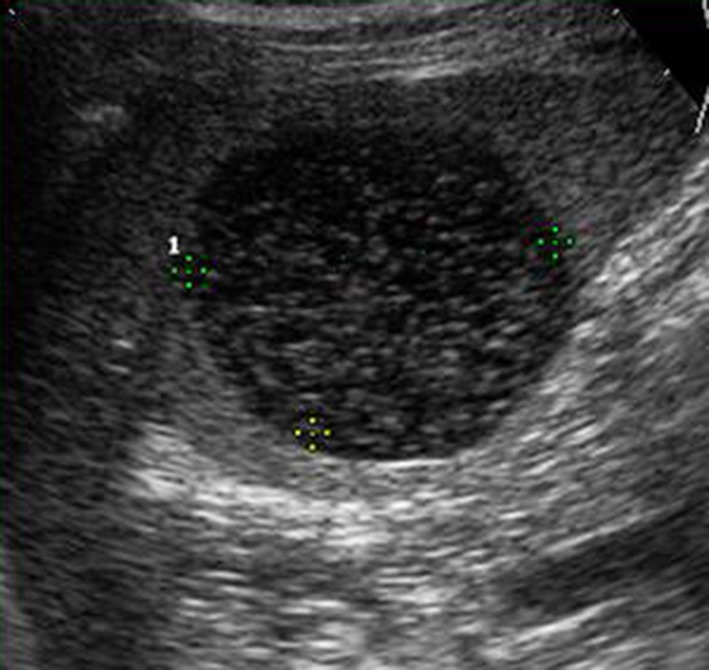
Left coronal scan. Hypo-echoic rounded, splenic lesion with floating internal echoes: dermoid splenic cyst
Lymphangioma is a relatively rare, congenital malformation caused by obstruction and subsequent dilatation of the lymphatic ducts; it is mainly located at the neck (75 %) and armpits (20 %) and is often associated with splenic lymphangioma. The lesion is rarely symptomatic, but if it is large (10–20 cm) it may cause pressure and bleeding due to coagulopathy and portal hypertension [15, 16]. It may be cystic or solid due to sclerosis of the cystic cavities. Histologically, it is classified into three subtypes: capillary, cavernous, and cystic. Cystic lymphangioma is the most frequent form, and lymphangioma is, therefore, often referred to as cystic lymphangioma.
Ultrasound imaging of multiloculated lymphangioma shows multiple hypo-echoic cysts of various dimensions with echoic septa. Capillary lymphangioma is rare and appears echoic and pseudo solid. Capillary and cavernous lymphangioma are difficult to differentiate from angioma. Splenic lymphangioma is usually a subcapsular lesion and color Doppler may show intrasplenic vessels with normal flow in the largest septa [12].
In the arterial phase, CEUS shows enhancement of the septa and the surrounding splenic tissue, with no or a modest washout in the parenchymal phase.
Splenic hydatid cyst is the third most common site of cystic echinococcosis after the liver and lung. These lesions are rare even in endemic regions, but in developing countries they account for two-thirds of splenic cysts (Table 2).
Table 2.
Geographic distribution of splenic echinococcosis (%)
| Germany | 0.50 |
| Iceland | 0.78 |
| Uruguay | 1.00 |
| Australia | 2.10 |
| Argentina | 2.14 |
| Italy/Caremani (2004) | 3.47 |
| Iran | 4.00 |
| India | 4.30 |
| Greece (2003) | 5.40 |
| Sudan/Njroga (2001) | 8.30 |
A splenic hydatid cyst is frequently associated with visceral involvement, and it indicates general dissemination of the disease, although it may be a sign of rupture of peritoneal cysts. Depending on the stage of the disease, Gray-scale US shows unilocular or multilocular cysts (type I–II), cysts with detachment of the wall or complex cysts (type III) (Fig. 5), and/or solid or calcified cysts (type IV–V).
Fig. 5.
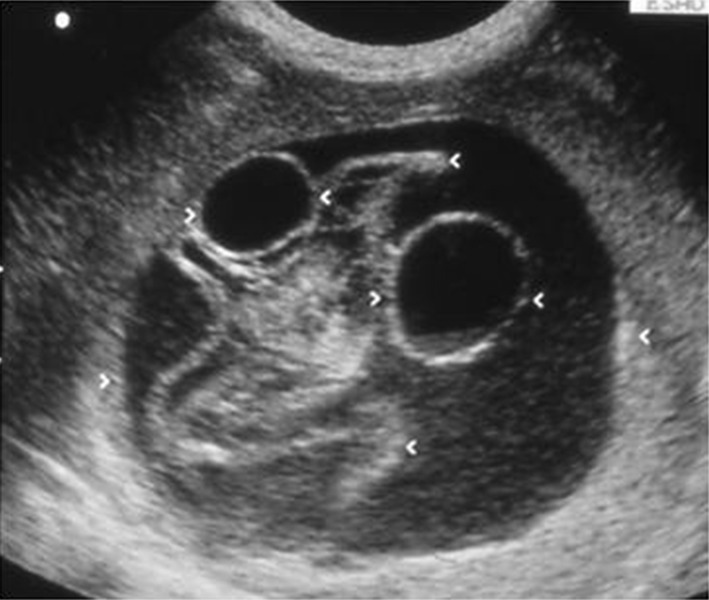
Right subcostal oblique scan. Anechoic rounded lesion containing two anechoic cysts and linear echoic structures: hydatid cyst with two daughter cysts and floating parasitic membranes
At Gray-scale US, solid hydatid cysts of the spleen (type IV) appear iso- or hyperechoic with well-defined margins. Color Doppler shows no vascular signals, and it may be difficult to differentiate these cysts from other solid lesions of the spleen. However, CEUS depicts lesions which do not absorb contrast agent, thereby indicating a non-vascular disease, confirming the clinical suspicion of parasitic lesions which are no longer active.
Splenic calcifications
In addition to echinococcosis, which is identified by the presence of complex lesions of large dimensions with wall calcifications or coarse echoic membranes (Fig. 6), calcifications are also found secondary to the episodes of disseminated tuberculosis involving the splenic tissue, i.e., tubercular lesions which have caused changes in the splenic tissue. In HIV infected patients, histoplasmosis, Pneumocystis carinii and Mycobacterium avium intracellulare are common causes of splenic calcifications. US shows multiple, disseminated, hyperechoic lesions in some cases with a small shadow cone [8]. Focal accumulation of hemosiderin and calcium deposition in the splenic parenchyma secondary to hemorrhage are called Gamna-Gandy bodies which are common in patients with cirrhosis and portal hypertension as well as in patients with schistosomiasis, splenic vein thrombosis, hemolytic anemia, hemochromatosis and secondary to trauma [2].
Fig. 6.
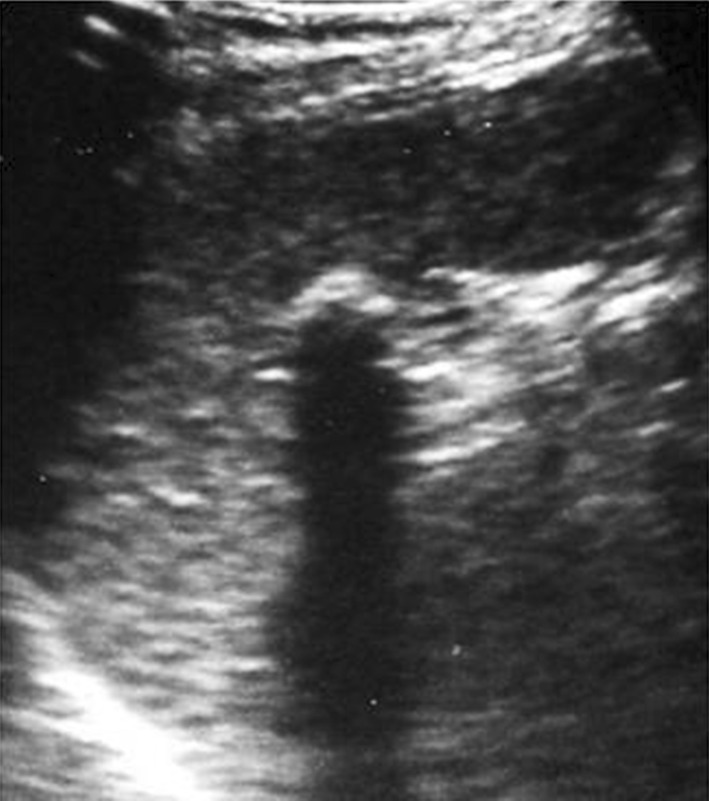
Left coronal scan. Hyperechoic lesion at the splenic hilum with acoustic shadow due to splenic calcification
Splenic abscess
Splenic abscess is a collection of pus commonly caused by hematogenous spread of infection. Drug addicts with bacterial endocarditis may have splenic abscesses, but other causes may be penetrating wounds or infectious complications of hematomas or infarctions [2]. The incidence of splenic abscess (bacterial, fungal, mycobacterial, Pneumocystis pneumonia) is growing due to the increasing number of patients with AIDS-related and non-AIDS-related immunodepression. The triad of clinical findings suggesting the presence of a splenic abscess is fever, pain in the left upper quadrant of the abdomen and leukocytosis. However, only 44 % of patients with splenic abscess present with these symptoms [17]. Splenic abscesses are rare, but mortality rate is high if diagnosis and treatment are delayed. If splenic abscess is suspected, US-guided percutaneous drainage is indicated to perform bacterial cultivation of the pus and achieve a correct diagnosis. This procedure is also the first step in the therapeutic management.
Gray-scale US imaging of a splenic abscess is not specific; the image shows a hypo-echoic lesion with a thick irregular wall (Fig. 7), but this aspect may vary depending on the etiology and the size of the lesion. Abscesses are therefore classified in three types according to the US pattern:
Hypo- or iso-echoic lesions, often solitary and larger than 2–3 cm in diameter with septa or areas of hyperechogenicity, maybe with reverberation artifacts due to the presence of gas (bacteria).
Lesions, sometimes multiple and smaller than 2 cm in diameter, hypo-echoic, with “target” appearance, “wheel within a wheel” appearance or hyperechoic (fungus, Pneumocystis carinii, Bartonella).
Multiple, homogenously hypo-echoic lesions 10–20 mm in diameter (mycobacteria).
Fig. 7.
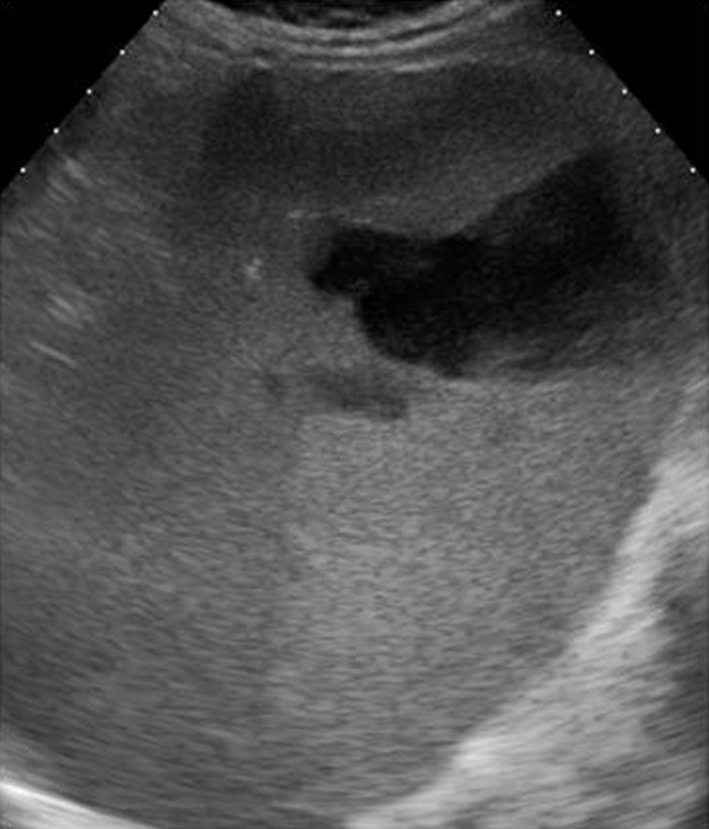
Left subcostal oblique scan. Anechoic lesion with triangular base facing the splenic capsule secondary to splenic infarction leading to formation of a splenic abscess
At CEUS, there is no uptake of contrast agent in the larger abscesses at any stage, but there may sometimes be ring enhancement and enhancement of the perilesional tissue and septa. The perilesional enhancement may be replaced in the late phase by a hypo-echoic area due to washout of contrast agent.
Also in small abscesses, no uptake of contrast agent is observed but there may be slight ring enhancement. Differential diagnosis between abscess and lymphoma may be difficult even at CEUS, especially in infectious lesions of small dimensions; in these cases clinical data and percutaneous US-guided drainage and bacterial culture may be useful [9, 10].
Angioma/hamartoma
Angioma is the most common benign lesion it is usually small, less than 2 cm in diameter. The incidence of angioma ranges from 10 to 14.56 % and prevalence at autopsy is 0.3–14 % [2, 18, 19]. This benign tumor derives from the sinusoidal epithelium and is characterized by proliferation of spaces containing blood and covered with endothelium, it may contain capillaries and/or cavernous vascular channels, and may be prone to thrombosis and hemorrhage [8]. Angioma is usually asymptomatic and it is often an incidental finding, but large peripheral angiomas may rupture and cause bleeding. US imaging will show two patterns depending on the type of angioma, if it is a capillary lesion or a lesion of the vascular channels, which also permits distinction at US imaging between capillary and cavernous angioma.
Capillary angioma is a predominantly iso-echoic (Fig. 8) or hyperechoic lesion, roundish with irregular, but well-defined margins and trabecular structure. It is solitary or multiple (Fig. 9) and is often small or medium sized, it accounts for 77.8 % of angiomas.
Fig. 8.
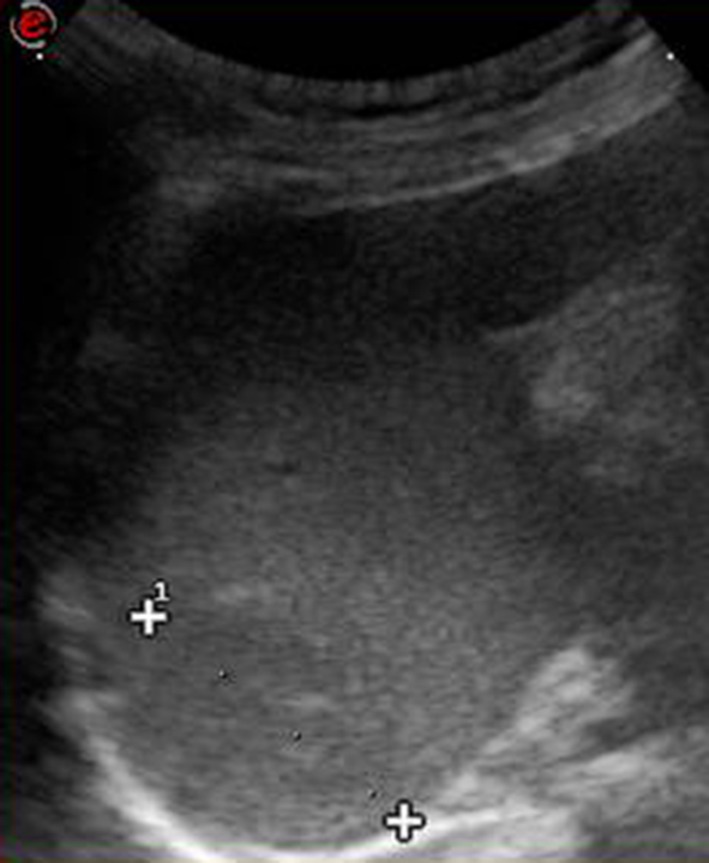
Left coronal scan. Lesion in the upper pole of the iso-echoic spleen (calipers) due to splenic hemangioma
Fig. 9.
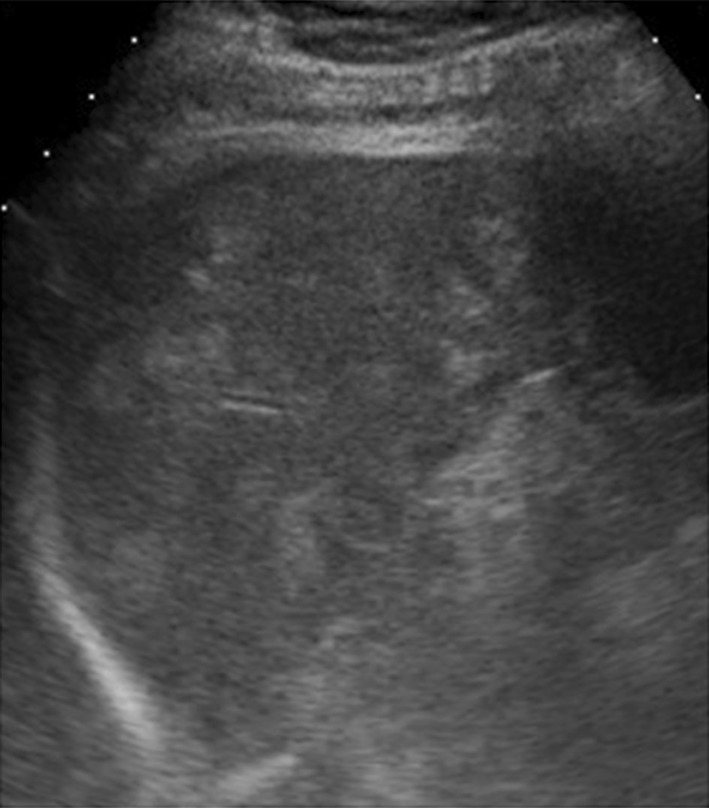
Left coronal scan. Small hyperechoic splenic lesions due to angiomatosis
Cavernous angioma appears inhomogeneous with a mixed pattern, sometimes with cysts and and/or calcifications. In the most common forms of this disease, Gray-scale US imaging suggests a diagnosis, but color Doppler does not add useful data, as there are no vascular signals in 81.5 % of cases [3].
In the arterial phase, CEUS shows iso-echoic, hyperechoic or globular enhancement and centripetal filling-in of the lesion in 59.2 % of cases, followed by homogeneous complete or incomplete enhancement of the splenic parenchyma in the parenchymal phase (due to fibrosis and/or thrombosis). In 29.6 % of cases angiomatous lesions are hypo-echoic, and in 11.1 % there is a washout in the parenchymal phase [3].
Hamartoma also referred to as focal nodular hyperplasia of the spleen, is a benign neoformation originating from the red pulp, and prevalence at autopsy is 0.13 %. Hamartoma is often smaller than 30 mm in diameter and it is composed of an aberrant mixture of normal spleen tissue with cystic sinusoidal dilation [12].
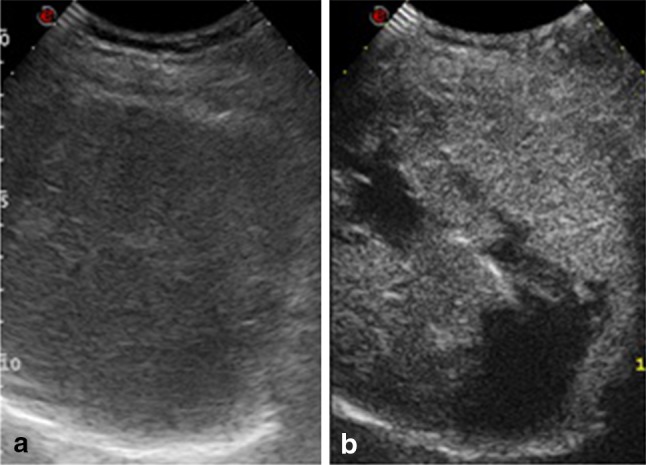
Ultrasound image is non-specific and characterized by hypo-echoic lesions (Fig. 10a), sometimes with multiple anechoic cystic changes with septa or an inhomogeneous appearance. Color Doppler may show hypervascular lesions, but a differential diagnosis should include angioma, metastasis, lymphoma, abscess, and hematoma.
Fig. 10.
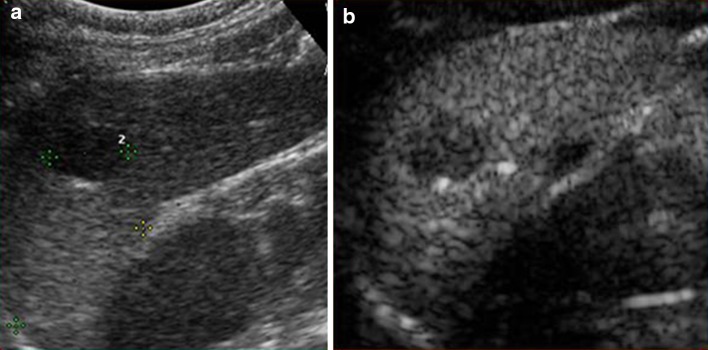
a Left oblique coronal scan. Hypo-echoic splenic lesion (calipers). b CEUS, parenchymal phase. After discrete arterial enhancement there is modest washout: splenic hamartoma
Contrast-enhanced US is useful for confirming the presence of a hypervascular lesion and may show intense and homogeneous centripetal or centrifugal enhancement and moderate washout (Fig. 10b) [20]. However, despite the use of all available imaging methods including CEUS, CT and MRI, final diagnosis is often angioma/hamartoma as it very difficult to distinguish between these two lesions.
Splenic metastasis
Splenic lesions are detected in 0.103–0.6 % of US examinations and only 1.3–4.85 % of these lesions are suspicious for metastatic disease. Splenic metastases have been disclosed in 2.3–12.9 % of autopsies performed in patients who died of cancer, and in 1.3 % of splenectomies performed for non-traumatic reasons [2, 21, 22]. Secondary neoplastic lesions limited to the spleen are considered very rare and US imaging identifies the primary tumor in the abdomen in 70–81 % of cases [23]. Splenic metastases are always a sign of advanced disease and often irrelevant from a prognostic point of view. The most frequent cause of splenic metastasis is breast cancer, melanoma, and lung cancer (21 %), but splenic metastasis may also be secondary to malignant tumors in the stomach (16 %), pancreas (12 %), liver (9 %), and colon (9 %).
On Gray-scale US image, splenic metastases appear hypo-echoic in 40–44 %, iso-echoic in 38–40 %, hyperechoic 10–40 %, and with “target appearance” in 10–20 %, and they may be both hypo-vascular (6–33.3 %) and hypervascular (66–66.6 %).
Contrast-enhanced US in hypo-vascular splenic metastases shows poor or absent enhancement in the arterial phase; there may be ring enhancement, but rapid washout in the parenchymal phase. Hypervascular metastases show an intense homogeneous or inhomogeneous enhancement in the arterial phase, but the lesion becomes rapidly hypo-echoic due to washout (Fig. 11a, b). In the detection of splenic metastases CEUS is 38 % more sensitive than Gray-scale US [1, 24, 25].
Fig. 11.
a, b CEUS, left coronal scan. Iso-echoic splenic lesion; a arterial phase, rapid, intense, and homogeneous enhancement; b venous phase, rapid washout and the lesion becomes hypo-echoic: hypervascular splenic metastasis
Splenic lymphoma
The spleen is involved in 30–40 % of cases of systemic lymphoma (Hodgkin’s disease 33 %, non-Hodgkin’s lymphoma 50 %), while primitive lymphoma of the spleen is rare (<1 %). Hematological malignancy accounts for 15.6 % of focal splenic lesions arising in the white pulp and then spreading to the red pulp [22, 27].
On Gray-scale US image, splenic lymphoma appears hypo-echoic (Fig. 12) in 60–70 % of cases, rarely showing “target appearance” (15.4 %) or heterogeneous pattern, which is typical of the recurrent lesions after therapy, while hyperechoic lesions are rare, accounting for only 8.8 % of hyperechoic splenic abnormalities, which usually do not exceed 4.2 % [1, 26, 27].
Fig. 12.
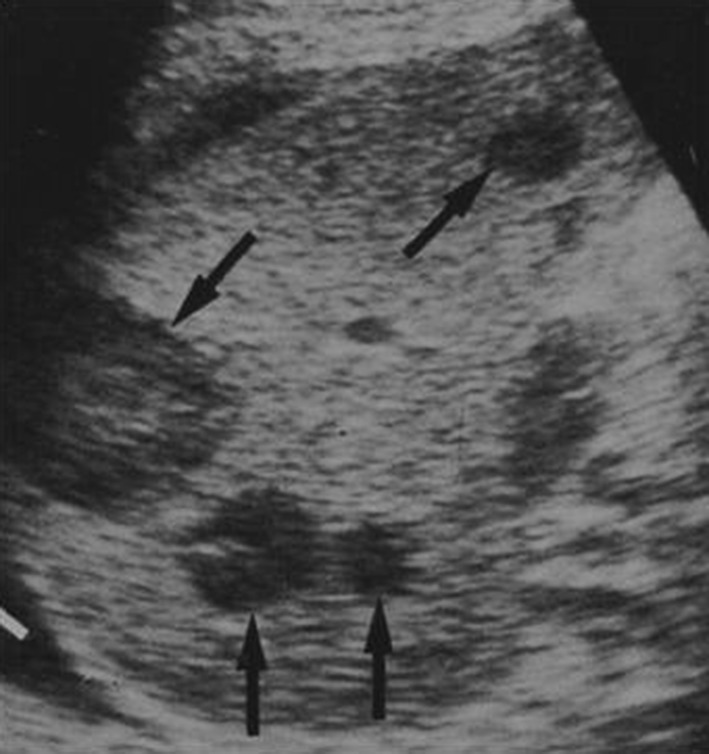
Left coronal scan. Inhomogeneous splenic appearance due to the presence of numerous hypo-echoic lesions: non-Hodgkin’s lymphoma of the spleen
In patients with splenic lymphoma, US shows abdominal lymphomatous lesions in 69–100 % of cases, while CT detects these lesions in 77–100 % of cases [1, 27–29]. US may show morphological features such as, diffuse miliary, or micronodular infiltration which is characteristic of low-grade malignant lymphomas, while macronodular lesions, also referred to as bulky lesions, are found in high-grade malignant lymphomas [28, 29].
In the arterial phase, CEUS shows lesions with iso-enhancement (5–6 s) in 37.5–46.35 % of cases, moderate hypo-enhancement in 50–53 % and hyper-enhancement only in 12.5 % compared to the surrounding parenchyma. In the parenchymal phase, there is a marked hypo-enhancement after only 60 s in 100 % of cases due to rapid washout [24, 30].
Differentiation between benign and malignant splenic lesions
The main difference between benign and malignant splenic focal lesions is evidenced at CEUS by the different dynamics of uptake and washout of sulfur hexafluoride contrast agent in the arterial and parenchymal phase. In the arterial phase, benign lesions show iso-hyper-enhancement followed in the parenchymal phase by slow, modest and incomplete washout in only 31 % of cases. The great intensity of enhancement in the arterial phase in benign splenic pathologies may reflect hypervascularity caused by an increased arterial blood flow, while modest washout might be due to the presence of spleen-like sinusoidal epithelium [31]. In the arterial phase, malignant focal splenic lesions present inhomogeneous enhancement which may be poor and in some cases peripheral or absent with areas of hypo-enhancement associated with a rapid and complete washout, indicating a low uptake of contrast medium. Elimination of microbubbles from malignant lesions is faster than from benign lesions. Reduced enhancement of malignant splenic lesions is probably due to neoangiogenesis, which creates a poor and slow flow. Rapid washout at the arteriovenous anastomoses is due to neoplastic vascularization, and persistent and punctiform enhancement is caused by the persistence of microbubbles in the microcirculation due to the loss of sinusoidal space.
A lesion which is constantly non-enhanced or iso-enhanced in the parenchymal phase is to be considered benign in 100 % of cases, whereas a lesion showing progressive hypo-enhancement is predictive of malignancy in 87 % of cases [31].
The diagnostic effectiveness of CEUS seems to be higher in the study of iso-hypo-echoic lesions than in hyperechoic lesions identified accidentally at Gray-scale US. Focal hyperechoic irregularities discovered incidentally at Gray-scale US imaging have a low-risk of being malignant, although CEUS yields false positive outcome in 15–22 % of cases, whereas iso-hypo-echoic neoformations incidentally identified at Gray-scale US with no history of malignancy have a risk of being malignant in 34.3 % of cases; in these lesions CEUS has a diagnostic accuracy of 91–94 % with a negative predictive value (NPV) of 100 %. [31].
Conclusions
EFSUMB 2011 guidelines consider CEUS indicated in focal splenic diseases:
To characterize splenic parenchymal inhomogeneity or suspected lesions.
To confirm suspected splenic infarction.
To confirm the presence of ectopic splenic tissue.
To detect splenic malignant lesions in oncologic patients when CT and/or MRI and PET are contraindicated or inconclusive.
To these guidelines, a number of important clinical indications for CEUS should be added:
Differential diagnosis between liver abscess and lymphoma.
Differential diagnosis between benign and malignant focal lesions, as CEUS has a sensitivity of 90 % and a specificity of 100 %.
Characterization of cystic and necrotic lesions.
To identify possible splenic lesions in patients with fever and/or pain in the left upper quadrant.
Suspected cystic echinococcosis.
To decide which patients should undergo US-guided biopsy.
Conflict of interest
None.
References
- 1.Wan YL, Cheung YC, Lui KW, Tseng JH, Lee TY. Ultrasonographic findings and differentiation of benign and malignant focal splenic lesions. Postgrad Med J. 2000;76:488–493. doi: 10.1136/pmj.76.898.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen MJ, Huang MJ, Chang WH, Wnng TE, Wang HY, Chu CH, et al. Ultrasonography of splenic abnormalities. Word J Gastroenterol. 2005;11:4061–4066. doi: 10.3748/wjg.v11.i26.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taibbi A, Bartolotta TV, Matraga D, Midiri M, Lagalla R. Splenic hemangiomas: contrast-enhanced sonographic findings. J Ultrasound Med. 2012;31:543–553. doi: 10.7863/jum.2012.31.4.543. [DOI] [PubMed] [Google Scholar]
- 4.Solbiati L, Bossi MC, Bellotti E, Ravetto C, Montali G. Focal lesions in the spleen: sonographic patterns and guided biopsy. AJR Am J Roentgenol. 1983;140:59–65. doi: 10.2214/ajr.140.1.59. [DOI] [PubMed] [Google Scholar]
- 5.Goerg C, Schwerk WB, Goerg K. Splenic lesions: sonographic patterns, follow-up, differential diagnosis. Eur J Radiol. 1991;13:59–66. doi: 10.1016/0720-048X(91)90058-4. [DOI] [PubMed] [Google Scholar]
- 6.Shetty CM, Lakhkar BN, Pereira NM, Koshy SM. Role of ultrasonography and computed tomography in the evaluation of focal splenic lesions. Abdom Imaging. 2005;15:183–190. [Google Scholar]
- 7.Wernecke K, Peters PE, Krüger KG. Ultrasonographic patterns of focal hepatic and splenic lesions in Hodgkin’s and non-Hodgkin’s lymphoma. Br J Radiol. 1987;60:655–660. doi: 10.1259/0007-1285-60-715-655. [DOI] [PubMed] [Google Scholar]
- 8.Benter T, Klühs L, Teichgräber U. Sonography of the spleen. J Ultrasound Med. 2011;30:1291–1293. doi: 10.7863/jum.2011.30.9.1281. [DOI] [PubMed] [Google Scholar]
- 9.Lim AK, Patel N, Eckersley RJ, Taylor-Rosbinson SD, Cosgrove DO, Blomley MJ. Evidence for spleen-specific uptake of a microbubble contrast agent: a quantitative study in healthy volunteers. Radiology. 2004;231:785–788. doi: 10.1148/radiol.2313030544. [DOI] [PubMed] [Google Scholar]
- 10.Görg C, Bert T. Second-generation sonographic contrast agent for differential diagnosis of perisplenic lesions. AJR Am J Roentgenol. 2007;186:621–626. doi: 10.2214/AJR.04.1900. [DOI] [PubMed] [Google Scholar]
- 11.Balzan SM, Riedner CE, Santos LM, Pazzinatto MC, Fontes PR. Posttraumatic splenic cysts and partial splenectomy: report of a case. Surg Today. 2001;31:262–265. doi: 10.1007/s005950170183. [DOI] [PubMed] [Google Scholar]
- 12.Giovagnoni A, Giorgi C, Goteri C. Tumors of the spleen. Cancer Imaging. 2005;5:73–77. doi: 10.1102/1470-7330.2005.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catalano O, Sandomenico F, Matarazzo I, Siani A. Contrast-enhanced sonography of the spleen. AJR Am J Roentgenol. 2005;184:1150–1156. doi: 10.2214/ajr.184.4.01841150. [DOI] [PubMed] [Google Scholar]
- 14.Shankar V, Ramachandra U. Epidermoid cyst of spleen. OJHAS. 2010;9:1–13. [Google Scholar]
- 15.Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, et al. The EFSUMB Guidelines and Recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33–59. doi: 10.1055/s-0031-1281676. [DOI] [PubMed] [Google Scholar]
- 16.Schininà V, Rizzi EB, Mazzuoli G, David V, Bibbolino C. US and CT findings in splenic focal lesions in AIDS. Acta Radiol. 2000;41:616–620. doi: 10.1080/028418500127346018. [DOI] [PubMed] [Google Scholar]
- 17.Changchien CS, Tsai TL, Hu TH, Chiou SS, Kuo CH. Sonographic patterns of splenic abscess: an analysis of 34 proven cases. Abdom Imaging. 2002;27:739–745. doi: 10.1007/s00261-002-0013-7. [DOI] [PubMed] [Google Scholar]
- 18.Willcox TM, Speer RW, Schlinkert RT, Sarr MG. Hemangioma of the spleen: presentation, diagnosis, and management. J Gastrointest Surg. 2000;4:611–613. doi: 10.1016/S1091-255X(00)80110-9. [DOI] [PubMed] [Google Scholar]
- 19.Andrews MW. Ultrasound of the spleen. World J Surg. 2000;24:183–187. doi: 10.1007/s002689910031. [DOI] [PubMed] [Google Scholar]
- 20.Görg C, Görg K, Bert T, Barth P. Colour Doppler ultrasound patterns and clinical follow-up of incidentally found hypoechoic, vascular tumours of the spleen: evidence for a benign tumour. Br J Radiol. 2006;79:319–325. doi: 10.1259/bjr/81529894. [DOI] [PubMed] [Google Scholar]
- 21.Kraus MD, Fleming MD, Vonderheide RH. The spleen as a diagnostic specimen: a review of 10 years’ experience at two tertiary care institutions. Cancer. 2001;91:2001–2009. doi: 10.1002/1097-0142(20010601)91:11<2001::AID-CNCR1225>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Compérat E, Bardier-Dupas A, Camparo P, Capron F, Charlotte F. Splenic metastases: clinicopathologic presentation, differential diagnosis and pathogenesis. Arch Pathol Lab Med. 2007;131:965–969. doi: 10.5858/2007-131-965-SMCPDD. [DOI] [PubMed] [Google Scholar]
- 23.Metser U, Miller E, Kessler A, Lerman H, Lievshitz G, Oren R, et al. Solid splenic masses: evaluation with 18F-FDG PET/CT. J Nucl Med. 2005;46:52–59. [PubMed] [Google Scholar]
- 24.von Herbay A, Barreiros AP, Ignee A, Westendorff J, Gregor M, Galle PR, et al. Contrast-enhanced ultrasonography with SonoVue: differentiation between benign and malignant lesions of the spleen. J Ultrasound Med. 2009;28:421–434. doi: 10.7863/jum.2009.28.4.421. [DOI] [PubMed] [Google Scholar]
- 25.Neesse A, Huth J, Kunsch S, Michl P, Bert T, Tebbe JJ, et al. Contrast-enhanced ultrasound pattern of splenic metastases––a retrospective study in 32 patients. Ultraschall Med. 2010;3:264–269. doi: 10.1055/s-0028-1109812. [DOI] [PubMed] [Google Scholar]
- 26.Mollejo M, Algara P, Mateo MS, Sánchez-Beato M, Lloret E, Medina MT, et al. Splenic small B-cell lymphoma with predominant red pulp involvement: a diffuse variant of splenic marginal zone lymphoma? Histopathology. 2002;40:22–30. doi: 10.1046/j.1365-2559.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- 27.Goerg C, Schwerk WB, Goerg K. Sonography of focal lesions of the spleen. AJR Am J Roentgenol. 1991;156:949–953. doi: 10.2214/ajr.156.5.2017957. [DOI] [PubMed] [Google Scholar]
- 28.Urrutia M, Mergo PJ, Ros LH, Torres GM, Ros PR. Cystic masses of the spleen: radiologic-pathologic correlation. Radiographics. 1996;16:107–129. doi: 10.1148/radiographics.16.1.107. [DOI] [PubMed] [Google Scholar]
- 29.Ishida H, Konno K, Ishida J, Naganuma H, Komatsuda T, Sato M, et al. Splenic lymphoma: differentiation from splenic cyst with ultrasonography. Abdom Imaging. 2001;26:529–532. doi: 10.1007/s00261-001-0006-y. [DOI] [PubMed] [Google Scholar]
- 30.Görg C. The forgotten organ: contrast enhanced sonography of the spleen. Eur J Radiol. 2007;64:189–201. doi: 10.1016/j.ejrad.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 31.Stang A, Keles H, Hentschke S, von Seydewitz CU, Dahlke J, Habermann C, et al. Incidentally detected splenic lesions in ultrasound: does contrast-enhanced ultrasonography improve the differentiation of benign hemangioma/hamartoma from malignant lesions? Ultraschall Med. 2011;6:582–592. doi: 10.1055/s-0031-1282034. [DOI] [PubMed] [Google Scholar]