Abstract
Pain is one of the most common causes of reduced productivity. The annual cost of health-related reductions in productivity has been estimated at approximately 225 billion dollars in the United States alone. Ultrasound-guided locoregional infiltration procedures have frequently been shown to offer economical, effective, lasting relief of pain. In-depth familiarity with the equipment (probes and needles) and techniques used to perform these procedures are fundamental for safe, effective treatment. In fact, depending on the characteristics of the patient and the clinical problem, the approach and technique may have to be modified to simplify the procedure and ensure better results. Up-to-date knowledge of the drugs used for these infiltrations (indications, how they are used) is equally important. Our aim is to provide an update on the techniques and materials used in interventional musculoskeletal ultrasonography based on a review of the most recent literature as well as on our personal experience.
Keywords: Interventional ultrasonography, Musculoskeletal pain, Pain management
Riassunto
Il dolore è una delle cause più frequenti di riduzione della produttività. Si stima che i costi della riduzione della produttività legata a problemi di salute siano di circa 225 miliardi di dollari per anno, nei soli Stati Uniti. Tra i trattamenti che hanno più frequentemente dimostrato di possedere caratteristiche di economicità, efficacia e durevolezza nella riduzione del dolore vi sono indubbiamente i trattamenti infiltrativi loco-regionali eco-guidati. E’ fondamentale avere una conoscenza approfondita delle apparecchiature (sonde e aghi) e delle diverse tecniche per raggiungere il sito di iniezione efficacemente ed in sicurezza. Infatti, in base alle caratteristiche del paziente e al problema clinico, può essere necessario cambiare approccio e tecnica al fine di raggiungere più semplicemente il miglior risultato. Non meno importante è un costante aggiornamento sulla corretta selezione e impiego delle sostanze da iniettare. Ci proponiamo pertanto di realizzare un update sulle tecniche e sui materiali utilizzati in ecografia interventistica muscoloscheletrica, alla luce sia della letteratura più recente che della nostra personale esperienza.
Introduction
Pain is one of the most frequent causes of reductions in productivity, reflected by sick leave and decreases in workers’ ability to carry out their jobs. The annual cost of reductions in productivity related to health problems has been estimated at approximately 225 billion dollars in the United States alone [1], and roughly 13 % of these costs are caused by pain. Musculoskeletal pain alone (degenerative or inflammatory in nature) accounts for approximately 7 % of the total [2].
Recent studies demonstrate that interventions aimed at limiting these decreases in productivity can produce a return of approximately 3 dollars for every dollar spent in reducing absences from the workplace [3]. These figures highlight the economic importance and strong social impact of the growing use of low-cost interventions capable of producing appreciable, long-lasting pain relief. Among the approaches found to offer economical, effective, lasting relief of pain, the most frequently cited are based on ultrasound-guided locoregional infiltration. In the scientific community, there is an ongoing debate on which drugs produce the best results for each disorder and site of involvement. A sonographer who intends to perform an US-guided infiltration procedure must, therefore, be capable of selecting the most appropriate drug for the task at hand. There is undoubtedly less controversy over the sonographic technique that should be used to guide the injection. However, selection of the transducer, needle, and the injection approach are of fundamental importance: use of an approach that is anatomically problematic or an inappropriate transducer or needle increases the risk of complications, prolongs procedure time, and reduces the effectiveness of the treatment.
In light of these reflections, the aim of this article is to provide an update on the techniques and materials used in interventional musculoskeletal ultrasonography based on a review of the most recent literature as well as on our personal experience.
Drugs used
Numerous drugs are currently used during interventional musculoskeletal ultrasound procedures. Their characteristics and the rationale for their use vary. These drugs can be used to achieve similar effects in different areas of the body or with different rationales depending on the site of use.
We do not intend to examine these drugs extensively. Instead, we will provide an overview of their mechanisms of action, the sites at which they are used, and the side effects they can cause. To this end, we have divided the injected substances into seven groups on the basis of their structural characteristics or mechanisms of action.
Corticosteroids (e.g., dexamethasone)
Sclerosing agents (e.g., polydocanol)
Hyaluronic acid
Autologous blood
Platelet-rich plasma (PRP)
Ozone
Normal saline (NaCl 0.9 %)
Corticosteroids
The corticosteroids have been used in the treatment of musculoskeletal pain since at least 1953 (Hollander, Philadelphia, 1953), and although their short-term efficacy is undisputed, their use in the long-term management of patients has always been controversial. The corticosteroids currently in the market are human hormone analogs that have been synthesized in a laboratory. In men, the corticosteroids are produced by the adrenal glands, and they are involved in the response to stress. The glucocorticoids control the metabolism of proteins, fats, and carbohydrates; they also exert anti-inflammatory activity involving blockade of the release of phospholipids, inhibition of the activation of the eosinophils, and many other mechanisms, including the reduction of vascularization. Only modest differences have been demonstrated between these drugs, and none is significantly superior to the others [4].
The rationale for their use in the control of the pain is obviously related to their anti-inflammatory activity, which attenuates the pain without affecting the mechanism that causes it. The pain relief is also partially related to the reduced formation of fibrosis, which indirectly increases the mobility of structures like tendons and reduces friction-related pain. There are no direct improvements on the functional aspects of the disorder being treated and the disability it causes [5].
The fact that the effects of corticosteroids are almost exclusively symptomatic is one of their main limitations; in addition, while their effects are quite powerful, they are also short lived. Compared with other substances, they produce better effects during the first 5 weeks of treatment. Thereafter, their performance declines progressively, and after 24 weeks, appreciable benefits are no longer observed [4]. The corticosteroids cause osteoporosis and increase the risk of tears involving tendons and bands; many authors maintain that they can also have negative effects on the cartilage although these effects have never been documented with certainty [4, 6]. Finally, it is important to consider that these drugs produce local immunosuppressive effects, which increase the risk of infectious complications (unless strict asepsis is maintained throughout the procedure) [7].
On the positive side, corticosteroid therapy has been used for years to treat diverse clinical problems, and it has repeatedly proved to be an effective solution for short-term relief of pain. These characteristics make them quite suitable for “one-shot treatment” (without significant improvements in performance with a second injection) of acute, severe pain [4]. The corticosteroids are indicated for treatment of degenerative disease of the shoulder, knee, and hip (generally in the form of intra-articular injections) [4–6], as well as inflammatory conditions (e.g., bursitis).
Sclerosing agents
The sclerosing agents (e.g., polydocanol, the one most widely used) exert selective effects on the intima that leads to thrombosis and closure of the vessel, and for this reason they have long been used in the treatment of varices and telangiectasias [8]. In 2002, the first data were published on the use of polydocanol for the treatment of the tendinopathies [9]. The rationale is based on the fact that pain is often related to neovascularization, although some studies show that neovascularization can actually be an indirect aid in the identification of sensitive nerve branches [10]. It is not clear whether polydocanol reduces pain directly by diminishing neovascularization or because treatment of neovascularized areas also produces sclerosis of the adjacent nerve endings. All studies, however, have shown that the drug produces immediate and sustained reduction of pain lasting up to 2 years [11]; functional improvement is also observed, and there are few side effects. Moreover, unlike corticosteroids, polydocanol also seems to act on the causes of the tendinopathy, reversing the degenerative process and producing what appears to be structural remodeling of the tendon [8].
Polydocanol is thus an excellent choice for the treatment of tendinopathy, regardless of the site of involvement; studies have been published on its use in patients with lesions of the Achilles tendon (Fig. 1), the patellar tendon, and the tendon of the supraspinatus muscle, as well as in those with tennis elbow [12]. Its only shortcoming is its limited efficacy in professional athletes, who frequently have to resort to more invasive interventions to obtain relief [11].
Fig. 1.
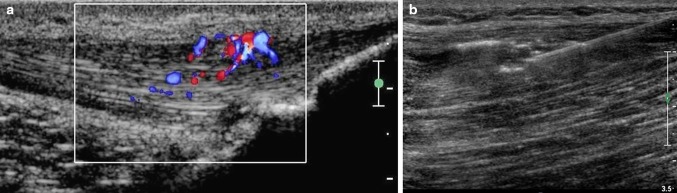
Use of sclerosing agents to treat tendinopathies. Longitudinal US scan of the Achilles tendon using a high-frequency transducer. Color Doppler is used to identify the area to be treated, which is the most highly vascularized zone (a). Longitudinal US scan of the Achilles tendon using a high-frequency linear transducer. Under US guidance, the needle is inserted in the previously identified area of the tendon (b)
Hyaluronic acid
Hyaluronic acid is a glycosaminoglycan with a non-branched polysaccharide chain; it is a fundamental component of connective tissue, where it contributes to the maintenance of hydration and plasticity. It is also secreted into the articular space by type B synoviocytes or fibroblasts, and exerts chondroprotective and lubricant effects. It has analgesic properties, which are related to its direct inhibition of nociceptors and its ability to bind substance P (a pain mediator) [13].
The hyaluronic acid currently used in medicine is a synthetic substance, which is available in various molecular weights (high molecular weights, i.e., approximately 500–730 kDa, are generally used for intra-articular injections) and concentrations. This often complicates attempts to standardize treatment protocols [14].
Since the mid-1980s, hyaluronic acid has been used to treat weight-bearing joints, especially the knee, mainly because of its “mechanical” effects (lubrication and separation of articular surfaces). Today, however, it is being used more and more frequently in non-weight-bearing joints, like those of the hand [15], and for treatment of advanced forms of subacromial impingement. Although the results have been promising, the only approved “on label” use is for treatment of the knee [15]; for other indications, more substantial data on efficacy and tolerability are needed.
Hyaluronic acid has certain side effects, including attacks of pseudo-gout with onset within 24–48 h after the initiation of treatment and gastrointestinal bleeding [15]. Serious complications of this type are extremely rare, while more frequent it is the sense of heaviness and clumsiness at the level of the treated joint.
Autologous blood
Autologous blood injections have long been used in the treatment of tendinopathies, but they are gaining new popularity particularly when combined with the technique of “dry needling”, which involves ultrasound-guided scarification of the degenerated segments of tendons and ligaments [16]. The mechanism by which this intervention improves the elastic characteristics and resistance of a tendon is probably related to the fact that the fenestrations created by the scarification allow local bleeding as well as the entry of autologous blood.
The blood contains moderate amounts of growth factors, such as fibroblast growth factor and transforming growth factor. These substances stimulate the production of granulation tissue, which becomes organized and increases the overall resistance of the tendon [17]. These growth factors are similar to those found in platelet-rich plasma (PRP), but autologous blood can be obtained more rapidly and economically than PRP.
One of the negative aspects of autologous blood injections is that the studies that have assessed its efficacy actually evaluated the combined effects of dry needling and autologous blood [16, 17]. It is thus impossible to say how much of the efficacy depends on the latter component. However, the results are extremely promising in almost all the studies that have been reported thus far [16, 17].
Platelet-rich plasma
Platelet-rich plasma is produced by centrifuging heparinized whole autologous blood for 15 min and separating the platelets from the other blood components. Later, the platelets are diluted with normal saline to obtain the optimal concentration [18].
The use of PRP is increasing rapidly in two directions: for the treatment of tendinopathies (Achilles, patellar) and, more recently, for intra-articular use (knee and hip). The rationale for its use in cases of tendinopathy is related to the fact that when the platelets reach the site of injury, they release a series of chemokines and growth factors that can stimulate repair processes and block catabolic processes in the tendon [18]. The growth factors include platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor (TGF-β1), and tissue inhibitors of metalloproteinases-1 and 2 (TIMP-1 and 2), which stimulate the growth and repair of the tendon and block inflammatory and catabolic processes [19]. However, while the positive effects of PRP on tendons have been demonstrated in the laboratory and in non-randomized studies, the most recent randomized, double-blind trials have not confirmed the in vivo effects of PRP [18]. In light of these findings and its high costs compared with (for example) with whole blood, the use of PRP thus needs to be re-evaluated.
As for its more recent use in intra-articular therapy, there are no data from randomized clinical trials to support this approach: only small studies without a control group [19]. Larger, more complex studies are needed before we can draw any conclusion on PRPs potentially beneficial effects in this context. However, it is important to recall that intra-articular injection of PRP results in persistently high platelet concentrations within the space of the joint capsule. Their release of growth factors is generally slow, and the effects of these factors are concentration-dependent. Consequently, the characteristics of intra-articular release could enhance the qualities of the PRP and render it more effective than it is when injected into a tendon [19].
Ozone
Ozone is a colorless gas with a characteristic odor; it is composed of three oxygen atoms. In nature, it is one of the components of the earth’s atmosphere. Its high reactivity gives it a short half-life. It can be produced artificially by subjecting diatomic oxygen to a high-voltage electrical discharge. It was first used during World War I for the treatment of gas gangrene [20].
Its current use in the field of musculoskeletal disorders is mainly confined to the treatment of small joints and localized diseases, such as Morton’s neuroma (Fig. 2), and disorders involving the interapophyseal facets of the vertebral column [21] or the temporomandibular joint [20]. Its mechanism of action is not yet fully known, but it seems to be linked to the gas’s ability to reduce free radical production caused by intense mechanical stress [22]. This reduction produces an anti-inflammatory effect, which is the basis of ozone’s clinical effectiveness. Its effects are naturally limited in large part to pain relief [21]; its ability to improve functional impairment is restricted to the pain-dependent component.
Fig. 2.
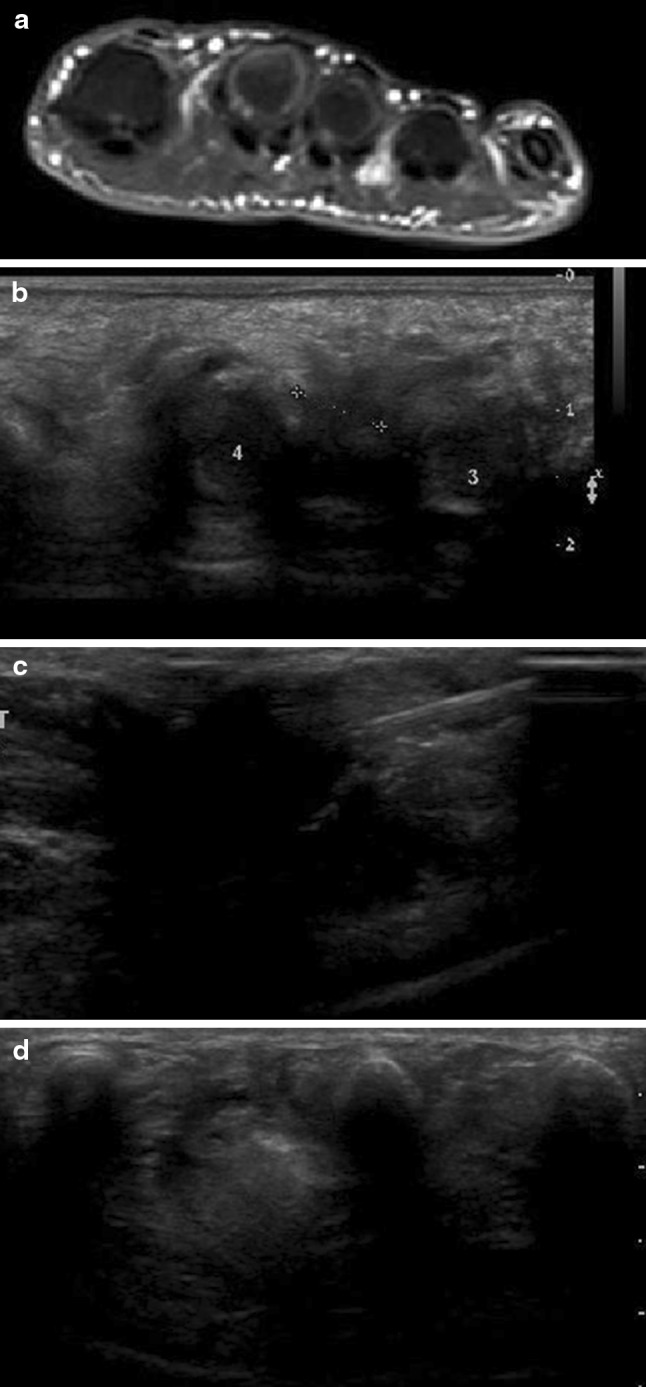
Morton’s neuroma. Axial STIR T2-weighted MRI scan. The neuroma is clearly visualized in the space between the third and fourth metatarsals (a). Scan performed along the short axis of the nerve with a high-frequency linear transducer. The neuroma is clearly visualized as a hypoechoic lesion located in the space between the metatarsal heads (b). The needle tip is inserted into the neuroma (c). After treatment, the neuroma appears hyperechoic (d)
Normal saline
Physiological saline is a 0.9 % w/v solution (that is approximately 9 g/L) of NaCl in purified water; it is essentially isotonic to blood and has no effect on the body other than that of increasing blood volume when injected intravenously. Its use during US-guided interventional musculoskeletal procedures is related to its ability to dissolve intratendinous calcifications, which leads not only to a reduction of the pain, but also to functional improvement. This technique is used frequently at the level of the rotator cuff, but it can be adapted to any site of the intratendinous calcification.
In the past, a single needle was inserted and used to inject the saline solution and to re-aspirate it, thereby producing a “flushing” effect that dissolved the calcification. Today, a dual-needle technique is widely used: the solution is injected with one needle and aspirated with the other, producing continuous lavage.
The normal saline per se has no side effects. Some authors maintain, however, that needle insertion can provoke tendon tears, and that use of two needles increases this risk [23], but there is no scientific evidence to support this view [24]. The results of some studies suggest that heating the saline solution to approximately 42 °C can improve performance and reduce the rate of complications (e.g., subacromial-deltoid bursitis), as compared with use of room temperature saline [25].
Sonographic technique
General aspects
When a US-guided injection is being planned, patient preparation is extremely important: maintenance of asepsis is fundamental for preventing post-procedure complications.
The injection site should be disinfected with an appropriate bactericidal solution (e.g., povidone iodine, Betadine®); in some cases, it may be necessary to shave the affected area prior to disinfection. The operating field is then delimited with sterile drapes, which also functions as a workspace. The choice of the transducer depends on the characteristics of the structure being imaged (for deep structures, it is sometimes necessary to use a convex transducer, while for superficial structures a linear probe is always used). The transducer should be appropriately disinfected and covered with a sterile probe cover, the operator should wear surgical gloves, and the gel used should also be sterile. Maintaining asepsis is easier if the operator is assisted by a second physician or a non-sterile nurse, who prepares the material and assists the doctor.
Needle advancement can be done free hand or with the aid of needle guides mounted on the transducer. These devices allow the operator to guide the needle along a predetermined track, which can also be visualized on the monitor. During the procedure, the transducer should be manipulated with the non-dominant hand and the needle with the dominant hand. This maximizes both sensitivity and precision. Needle selection (Fig. 3) will vary based on the depth of the structure being imaged. The gauge will depend on the dimensions of the structure. Longer, smaller-bore needles are more likely to deviate from the planned trajectory, thus rendering the procedure more difficult.
Fig. 3.
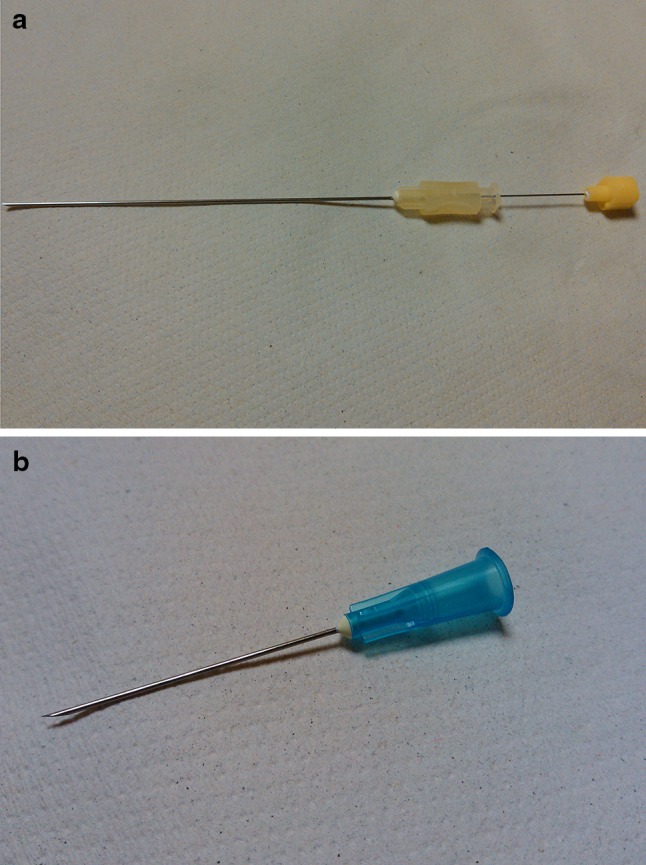
Needles used during US-guided musculoskeletal interventional procedures. 20-gauge needle (a), 23-gauge needle (b)
Once the structure of interest has been reached, a small bolus of normal saline can be injected: sonographic visualization of the flow of this liquid near or within (e.g., intra-articular flow) the structure of interest confirms that the needle has been correctly positioned (Fig. 4). When the position has been verified, the chosen drug can be injected.
Fig. 4.
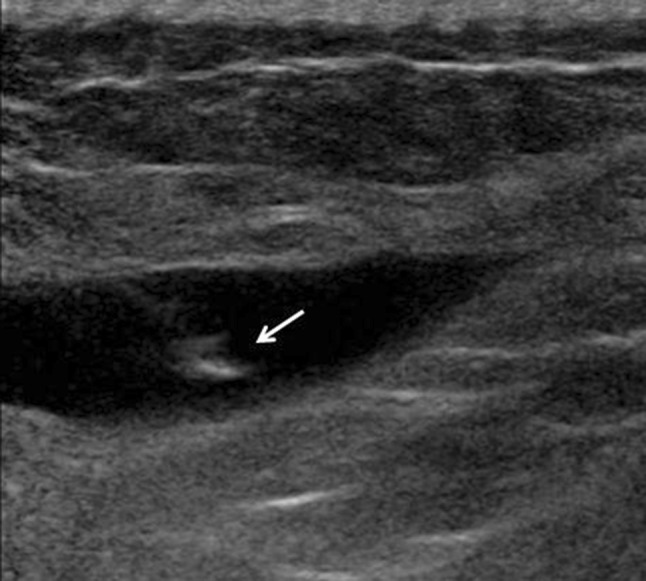
Intralesional flow. US reveals an intramuscular hematoma whose content appears hypoechoic. Injection of normal saline is associated with a hyperechoic ‘spurt’ of fluid within the lesion (arrow), which confirms that the needle tip has been correctly placed
Specific aspects
Various types of structures can be treated in patients with musculoskeletal pain: joints, tendons, nerves, bursae, etc. For each structure, there is a specific technique based on the use of easy-to-locate anatomical landmarks, which simplifies the procedure and reduces the risk of complications. The approaches used to access the structures of major interest are described below.
Wrist and hand
Median nerve: the patient is placed in a seated or supine position. The forearm is supinated and the wrist placed on a roll of gauze to maintain a position of mild flexion. The procedure is performed with a high-frequency (15–18 mHz) linear probe and small-bore needles (25 gauge). A transverse scan is done to locate the median nerve where it enters the carpal tunnel. Anatomical anomalies (e.g., bifid median nerve, persistent median artery) are then identified [26], since they can influence the procedure. The vascular study is done with Power Doppler to ensure visualization even of the smaller vessels. The needle is then inserted at the level of the crease in the wrist, ulnar to the palmaris longus tendon and at an angle of approximately 30°. The tip penetrates the flexor retinaculum and part of the liquid is injected along the ulnar margin of the nerve. Generally, this procedure expands the space between the nerve and the retinaculum, so that tissues adjacent to the nerve can also be infiltrated (the perineural or peritendinous approach should be used routinely, since it improves the efficacy of the procedure). The operator should then verify that the injected liquid is properly and uniformly distributed around the nerve [27, 28].
-
Distal radioulnar joint: the patient is placed in a seated or supine position. The forearm is pronated and the wrist placed on a roll of gauze to maintain a position of mild flexion. The procedure is performed with a high-frequency (15–18 MHz) linear probe and small-bore needles (25 gauge).
The Lister tubercle and the ulnar styloid are identified, and the transducer is placed with its axis parallel to the line that connects these two structures. This provides good cross-sectional visualization of the osseofibrous tunnels. The needle is advanced along the ulnar aspect of the extensor digiti minimi, which runs through the fifth compartment. This reduces the risk of injury to neurovascular structures (e.g., the interosseous artery, the cutaneous branch of the ulnar nerve) and gives access to the largest recess of the distal radioulnar joint, which is near the head of the ulna [29].
Retinaculum of the first osseofibrous tunnel. The patient is seated with the forearm maximally pronated. The procedure is performed with a high-frequency (15–18 MHz) linear probe and small-bore needles (27 gauge). The thickened retinaculum is identified and the needle advanced from the ulnar to the radial side, parallel to the retinaculum itself. When the tip reaches the retinaculum, the drug is injected (Fig. 5).
Fig. 5.
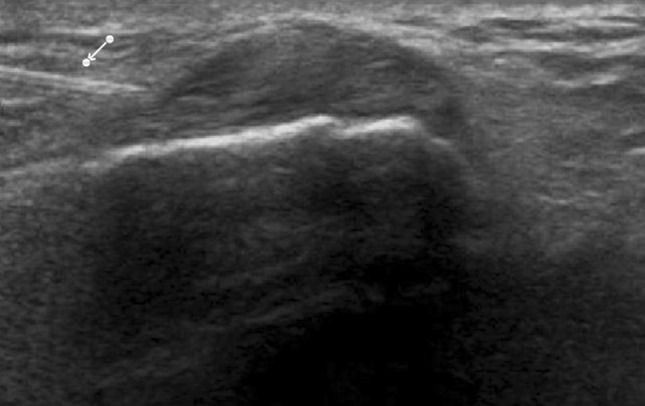
de Quervain syndrome. US shows marked hyperechoic thickening of the extensor retinaculum. The needle tip (arrow) can be visualized as it advances to the injection site
Elbow
-
4.
Ulnar nerve: the patient is placed in the supine position with the affected arm above the head and flexed at an angle of 70°–90°. The procedure is performed with a high-frequency (15–18 MHz) linear probe and intermediate-bore needles (24 gauge).
The ulnar nerve can be accessed inside the cubital canal (the area most frequently affected) or immediately proximal to the canal. With the ultrasound transducer positioned transversally to the cubital canal, the nerve is visualized as it passes between the medial epicondyle and the olecranon. The operator proceeds with the infiltration of tissues adjacent to the site of injury (represented by focal thickening) [30].
-
5.
Lateral epicondylitis (tennis elbow): The patient is placed in a seated position with the affected arm flexed at an angle of 90°. The procedure is performed with a high-frequency (15–18 MHz) linear probe and intermediate-bore needles (24 gauge).
A longitudinal scan is done at the level of the lateral epicondyle of the elbow, the common extensor tendon is visualized along its long axis, and the adjacent soft tissues are infiltrated (Fig. 6) [31].
Fig. 6.
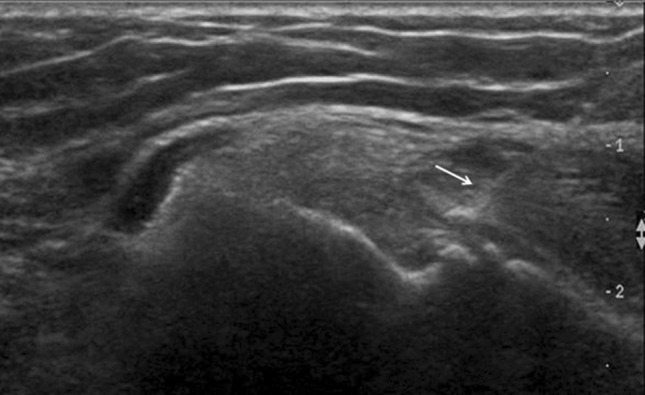
Lateral epicondylitis. US-guided infiltration. The needle tip (arrow) is positioned in the peritendinous soft tissues and the contents of the syringe injected
Shoulder
-
6.
Subdeltoid bursa: the patient is seated, with the arm behind the back, the elbow flexed 90°, and the dorsal aspect of the hand pressed against the back. The procedure is performed with a multifrequency (9–18 MHz) linear transducer and short (5 cm), 20-gauge needles.
Scanning parallel to the supraspinatus muscle, one encounters the subacromial space, which contains the supraspinatus and the humeral head; the probe is moved laterally until the deltoid muscle is seen. When distended, the subdeltoid bursa is depicted as a hypoechoic structure located between the rotator cuff and the deltoid (Fig. 7) [32].
-
7.
Shoulder joint: when a posterior approach is used, the patient is seated, with the arm abducted and the elbow flexed at 90°. The procedure is performed with a multifrequency (9–18 MHz) linear transducer and intermediate-length (7 cm), 18–20 gauge needles.
The glenohumeral joint capsule can be accessed with different approaches (most of which are derived from arthrography [33]), and the procedure has several variants. The anterolateral approach, for example, involves advancing the needle between the biceps tendon and the subscapularis muscle. Using the posterior approach, the probe is placed on the posterior aspect of the shoulder, along a plane perpendicular to the glenohumeral joint rim. The transducer is moved from the acromion caudally and medially until the articular rim is visualized. The needle is advanced until it touches the humeral head. A small bolus of physiological saline is injected to separate the capsule from the bone plane and make sure the needle has reached the joint [34].
-
8.
The supraspinatus tendon: sonographic visualization of the supraspinatus tendon should not be a problem for a trained sonographer (Fig. 8). The only difficulty is related to the fact that these procedures are often performed to treat calcifications, so the tip of the needle must be positioned in or near the calcification (Fig. 9). Moreover, it is often better to insert two needles (one for injecting, one for aspirating) if lavage with normal saline is being planned [35].
In the treatment of enthesitis, the tip of the needle should preferably be placed next to the tendon (Fig. 8), rather than inside the tendon.
Fig. 7.
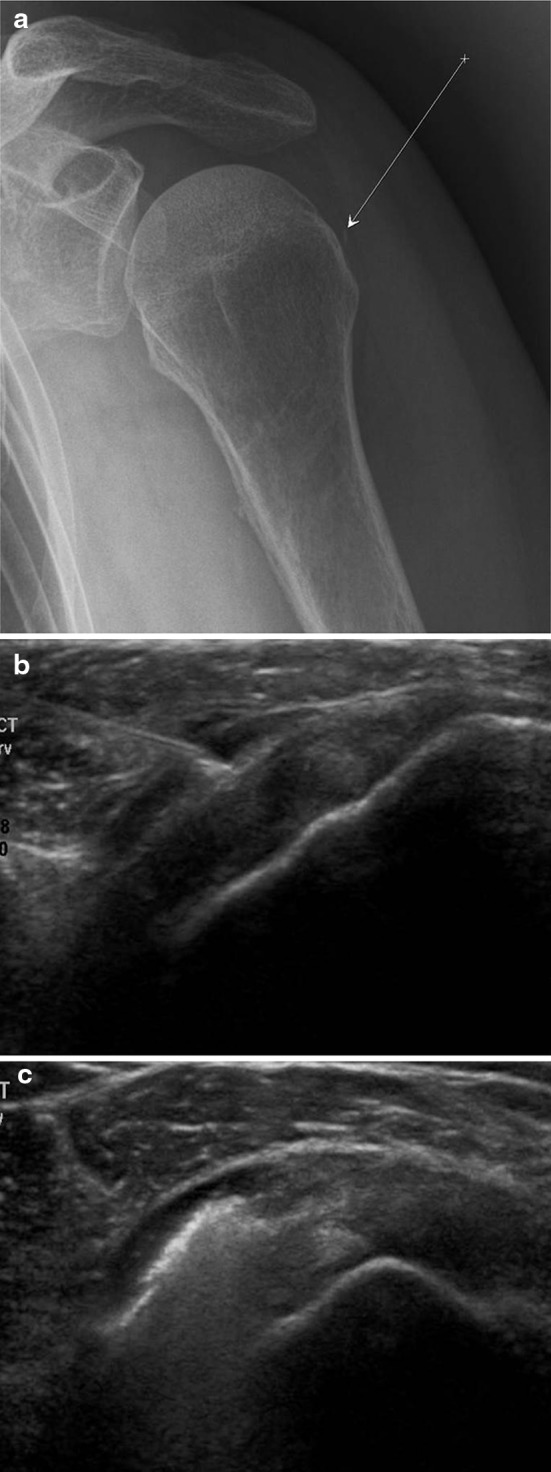
US-guided infiltration of the subdeltoid bursa. Anteroposterior radiograph: the arrow identifies the trajectory of the needle and the arrowhead the bursa (a). Coronal US scan performed with a high-frequency linear array transducer. The needle is advanced into the bursa, which is located between the deltoid muscle and the rotator cuff (b). Coronal US scan performed with a high-frequency linear array transducer. The image confirms uniform distribution of the injected substance within the bursa (c)
Fig. 8.
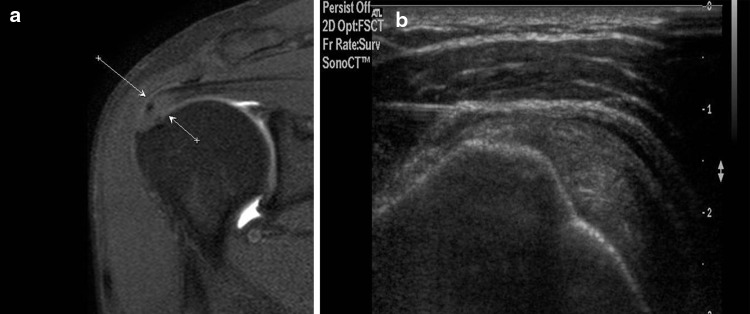
US-guided infiltration of enthesitis of the supraspinatus tendon. Coronal MRI scan (proton density weighted with fat suppression): the arrows indicate an area of degenerative changes at the insertion of the supraspinatus tendon (a). US scan along the long axis of the tendon with a high-frequency linear array transducer (b). The tip of the needle is visualized as it is advanced toward the degenerated zone of the tendon
Fig. 9.
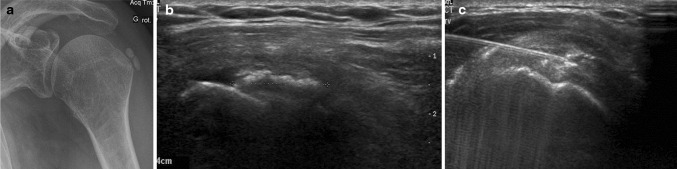
Calcifications of the supraspinatus tendon. Anteroposterior radiograph reveals gross calcifications on the tendon of the supraspinatus muscle (a). Sonographic scan along the long axis of the tendon with a high-frequency linear transducer. The calcifications are delimited by the calipers (b). The needle is placed near the calcification and the area flushed with normal saline (c)
Hip
-
9.
Coxofemoral joint: the patient lies on his back with the hip in a neutral position or slightly flexed (to maximize comfort during the procedure). Linear or convex intermediate-frequency (5–9 MHz) or multifrequency (9–18 MHz) transducers are used, depending on the habitus of the patient, and long needles (10 cm), approximately 20 gauge.
The anterior superior iliac spine is identified, and the transducer is placed on the sagittal plane with the cranial end at the level of the spine itself. The transducer is moved medially and caudally until the femoral head is visualized. The lower end of the transducer is rotated laterally until the segment between the head and the neck of the femur is visualized (a single rotation of approximately 30° is generally sufficient). At this level one encounters the anterior synovial recess, which may or may not be distended with liquid. Before making the intra-articular injection, the operator must evaluate the position of the femoral neurovascular bundle (best visualized in the axial plan and with the aid of Doppler). Using the lateral approach, the operator will in most cases be able to delineate a trajectory that avoids these structures. When the joint has been reached (Fig. 10) proper needle placement can be confirmed by injecting a small amount of physiological saline (which will be visualized as intra-articular flow) [36, 37].
Fig. 10.
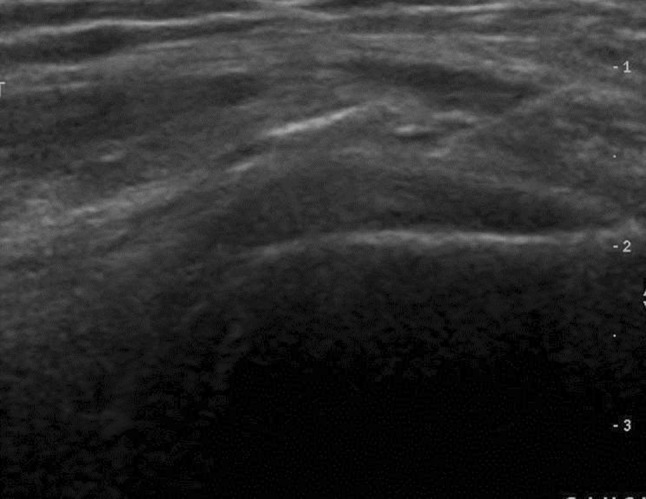
Coxofemoral joint. Sonography: longitudinal scan with a 9 MHz linear transducer. Detail of the image showing the tip of the needle as it advances toward the anterior synovial recess
Knee
-
10.
The joint capsule of the knee: the patient lies on his back or is seated with the knee extended and externally rotated, so that the medial surface is exposed (additional external rotation can be avoided by placing a rolled-up towel under the lateral aspect of the knee). Linear multifrequency (9–18 MHz) transducers are generally used with 20-gauge needles.
On the longitudinal scan, the medial border of the patella is identified; the needle is inserted medial to the junction of the middle and upper thirds of the patellar margin. Subsequently, the needle is rotated and advanced cranially and laterally toward the suprapatellar recess [38].
-
11.
Tendon of the popliteal muscle: with the patient lying on his back or seated, the knee is flexed slightly (20–30°) and internally rotated to expose the lateral surface. Linear multifrequency (9–18 MHz) transducers are generally used with short (no more than 5 cm), small-bore (around 25 gauge) needles.
The lateral femoral condyle is located by palpation and the probe placed over it to visualize the lateral collateral ligament. At this point, the transducer is rotated in the axial plan and moved caudally until the popliteal sulcus is visualized on the lateral aspect of the femoral condyle. The roof of the sulcus is the lateral collateral ligament. The tendon of the popliteal muscle lies within the sulcus. The needle is advanced toward the tendon and the tip inserted into the tendon sheath. The tendon is visualized along both the short and long axes during the injection to ensure 360° control over the position of the needle [39].
Ankle
-
12.
Peroneal tendons: the patient lies on his side with the medial aspect of the ankle resting on a roll of gauze so that it is in a slightly inverted, plantar-flexed position. Linear multifrequency (9–18 MHz) transducers are generally used with short (no more than 5 cm), small-bore (around 25 gauge) needles.
The lateral malleolus is palpated and the transducer positioned immediately behind it in the axial plane. Starting from the apex of the fibula, the transducer is moved cranially for approximately 4–5 cm until the peroneal tendons are visualized on transverse section. The needle is inserted anteriorly and advanced along an anteroposterior trajectory to the tendon sheath, where the infiltration is performed [40].
-
13.
Posterior talocalcaneal joint: The patient is placed in the prone position with the foot dorsiflexed and the toes resting on the table. The procedure is performed with a multifrequency (9–18 MHz) linear transducer and intermediate-length (7 cm), 18–20 gauge needles.
The probe is placed on a sagittal plane, parallel and slightly lateral to the Achilles tendon; the following structures can thus be identified (moving caudally: the posterior tibial margin, the posterior talus (with the os trigonum), and the posterior calcaneus. Between the last two structures, one can see the posterior articular recess when it is pathologically distended. The needle is inserted at the caudal edge of the transducer and advanced to the recess through the fat of the triangle of Kager. The sural nerve and the small saphenous vein are in an anterior position and do not cross the trajectory of the needle [41]. The posterolateral approach to this joint is preferred in our center, but others can also be used, including the posteromedial approach, which is also useful for the Achilles tendon, and the anterolateral approach, which is also useful for the tibiotalar joint (Fig. 11) [41].
Fig. 11.
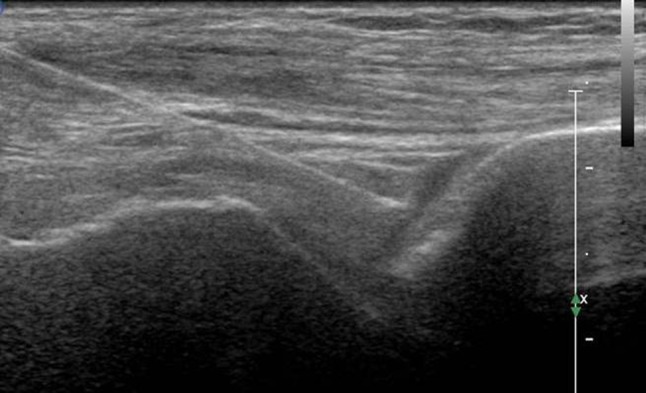
Tibiotalar joint. Anterior longitudinal scan with a high-frequency linear transducer. The tip of the needle is placed at the level of the joint, identifying the space between the tibia and the talus
Conclusions
As this review of our experience and the most recent literature shows, sonographers must continuously improve their knowledge of the materials and techniques used for interventional musculoskeletal US. In-depth familiarity with the equipment (transducers and needles) and techniques are fundamental for safe, effective administration of these injections. In fact, depending on the characteristics of the patient and the clinical problem, the approach and technique may have to be changed to ensure better results with a simpler procedure [41]. It is equally important to be aware of the possible complications of each procedure [42] and current indications for the substances being injected and how they are used. The task is complicated by the abundance in the literature of poorly designed or confusing studies and clinical trials that are of little significance.
However, given the increasing importance of US-guided locoregional treatments for the musculoskeletal pain, these are difficulties that must be overcome. And in our opinion, this is what makes articles like this necessary. In fact, the information must be joined together and simplified to improve patient compliance and outcomes, thus promoting a faster, more complete resumption of occupational or sports-related activities.
Conflict of interest
None.
References
- 1.Stewart WF, Ricci JA, Chee E, Morganstein D. Lost productive work time costs from health conditions in the United States: results from the American Productivity Audit. J Occup Environ Med. 2003;45(12):1234–1246. doi: 10.1097/01.jom.0000099999.27348.78. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R (2003) Lost productive time and cost due to common pain conditions in the US workforce. JAMA 12;290(18):2443–2454 [DOI] [PubMed]
- 3.Aldana SG. Financial impact of health promotion programs: a comprehensive review of the literature. Am J Health Promotion. 2001;15(5):296–320. doi: 10.4278/0890-1171-15.5.296. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G (2006) Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 19;(2):CD005328 [DOI] [PubMed]
- 5.Gialanella B, Prometti P (2011) Effects of corticosteroids injection in rotator cuff tears. Pain Med 12(10):1559–1565. doi: 10.1111/j.1526-4637.2011.01238.x [DOI] [PubMed]
- 6.Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265–2268. [PubMed] [Google Scholar]
- 7.Bortolotto C, Gregoli B, Coscia DR, Draghi F. Septic complications involving hand and wrist in patients with pre-existing rheumatoid arthritis: the role of magnetic resonance imaging and sonography. J Ultrasound. 2012;15(2):115–120. doi: 10.1016/j.jus.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lind B, Ohberg L, Alfredson H (2006) Sclerosing polidocanol injections in mid-portion Achilles tendinosis: remaining good clinical results and decreased tendon thickness at 2-year follow-up. Knee Surg Sports Traumatol Arthrosc 14(12):1327–1332 [DOI] [PubMed]
- 9.Ohberg L, Alfredson H (2002) Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med 36(3):173–175; discussion 176–177 [DOI] [PMC free article] [PubMed]
- 10.Alfredson H, Harstad H, Haugen S, Ohberg L (2006) Sclerosing polidocanol injections to treat chronic painful shoulder impingement syndrome-results of a two-centre collaborative pilot study. Knee Surg Sports Traumatol Arthrosc 14(12):1321–1326 [DOI] [PubMed]
- 11.Hoksrud A, Bahr R (2011) Ultrasound-guided sclerosing treatment in patients with patellar tendinopathy (jumper’s knee). 44-month follow-up. Am J Sports Med 39(11):2377–2380. doi:10.1177/0363546511417097 [DOI] [PubMed]
- 12.Zeisig E, Ohberg L, Alfredson H (2006) Sclerosing polidocanol injections in chronic painful tennis elbow-promising results in a pilot study. Knee Surg Sports Traumatol Arthrosc 14(11):1218–1224 [DOI] [PubMed]
- 13.Tagliafico A, Serafini G, Sconfienza LM, Lacelli F, Perrone N, Succio G, et al (2011) Ultrasound-guided viscosupplementation of subacromial space in elderly patients with cuff tear arthropathy using a high weight hyaluronic acid: prospective open-label non-randomized trial. Eur Radiol 21(1):182–187. doi:10.1007/s00330-010-1894-4 [DOI] [PubMed]
- 14.Petrella RJ, Petrella M. A prospective, randomized, double-blind, placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol. 2006;33(5):951–956. [PubMed] [Google Scholar]
- 15.Mandl LA, Hotchkiss RN, Adler RS, Lyman S, Daluiski A, Wolfe SW, et al. Injectable hyaluronan for the treatment of carpometacarpal osteoarthritis: open label pilot trial. Curr Med Res Opin. 2009;25(9):2103–2108. doi: 10.1185/03007990903084016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James SL, Ali K, Pocock C, Robertson C, Walter J, Bell J, et al (2007) Ultrasound guided dry needling and autologous blood injection for patellar tendinosis. Br J Sports Med 41(8):518–521; discussion 522 [DOI] [PMC free article] [PubMed]
- 17.Suresh SP, Ali KE, Jones H, Connell DA (2006) Medial epicondylitis: is ultrasound guided autologous blood injection an effective treatment? Br J Sports Med 40(11):935–939; discussion 939 [DOI] [PMC free article] [PubMed]
- 18.de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, et al (2010) Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA 13;303(2):144–149 [DOI] [PubMed]
- 19.Sánchez M, Guadilla J, Fiz N, Andia I (2012) Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology (Oxford) 51(1):144–150. doi:10.1093/rheumatology/ker303 [DOI] [PubMed]
- 20.Daif ET (2012) Role of intra-articular ozone gas injection in the management of internal derangement of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol 113(6):e10–4. doi:10.1016/j.tripleo.2011.08.006 [DOI] [PubMed]
- 21.Al-Jaziri AA, Mahmoodi SM. Painkilling effect of ozone-oxygen injection on spine and joint osteoarthritis. Saudi Med J. 2008;29(4):553–557. [PubMed] [Google Scholar]
- 22.Milam SB, Zardeneta G, Schmitz JP. Oxidative stress and degenerative temporomandibular joint disease: a proposed hypothesis. J Oral Maxillofac Surg. 1998;56:214–223. doi: 10.1016/S0278-2391(98)90872-2. [DOI] [PubMed] [Google Scholar]
- 23.Aina R, Cardinal E, Bureau NJ, Aubin B, Brassard P. Calcific shoulder tendinitis: treatment with modified US-guided fine-needle technique. Radiology. 2001;22(2):455–461. doi: 10.1148/radiol.2212000830. [DOI] [PubMed] [Google Scholar]
- 24.Serafini G, Sconfienza LM, Lacelli F, Silvestri E, Aliprandi A, Sardanelli F. Rotator cuff calcific tendonitis: short-term and 10-year outcomes after two-needle US-guided percutaneous treatment—non-randomized controlled trial. Radiology. 2009;252(1):157–164. doi: 10.1148/radiol.2521081816. [DOI] [PubMed] [Google Scholar]
- 25.Sconfienza LM, Bandirali M, Serafini G, Lacelli F, Aliprandi A, Di Leo G, et al (2012) Rotator cuff calcific tendinitis: does warm saline solution improve the short-term outcome of double-needle US-guided treatment? Radiology 262(2):560–566. doi:10.1148/radiol.11111157 [DOI] [PubMed]
- 26.Presazzi, C. Bortolotto, M. Zacchino, L. Madonia, F. Draghi (2011) Carpal tunnel: Normal anatomy, anatomical variants and ultrasound technique. J Ultrasound 14:40–46 [DOI] [PMC free article] [PubMed]
- 27.MacLennan A, Schimizzi A, Meier KM, Barron OA, Catalano L, Glickel S. Comparison of needle position proximity to the median nerve in 2 carpal tunnel injection methods: a cadaveric study. J Hand Surg Am. 2009;34(5):875–879. doi: 10.1016/j.jhsa.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Smith J, Wisniewski SJ, Finnoff JT, Payne JM. Sonographically guided carpal tunnel injections: the ulnar approach. J Ultrasound Med. 2008;27(10):1485–1490. doi: 10.7863/jum.2008.27.10.1485. [DOI] [PubMed] [Google Scholar]
- 29.Smith J, Rizzo M, Sayeed YA, Finnoff JT. Sonographically guided distal radioulnar joint injection: technique and validation in a cadaveric model. J Ultrasound Med. 2011;30(11):1587–1592. doi: 10.7863/jum.2011.30.11.1587. [DOI] [PubMed] [Google Scholar]
- 30.Alblas CL, van Kasteel V, Jellema K (2012) Injection with corticosteroids (ultrasound guided) in patients with an ulnar neuropathy at the elbow, feasibility study. Eur J Neurol 19(12):1582–1584. doi:10.1111/j.1468-1331.2012.03676.x [DOI] [PubMed]
- 31.Zhu J, Hu B, Xing C, Li J. Ultrasound-guided, minimally invasive, percutaneous needle puncture treatment for tennis elbow. Adv Ther. 2008;25(10):1031–1036. doi: 10.1007/s12325-008-0099-6. [DOI] [PubMed] [Google Scholar]
- 32.Chen MJ, Lew HL, Hsu TC, Tsai WC, Lin WC, Tang SF, et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am J Phys Med Rehabil. 2006;85(1):31–35. doi: 10.1097/01.phm.0000184158.85689.5e. [DOI] [PubMed] [Google Scholar]
- 33.Choudur HN, Ellins ML (2011) Ultrasound-guided gadolinium joint injections for magnetic resonance arthrography. J Clin Ultrasound 39(1):6–11. doi:10.1002/jcu.20753 [DOI] [PubMed]
- 34.Lee HJ, Lim KB, Kim DY, Lee KT. Randomized controlled trial for efficacy of intra-articular injection for adhesive capsulitis: ultrasonography-guided versus blind technique. Arch Phys Med Rehabil. 2009;90(12):1997–2002. doi: 10.1016/j.apmr.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Farin PU, Räsänen H, Jaroma H, Harju A. Rotator cuff calcifications: treatment with ultrasound-guided percutaneous needle aspiration and lavage. Skeletal Radiol. 1996;25(6):551–554. doi: 10.1007/s002560050133. [DOI] [PubMed] [Google Scholar]
- 36.Smith J, Hurdle MF. Office-based ultrasound-guided intra-articular hip injection: technique for physiatric practice. Arch Phys Med Rehabil. 2006;87(2):296–298. doi: 10.1016/j.apmr.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Smith J, Hurdle MF, Weingarten TN. Accuracy of sonographically guided intra-articular injections in the native adult hip. J Ultrasound Med. 2009;28(3):329–335. doi: 10.1016/j.ultrasmedbio.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Berkoff DJ, Miller LE, Block JE (2012) Clinical utility of ultrasound guidance for intra-articular knee injections: a review. Clin Interv Aging 7:89–95. doi:10.2147/CIA.S29265 [DOI] [PMC free article] [PubMed]
- 39.Smith J, Finnoff JT, Santaella-Sante B, Henning T, Levy BA, Lai JK. Sonographically guided popliteus tendon sheath injection: techniques and accuracy. J Ultrasound Med. 2010;29(5):775–782. doi: 10.7863/jum.2010.29.5.775. [DOI] [PubMed] [Google Scholar]
- 40.Muir JJ, Curtiss HM, Hollman J, Smith J, Finnoff JT. The accuracy of ultrasound-guided and palpation-guided peroneal tendon sheath injections. Am J Phys Med Rehabil. 2011;90(7):564–571. doi: 10.1097/PHM.0b013e31821f6e63. [DOI] [PubMed] [Google Scholar]
- 41.Smith J, Finnoff JT, Henning PT, Turner NS. Accuracy of sonographically guided posterior subtalar joint injections: comparison of 3 techniques. J Ultrasound Med. 2009;28(11):1549–1557. doi: 10.7863/jum.2009.28.11.1549. [DOI] [PubMed] [Google Scholar]
- 42.Draghi F, Robotti G, Jacob D, Bianchi S. Interventional musculoskeletal ultrasonography: precautions and contraindications. J Ultrasound. 2010;13:126–133. doi: 10.1016/j.jus.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


