Abstract
Background
Doxorubicin treatment is known to cause muscular weakness. However, the cellular mechanisms have not been elucidated. We aimed to determine the effects of acute doxorubicin treatment on proteome lysine acetylation status, an indication of the apoptotic and inflammatory environment, and the expression and activation of various apical caspases involved in the initiation of apoptosis.
Methods
Six-week-old male F344 rats were injected intraperitoneally with 20 mg/kg of doxorubicin or saline. Once the treatment was administered, both groups of animals were fasted with no food or water until sacrifice 24 h posttreatment.
Results
Doxorubicin treatment affected neither the proteome lysine acetylation status nor the expression of sirtuin 1, sirtuin 3, SOD1, or SOD2 in soleus of fasted animals. Doxorubicin treatment also did not affect the expression or activation of procaspase-1, procaspase-8, procaspase-9, or procaspase−12.
Conclusion
We suggest that doxorubicin does not exert a direct effect on these catabolic parameters in skeletal muscle in vivo.
Keywords: Soleus, Sirtuin, Doxorubicin, Caspase, In vivo, Apoptosis
Introduction
Doxorubicin is one of the most effective anti-cancer agents to treat solid tumors and hematological malignancies, but with known deleterious side effects which limits its use [1]. Doxorubicin is known to cause cardiac, hepatic, and nephrotoxicity with the underlying mechanisms involving mitochondrial dysfunction, oxidative stress, and apoptosis [2–5].
Doxorubicin treatment is also known to cause muscular weakness [6]. However, the mechanisms of these effects have not been elucidated. Previous studies have shown that doxorubicin treatment in animals stimulate oxidative stress, proteolysis, autophagy, and weakness in limb skeletal muscle [7–10]. However, it is not clear if doxorubicin treatment induces apoptosis in vivo in skeletal muscle as it has been shown to do in other tissues. Only one study has reported that doxorubicin treatment in vivo increased a marker of nuclear apoptosis [9], but there has been no published reports identifying the possible initiating apical caspase(s) responsible. Furthermore, recent studies have shown that cellular acetylation status is an important regulator of a pro-survival and anti-inflammatory environment and that cellular stress can alter the lysine acetylation status favoring an apoptotic and inflammatory environment [11, 12]. Normalization of the acetylation status can prevent cell death and activation of apoptotic pathways [11, 12]. Currently, the effect of doxorubicin on proteome lysine acetylation status, or expression of sirtuin lysine deacetylases in skeletal muscle, is not known. Thus, we aim to determine the effects of acute doxorubicin treatment on proteome lysine acetylation status, sirtuin expression, and the expression and activation of various apical caspases involved in the initiation of apoptosis. Soleus muscle was chosen to be analyzed since previous research has shown that the effects of doxorubicin treatment in vivo are more pronounced in soleus compared to other limb muscles [8]. Furthermore, soleus muscle expresses higher levels of procaspase-12, a caspase known to be involved in doxorubicin-induced cardiotoxicity, and thus may be more susceptible to this mode of apoptosis compared to other skeletal muscles [13].
Material and methods
Animals and experimental design
Six-week-old male F344/Crl rats (weighing 100–120 g) were obtained from Charles River (Wilmington, MA). All procedures were approved by the institutional Research Review Board. Animals were randomly divided into groups and were housed in a temperature-controlled (22 ± 2 °C) and light-controlled facility. Animals were injected intraperitoneal (IP) with 20 mg/kg of doxorubicin (Sigma, Saint Louis, MO) or 0.9 % NaCl during the light phase of daily cycle. This dose of doxorubicin is equal to human clinical doses that are pharmacologically scaled for use in rats [14, 15]. Once the treatment was administered, both groups of animals were fasted with no food or water until sacrificed 24 h later. It has been shown that doxorubicin treatment leads to an approximate 50–70 % reduction in food and water intake within 24 h of drug administration and continues for several days [7]. Thus, we fasted both groups of animals to control for this external variable. Animals were sacrificed via cervical dislocation after exposure to ether in an enclosed chamber to render them unconscious. Tissues were immediately excised, rinsed in saline, snap frozen in liquid nitrogen, and stored at −80 °C for future analysis.
Tissue analysis
Soleus muscle was homogenized (Power Gen 125, Fisher Scientific, Pittsburgh, PA) in ice-cold phosphate-buffered saline (137 mM NaCl, 2.68 mM KCl, 10 mM Na2HPO4, 1.75 mM KH2PO4, 5 mM EDTA; 1:10 w/v) supplemented with 10 μl/ml of Halt Protease Inhibitor Cocktail and 10 μl/ml of Halt Phosphatase Inhibitor Cocktail (Pierce Biochemicals, Rockford, IL). Tissue homogenate was centrifuged at 660×g for 10 min at 4 °C. The supernatant was used for biochemical analysis. Protein concentration was determined using the Bicinchoninic Acid Protein Assay Kit (Sigma, Saint Louis, MO).
Protein content of superoxide dismutase 1 (SOD1), 2 (SOD2), sirtuin 1 (sirt1), sirtuin 3 (sirt3), procaspase-1, procaspase-8, procaspase-9, procaspase-12, and protein lysine acetylation were determined by Western blot analysis. Proteins were separated on tris-glycine 12 % separating polyacrylamide PAGEr Gold Precast Gels (Lonza, Rockland, ME) under denaturing conditions (samples prepared in Laemmli buffer supplemented with beta-mercaptoethanol) and transferred to nitrocellulose membranes using standard wet blotting techniques. Nitrocellulose membranes were blocked for 1 h at room temperature using a PBS blocking solution containing 5.0 % powdered milk. Membranes were incubated overnight at 4 °C in primary antibody with a dilution of 1:1,000 (all antibodies purchased from Santa Cruz Biotechnology, Inc, Santa Cruz, CA: sc-271014, sc-137254, sc-56036, sc-166320, sc-81663, sc-21747, sc-15404, sc-99143, sc-32268). Membranes were incubated with secondary HRP-linked antibody, with a dilution of 1:10,000, for 2 h at room temperature. Protein bands were visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biochemicals) and the Kodak IS4000R Imaging System (Carestream Health, Inc., New Haven, CT). Values are expressed as arbitrary units of densitometry calculated by multiplying the area of each band by its average densitometry. The local background was first subtracted from the average densitometry for each band. Ponceau staining (Pierce Biochemicals) of the nitrocellulose membranes was used to assure equal loading of protein.
Statistical analysis
A Student’s t test was used for statistical analysis between groups. p < 0.050 was considered statistically significant. A Levene’s test was used to test homogeneity of variances. All data is expressed mean ± SEM, control group (n = 7) vs doxorubicin group (n = 8), respectively. All arbitrary densitometry units and p values are shown in Table 1.
Table 1.
Summary of densitometry values (arbitrary units) for all proteins analyzed
| Control | Dox | p value | |
|---|---|---|---|
| SOD1 | 744,632 ± 111,317 | 719,536 ± 83,506 | 0.86 |
| SOD2 | 2,064,940 ± 195,096 | 2,110,880 ± 154,082 | 0.85 |
| Procaspase-9 | 182,497 ± 25,124 | 181,011 ± 23,117 | 0.97 |
| Procaspase-12 | 319,006 ± 63,557 | 274,257 ± 44,489 | 0.57 |
| Procaspase-1 | 44,898 ± 11,248 | 28,551 ± 6,212 | 0.23 |
| Procaspase-8 | 152,319 ± 39,571 | 127,188 ± 28,324 | 0.61 |
| Sirt1 | 105,801 ± 11,444 | 100,981 ± 12,270 | 0.78 |
| Sirt3 | 24,688 ± 3,257 | 19,170 ± 4,250 | 0.33 |
Results
Protein content of SOD1 and SOD2
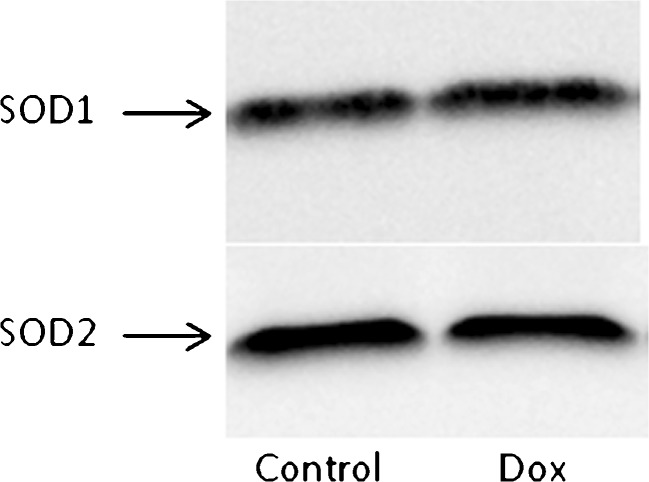
SOD1 and SOD2 are enzymatic antioxidants which function in the cytosolic and mitochondrial compartments, respectively. Acute doxorubicin treatment did not affect the expression of either SOD1 or SOD2. Representative Western blots are shown in Fig. 1.
Fig. 1.
Representative Western blot of SOD1 and SOD2 in soleus
Protein content and activation of procaspases
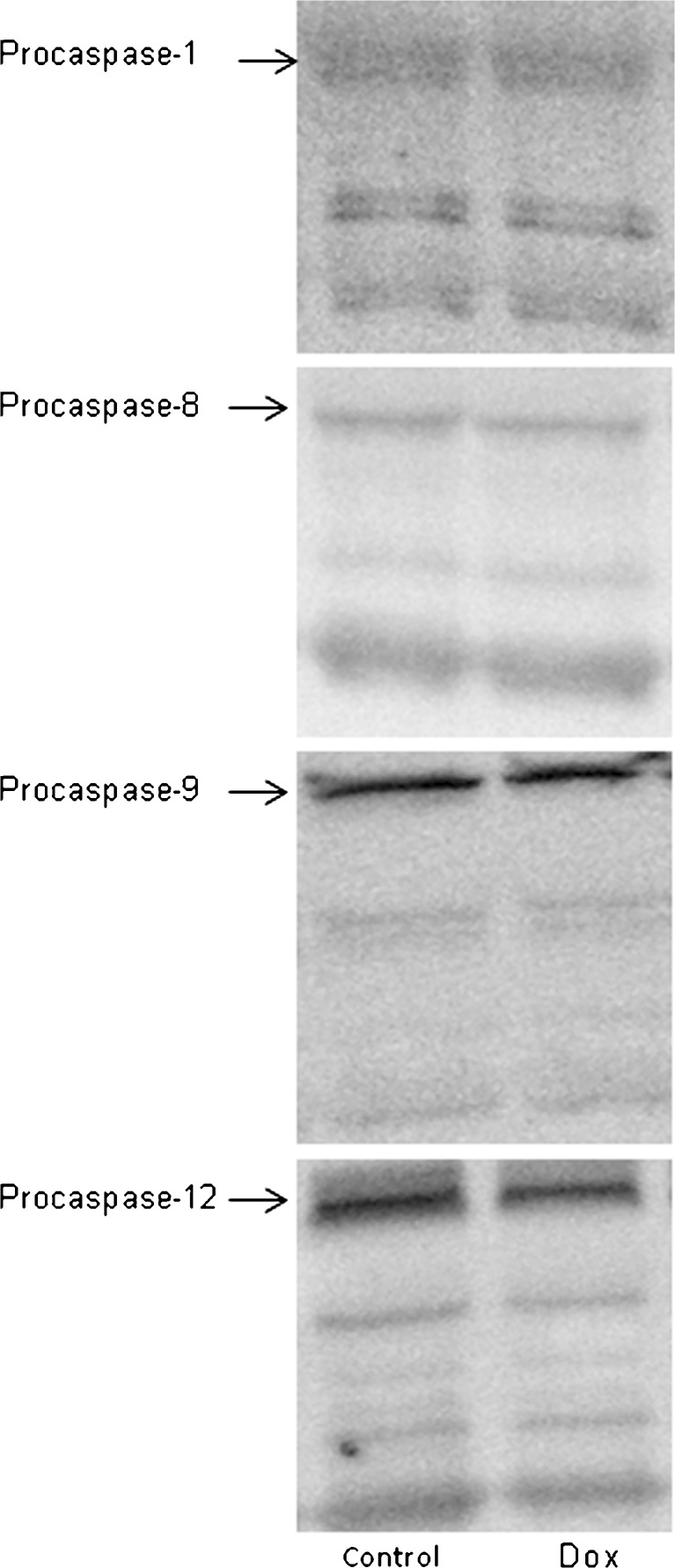
In order to assess the effects of doxorubicin on the activation of the mitochondrial-mediated, sarcoplasmic reticulum-mediated, and receptor-mediated apoptotic signaling pathways, expression, and activation of procaspase-9, procaspase-12, and procaspase-8 were analyzed, respectively. Procaspase-1, a caspase involved in inflammation, was also analyzed. Acute doxorubicin treatment had no effect on expression or activation of these caspases, suggesting that doxorubicin does not induce activation of these apoptotic and inflammatory pathways in skeletal muscle. Representative Western blots of each caspase are shown in Fig. 2.
Fig. 2.
Representative Western blot of caspases in soleus. Arrows indicate the full-length form of each caspase. Bands below each procaspase may include potential cleavage products. No difference between groups was observed for any of these lower molecular weight species
Proteome lysine acetylation status and expression of sirtuins
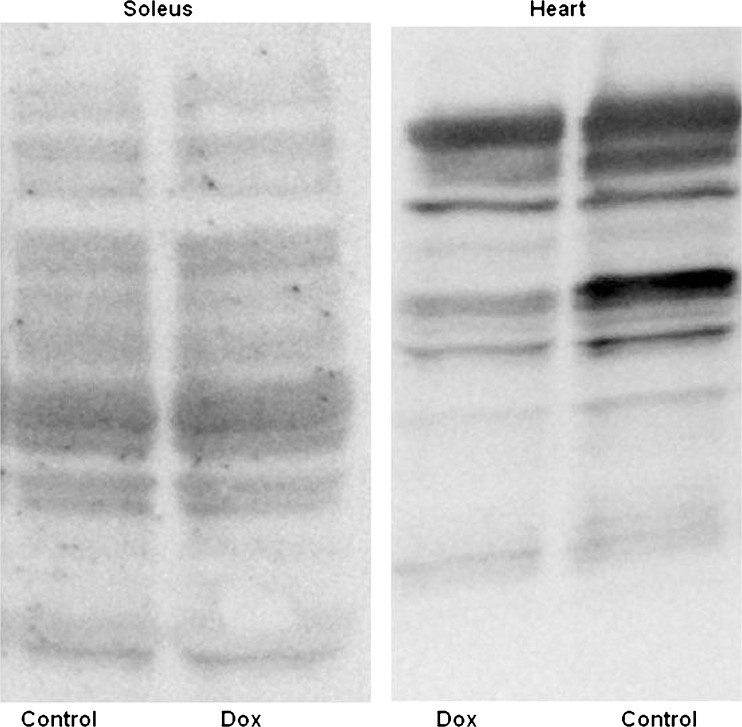
Acute doxorubicin treatment did not have an effect on proteome lysine acetylation status in soleus muscle. However, acute doxorubicin treatment did affect proteome lysine acetylation status of the heart (see Fig. 3) and liver (not shown) causing significant deacetylation (data on heart and liver will be published elsewhere). Doxorubucin treatment did not affect the expression of sirt1 and sirt3, lysine deacetylases, in soleus muscle. Since doxorubicin did not affect lysine acetylation status, it is not unexpected that expression of sirt1 and sirt3 were also unaffected. Representative Western blots of sirt1 and sirt3 are shown in Fig. 4.
Fig. 3.
Protein lysine acetylation in soleus and heart. Representative Western blot showing deacetylation in heart of doxorubicin-treated animals, with no effect in soleus muscle of the same animals
Fig. 4.
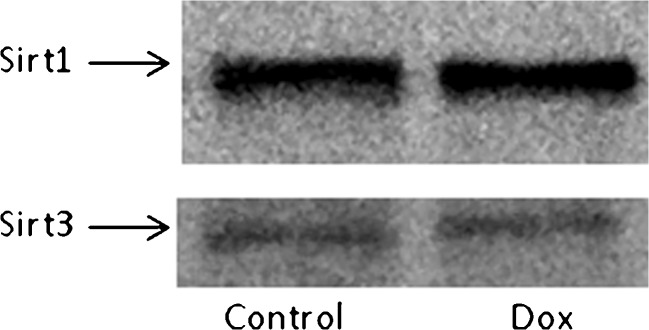
Representative Western blot of sirt1 and sirt3 in soleus
Discussion
Acute doxorubicin treatment in vivo did not affect proteome lysine acetylation status, or expression of sirt1, sirt3, SOD1, and SOD2. Twenty-four hours of doxorubicin exposure also did not affect expression or activation of caspase-1, caspase-8, caspase-9, or caspase−12. No other studies have reported the effects of doxorubicin treatment in skeletal muscle on these variables, except for SOD1 and SOD2. It was previously reported that doxorubicin treatment increased expression of SOD1, but not SOD2 in soleus muscle [9]. The difference in our results may be due to differences in experimental design and/or possibly due to the difference in strain of rat.
Previously published studies have shown that acute doxorubicin treatment in vivo can lead to oxidative stress and proteolysis [9], autophagy [10], and weakness in limb skeletal muscle [7, 8]. However, it cannot be determined whether these effects were due to the direct effects of doxorubicin or due to mal consumption of food and water, which were not controlled in these studies. Animals treated with doxorubicin significantly reduce their food and water intake by more than half within the first 24 h of treatment and lasts for several days [7]. Anorexia is known to stimulate catabolic pathways and proteolysis in skeletal muscle [16]. Proteolysis involves activation of various proteolytic systems, including the calpain and ubiquitin–proteasome systems as well as central components of the apoptotic machinery, specifically caspase-3 [17]. Anorexia also stimulates autophagy in order for the cell to recycle cellular constituents during times of low nutrient and energy status [18, 19]. Interestingly, it has been shown that the production of reactive oxygen species and, consequently, DNA damage is required for the optimal induction of autophagy in response to starvation [20]. Others have also shown that fasting can deplete glutathione [21] and stimulate oxidative stress [22, 23]. Fasting also alters cell signaling pathways which regulate cell survival and cell death [18]. Water deprivation and dehydration stimulates release of stress hormones, such as cortisol, and activation of the RAAS, known to also have adverse catabolic effects on skeletal muscle [24–26]. Thus, it is difficult to discern the effects of doxorubicin on cellular signaling and catabolic pathways in skeletal muscle without controlling for food and water intake. Zima et al. studied the effects of doxorubicin on skeletal muscle protease activities while controlling for food intake [27]. It was reported that doxorubicin treatment in these animals did not increase activity of various lysosomal and cytosolic proteases at 2.5 or 24 h posttreatment. This study is in agreement with our results in that doxorubicin had no effect on skeletal muscle catabolic parameters analyzed in fasted animals at 24 h posttreatment.
As a positive control, we assessed changes in lysine acetylation status in the heart and liver; tissues that are well described to be adversely affected by acute doxorubicin treatment [1]. Doxorubicin induced significant lysine deacetylation in both tissues thereby confirming that doxorubicin likely has a direct adverse effect on the heart and liver, but not skeletal muscle. Lysine acetylation is a common posttranslational modification regulating cellular signaling at multiple levels [28]. Lysine acetylation is known to regulate function of histones, transcription factors, cytoskeletal components, proteins affecting the ubiquitin–proteasome system, metabolic enzymes involved in a variety of energy-producing processes, and enzymes involved in oxidative stress defense system [28]. Since lysine acetylation regulates numerous processes and that proteome lysine acetylation status appears to be a marker of generalized cellular stress [11], the data support that doxorubicin may not directly affect skeletal muscle in vivo. Doxorubicin pharmacokinetics after intraperitoneal administration show that the maximal concentration reached in skeletal muscle is significantly less than heart, liver, and kidney [29]. Furthermore, the reduced metabolite of doxorubicin, doxorubicinol, has been shown to be more toxic to tissues than doxorubicin [30]. Carbonyl reductase 1 reduces doxorubicin into doxorubicinol and its expression has been shown to be minimal in skeletal muscle compared to heart, liver, and kidney [31]. These may be possible explanations supporting our results showing the lack of a direct effect, at least on markers assessed in this study.
A limitation to our study is the lack of an ad libitum-fed control group. This would have allowed us to specifically determine the effects of food and water deprivation on these parameters in skeletal muscle. However, our aim was to remove this variable from our experimental design so we could confidently state that the results that we found were not due to the effects of food and water deprivation.
In conclusion, doxorubicin treatment affected neither the proteome lysine acetylation status or the expression of sirt1, sirt3, SOD1, SOD2, nor the expression and activation of various procaspases in soleus muscle of fasted animals. We suggest that doxorubicin may not exert a direct effect on skeletal muscle in vivo, but rather doxorubicin treatment causes muscle atrophy and weakness via indirect mechanisms such as mal consumption of food and water as well as peripheral tissue toxicity. More studies with an experimental design controlling for food and water deprivation, focusing on the effects of doxorubicin on other catabolic parameters, are needed to confirm this hypothesis.
Acknowledgments
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle 2010;1:7–8 (von Haehling S, Morley JE, Coats AJ and Anker SD).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Carvalho C, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–85. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 2.Pointon AV, et al. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS One. 2010;5:e12733. doi: 10.1371/journal.pone.0012733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Childs AC, et al. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62:4592–8. [PubMed] [Google Scholar]
- 4.Chen Y, et al. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147–56. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 5.Jang YM, et al. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett. 2004;577:483–90. doi: 10.1016/j.febslet.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 6.Gilliam LA, St Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal. 2011;15:2543–63. doi: 10.1089/ars.2011.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilliam LA, et al. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol. 2009;107:1935–42. doi: 10.1152/japplphysiol.00776.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hydock DS, et al. Characterization of the effect of in vivo doxorubicin treatment on skeletal muscle function in the rat. Anticancer Res. 2011;31:2023–8. [PubMed] [Google Scholar]
- 9.Smuder AJ, et al. Exercise protects against doxorubicin-induced oxidative stress and proteolysis in skeletal muscle. J Appl Physiol. 2011;110:935–42. doi: 10.1152/japplphysiol.00677.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smuder AJ, et al. Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. J Appl Physiol. 2011;111:1190–8. doi: 10.1152/japplphysiol.00429.2011. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Alam HB. Modulation of acetylation: creating a pro-survival and anti-inflammatory phenotype in lethal hemorrhagic and septic shock. J Biomed Biotechnol. 2011;2011:523481. doi: 10.1155/2011/523481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Alam HB. Creating a pro-survival and anti-inflammatory phenotype by modulation of acetylation in models of hemorrhagic and septic shock. Adv Exp Med Biol. 2012;710:107–33. doi: 10.1007/978-1-4419-5638-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dirks-Naylor AJ, Kouzi SA. Constitutive protein content of procaspases in murine tissue. Central Eur J Biol. 2012;7:957–963. doi: 10.2478/s11535-012-0100-x. [DOI] [Google Scholar]
- 14.Freireich EJ, et al. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–44. [PubMed] [Google Scholar]
- 15.Glass B, et al. Dose-escalated CHOP plus etoposide (MegaCHOEP) followed by repeated stem cell transplantation for primary treatment of aggressive high-risk non-Hodgkin lymphoma. Blood. 2006;107:3058–64. doi: 10.1182/blood-2005-04-1570. [DOI] [PubMed] [Google Scholar]
- 16.Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006;22:830–44. doi: 10.1016/j.nut.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Du J, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–23. doi: 10.1172/JCI200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogata T, et al. Fasting-related autophagic response in slow- and fast-twitch skeletal muscle. Biochem Biophys Res Commun. 2010;394:136–40. doi: 10.1016/j.bbrc.2010.02.130. [DOI] [PubMed] [Google Scholar]
- 19.Kanamori H, et al. Functional significance and morphological characterization of starvation-induced autophagy in the adult heart. Am J Pathol. 2009;174:1705–14. doi: 10.2353/ajpath.2009.080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Vargas JM, et al. ROS-induced DNA damage and PARP-1 are required for optimal induction of starvation-induced autophagy. Cell Res. 2012;22:1181–98. doi: 10.1038/cr.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Simplicio P, et al. Antioxidant status in various tissues of the mouse after fasting and swimming stress. Eur J Appl Physiol Occup Physiol. 1997;76:302–7. doi: 10.1007/s004210050252. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen M, et al. Effects of fasting on oxidative stress in rat liver mitochondria. Free Radic Res. 2006;40:339–47. doi: 10.1080/10715760500250182. [DOI] [PubMed] [Google Scholar]
- 23.Marczuk-Krynicka D, et al. The effect of brief food withdrawal on the level of free radicals and other parameters of oxidative status in the liver. Med Sci Monit. 2003;9:BR131–5. [PubMed] [Google Scholar]
- 24.Song YH, et al. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–8. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Judelson DA, et al. Effect of hydration state on resistance exercise-induced endocrine markers of anabolism, catabolism, and metabolism. J Appl Physiol. 2008;105:816–24. doi: 10.1152/japplphysiol.01010.2007. [DOI] [PubMed] [Google Scholar]
- 26.Taylor PJ, et al. Simultaneous measurement of aldosterone and cortisol by high-performance liquid chromatography-tandem mass spectrometry: application to dehydration-rehydration studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1195–8. doi: 10.1016/j.jchromb.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Zima T, et al. Effects of doxorubicin (adriamycin) and [(+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)]propane (ICRF-187) on skeletal muscle protease activities. Toxicol Appl Pharmacol. 2001;171:135–40. doi: 10.1006/taap.2000.9084. [DOI] [PubMed] [Google Scholar]
- 28.Spange S, et al. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009;41:185–98. doi: 10.1016/j.biocel.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Johansen PB. Doxorubicin pharmacokinetics after intravenous and intraperitoneal administration in the nude mouse. Cancer Chemother Pharmacol. 1981;5:267–70. doi: 10.1007/BF00434396. [DOI] [PubMed] [Google Scholar]
- 30.Olson RD, et al. Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc Natl Acad Sci U S A. 1988;85:3585–9. doi: 10.1073/pnas.85.10.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassner N, et al. Carbonyl reductase 1 is a predominant doxorubicin reductase in the human liver. Drug Metab Dispos. 2008;36:2113–20. doi: 10.1124/dmd.108.022251. [DOI] [PubMed] [Google Scholar]