Abstract
Background
We recently validated in cross-sectional studies a new method to determine total body creatine pool size and skeletal muscle mass based on D3-creatine dilution from an oral dose and detection of urinary creatinine enrichment by isotope ratio mass spectrometry (IRMS). Routine clinical use of the method in aging and disease will require repeated application of the method, with a more widely available technology than IRMS, to enable determination of change in skeletal muscle mass in longitudinal studies. We therefore adapted the method to liquid chromatography-tandem mass spectrometry (LC-MS/MS) technology, and sought to establish proof of concept for the repeated application of the method in a longitudinal study. Because the turnover of creatine is slow, it was also critical to determine the impact of background enrichment from an initial dose of oral D3-creatine on subsequent, longitudinal measurements of change in muscle mass.
Methods
Rats were given an oral tracer dose of D3-creatine (1.0 mg/kg body weight) at 10 and 17 weeks of age. LC-MS/MS was used to determine urinary D3-creatine, and urinary D3-creatinine enrichment, at time intervals after D3-creatine administration. Total body creatine pool size was calculated from urinary D3-creatinine enrichment at isotopic steady state 72 h after administration of D3-creatine tracer.
Results
At 10 weeks of age, rat lean body mass (LBM) measured by quantitative magnetic resonance correlated with creatine pool size (r = 0.92, P = 0.0002). Over the next 7 weeks, the decline in urinary D3-creatinine enrichment was slow and linear, with a rate constant of 2.73 ± 0.06 %/day. Subtracting background urinary D3-creatinine enrichment from the elevated enrichment following a second dose of D3-creatine at 17 weeks permitted repeat calculations of creatine pool size. As at 10 weeks, 17-week LBM correlated with creatine pool size (r = 0.98, P <0.0001). In addition, the change in creatine pool size was correlated with the change in LBM during the 7 weeks of rat growth between measurements (r = 0.96, P <0.0001).
Conclusion
The LC-MS/MS-based D3-creatine dilution method can be applied repeatedly to measure total body creatine skeletal muscle mass change in longitudinal study.
Keywords: Body composition, Total body skeletal muscle mass, Total body creatine pool size, Stable isotope dilution
Introduction
The measurement of total body skeletal muscle mass has been problematic for a number of reasons. At present time, there is no method available that can directly assess skeletal muscle mass or its change, during aging, inactivity, disease, or exercise. We recently described a new method, D3-creatine dilution, for determination of total body creatine pool size and skeletal muscle mass. The D3-creatine dilution method takes advantage of a number of aspects of creatine biology, and builds on principles underlying the 24-h urinary creatinine method for determination of skeletal muscle mass [1]. Up to 98 % of total body creatine is found in skeletal muscle [2]. However, muscle has no mechanism for creatine synthesis. Creatine is synthesized in the kidney and liver and transported against a large concentration gradient into the sarcoplasm. The turnover of the sarcoplasmic creatine pool is slow and determined by the non-enzymatic, irreversible conversion of creatine to creatinine, which is excreted in urine (reviewed in [3, 4]). We [5] demonstrated that in rats, an oral tracer dose of D3-creatine is completely bioavailable, transported into skeletal muscle with minimal loss in urine, and the enrichment of urinary D3-creatinine provides an accurate and precise measure of total body creatine pool size. In cross-sectional studies of skeletal muscle accrual and atrophy in rats, this measure of creatine pool size was strongly related to the independent measures lean body mass (by quantitative magnetic resonance) and dissected skeletal muscle mass. Because there is no clinical tool to directly measure muscle mass at the present time, this method holds considerable promise for use in diagnosis of muscle wasting conditions and monitoring therapy targeted to skeletal muscle.
One objective of this study was to examine the use of more readily available liquid chromatography-tandem mass spectrometry (LC-MS/MS) technology rather than isotope ratio mass spectrometry (IRMS) for determination of urine creatinine enrichment. In the previous study, IRMS was used to measure deuterium enrichment in creatinine [5]. However precise IRMS may be, this technology is not sufficiently facile and available for routine higher throughput use; potentially limiting this method for clinical use.
The second objective of this study was to validate this method in a longitudinal study. Longitudinal application of the method requires confirmation that after an initial tracer dose of D3-creatine, the decline in urinary D3-creatinine enrichment is sufficiently slow and stable that at any later time, the background enrichment could be subtracted from the enrichment level measured after a subsequent tracer dose for determination of change in body creatine pool size. We used an independent and validated assessment of lean body mass measured by quantitative magnetic resonance (QMR), which in rats during the age range of growth studied here, is an excellent approximation of skeletal muscle mass [5], as a standard against which to compare the new creatine dilution method for determination of skeletal muscle mass.
We hypothesized that the LC-MS/MS analyses provide similar results as our initial IRMS cross-sectional studies, and that the repeated use of the D3-creatine dilution method in the same animals provides an accurate measure of change in total body creatine pool size.
Methods
Methods overview
Three key steps were involved in testing the study hypothesis of repeated application of the D3-creatine dilution method in a longitudinal study. The first was to determine whether LC-MS/MS could be used to determine urinary creatinine enrichment for creatine pool size determinations. The second was to determine the creatine turnover rate and confirm that it was slow and stable such that at any 72-h time period during isotopic steady state after administration of a second tracer dose, background enrichment from a previous tracer dose could be subtracted from the new enrichment for calculation of the new creatine pool size. The third was to repeat the application of the D3-creatine dilution method in the same rats at 10 and 17 weeks of age, and investigate the relationship between the change in creatine pool size measured by D3-creatine dilution and the change in an independent estimate of skeletal muscle mass, lean body mass measured by QMR.
Chemical and reagents
D3-creatine for in vivo use was synthesized by GlaxoSmithKline (GSK) Isotope Chemistry as creatine-D3-(Methyl-D3) monohydrate, 99 % pure, and was dissolved in pure H2O immediately before dosing. Reference standards of D3-Creatine (monohydrate) and D3-creatinine were purchased from CDN Isotopes, Montreal Canada. Stable isotopically labeled internal standards for both creatine and creatinine were obtained from GSK Isotope Chemistry.
Rats
Male Crl:CD (SD) rats (Charles River) were used. Rats were acclimatized for 1 week before study initiation. All studies were conducted after review by the GlaxoSmithKline Institutional Animal Care and Use Committee and in accordance with the GlaxoSmithKline Policy on the Care, Welfare, and Treatment of Laboratory Animals.
D3-creatine dilution protocol
The day prior to study, rat body weight and lean body mass (LBM) were determined. LBM was determined in conscious rats using QMR on an EchoMRI-700 (Echo Medical Systems, Houston, TX). The day of the study, rats were given an oral dose of D3-creatine (1.0 mg/kg body weight) at 10 am. The rats were immediately placed in single house metabolic cages, with ad libitum normal chow and water, for collection of urine over the next 72 h. Urine samples were collected at 10 a.m. to 4 p.m. and 4 p.m. to 10 a.m. intervals, clarified by centrifugation, and stored at −80 C. D3-creatine was quantified in all urine samples to determine the amount of tracer spillage in all urine from 0–72 h as percentage of the dose.
Assessment of D3-creatine spillage in urine
The urine samples were analyzed for D3-creatine by ultra-high performance LC-MS/MS using positive mode turbo ion spray, as previously described [5].
Assessment of urinary D3-creatinine enrichment by LC-MS/MS and calculation of total body creatine pool size
Urinary D3-creatine, creatinine, and D3-creatinine were determined by LC-MS/MS. Stock solutions of D3-creatinine are prepared at 1.0 mg/mL in water and confirmation of equivalence is performed. A 200-μL aliquot of the internal standard in acetonitrile was added to each well. A 40-μL aliquot of urine sample or standard was then transferred to the appropriate well. The plate was sealed and vortex mixed for approximately 3 min. The plate was centrifuged at approximately 3,000×g for 5 min. Supernatant was transferred to a clean 96-well plate and then injected onto the LC-MS/MS system (Acquity UPLC, Waters Corp., Milford, Ma.; Sciex API 5000, Applied Biosystems, Foster City, Ca.) for analysis. Urinary D3-creatinine enrichment was expressed as mole percent excess (MPE), where:
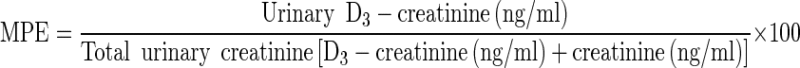 |
We then used the LC-MS/MS analyses to determine urinary spillage of D3-creatine during the 72 h after a single tracer dose of D3-creatine to define the tracer dose that was available for uptake into tissues, This and the urinary D3-creatinine enrichment at 72 h were used to calculate total-body creatine pool size as follows:
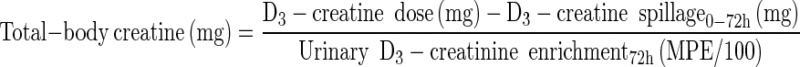 |
Statistics
The data were analyzed in SAS 9.2 (SAS Institute, Cary, NC) and figures were created in JMP 9.0.1 (SAS Institute, Cary, NC). All results are provided as mean +/− SE. Multiple observations were collected from each rat, and therefore, P values were derived by fitting a linear-mixed model with fixed main effect for age and a random effect for rat. Linear regression and Pearson’s correlation coefficient were used to assess linear relationships and the strength of the relationships in the cross-sectional validation of the new LC-MS/MS-based D3-dilution method, and within-group and within-rat comparisons in the longitudinal application of the D3-creatine dilution method. Clustering of groups can lead to artificially large correlation coefficients, so to adjust for grouping effects, we also calculated age (and treatment) effect adjusted partial correlation coefficients, as previously described [5]. In addition to calculating the correlation coefficient, the null hypothesis Ho: no linear relationship exists between lean body mass and total-body creatine pool size was tested and the P values were provided.
Results
Validation of an LC-MS/MS-based method for determination of total-body creatine pool size and skeletal muscle mass by D3-creatine dilution
We used archived urine samples from the previously reported IRMS-based D3-dilution method in a cross-sectional study of dexamethasone-induced muscle atrophy in rats to validate the new LC-MS/MS-based method [5]. As previously reported, any D3-creatine urinary spillage occurs mainly in the first 24 h after the tracer dose of D3-creatine used, and total urinary D3-creatine spillage following the 0.475-mg dose of D3-creatine per rat was less than 0.5 % from 0–72 h [5]. The change in the numerator of the total-body creatine equation above is negligible at this low level of urinary spillage, and urinary spillage was not included in the previously reported IRMS data [5], but for completeness and consistency is included with the LC-MS/MS-based calculations in the present study.
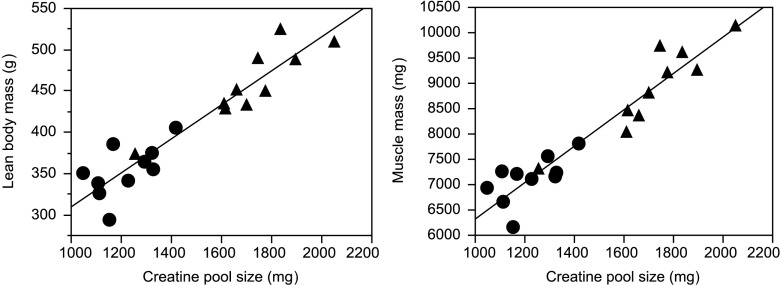
Similar results were obtained with the new LC-MS/MS method compared to the previously reported IRMS-based method: both methods show the same reduction in total-body creatine pool size induced by dexamethasone compared to vehicle-treated controls (IRMS, 1,255 ± 41 vs. 1,853 ± 72 mg, 32 % reduction, P <0.001; LC-MS/MS, 1,221 ± 39 vs. 1,715 ± 67 mg, 29 % reduction, P <0.001). As with the IRMS-based method, total-body creatine pool size calculated by the LC-MS/MS method also strongly correlated with independent measures of muscle mass (LBM determined by QMR or hind limb skeletal muscle mass determined by dissection of individual muscles) (Fig. 1), and the correlation between total-body creatine pool size measured with the LC-MS/MS method or the IRMS method was highly significant (rall rats = 0.96; rall rats treatment adjusted = 0.86, data not shown; P <0.0001).
Fig. 1.
Correlation between body creatine pool size (measured by D3-creatine dilution) and lean body mass (left; measured by quantitative magnetic resonance; rall rats = 0.9392, P <0.0001; rall rats treatment adjusted = 0.8075, P <0.0001) or skeletal muscle mass (right; measured as the sum of right and left mass of gastrocnemius, tibialis anterior, extensor digitorum longus, and soleus for each rat; rall rats = 0.9520, P <0.0001; rall rats treatment adjusted = 0.8551, P <0.0001) for groups of 10 rats at 22 weeks of age, treated the previous 2 weeks subcutaneously once daily with either saline (triangles) or dexamethasone (circles; 2 mg/ml for first week, 0.1 mg/kg for second week)
Demonstration of slow and linear decline in urinary D3-creatinine following a single dose of D3-creatine during isotopic steady state
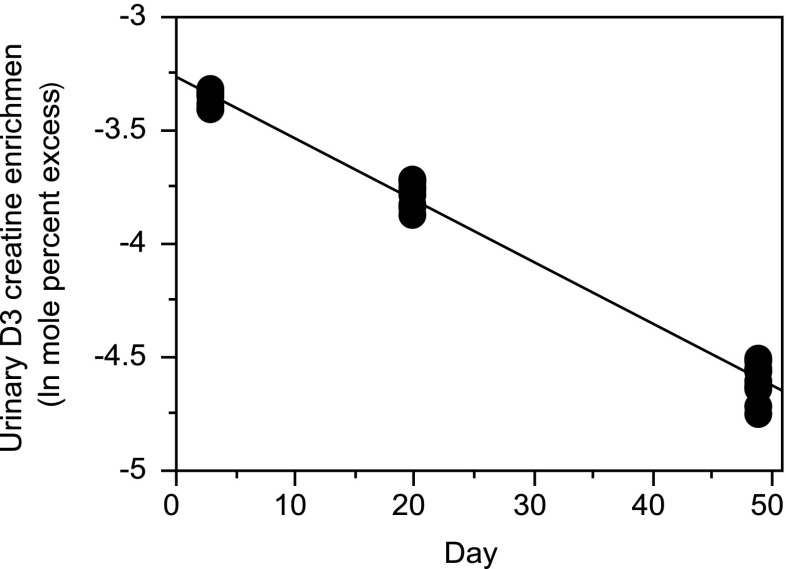
Our previous report showed that isotopic steady state is reached 24–48 h after a single tracer dose of D3-creatine, but we had not looked beyond 72 h [5]. In the present study, the decline in urinary D3-creatinine MPE from day 3–49 was examined after oral administration of D3-creatine tracer (1.0 mg/kg body weight). The turnover rate constant (k) for body creatine was calculated after administration of a creatine stable isotope as the slope of a plot of the natural log of urinary stable creatinine isotope enrichment over time [6]. Figure 2 shows that the turnover was slow and linear, with a rate constant of 2.73 ± 0.06 %/day.
Fig. 2.
Decrease in natural log of urinary D3-creatinine enrichment over time in groups of 10 rats given a single oral tracer dose of 1 mg/kg body weight of D3-creatine 72 h earlier. The slope is −0.0273 ± 0.000565/day
This result further suggest that at any point in time during isotopic steady state after a tracer dose of D3-creatine, there is a sufficiently stable background of urinary D3-creatinine enrichment that this background can be subtracted from a new elevated enrichment at steady state after a second tracer dose in order to determine the enrichment due solely to the second tracer dose. In practice, we hypothesized that for longitudinal application of the D3-creatine dilution method, a background urinary D3-creatinine enrichment could be determined in a spot urine sample taken the day prior to administration of a second D3-creatine tracer dose. This background could be subtracted from the new steady state enrichment level obtained in a spot urine sample taken 3 days after the second tracer dose. The new creatine pool size could then be determined from the enrichment difference attributable to the second tracer dose. This enrichment difference is then divided into the second D3-creatine tracer dose, after subtracting any urinary spillage following the second D3-creatine tracer dose, as described in Materials and Methods.
Demonstration of the longitudinal application of the D3-cr dilution method: increase in creatine pool size in growing rats from 10–17 weeks of age
To test our hypothesis for longitudinal application of the D3-creatine dilution method, we used a cohort of growing rats. Creatine pool size was determined from the urinary D3-creatinine enrichment obtained in a spot urine sample taken 3 days after three different tracer doses of D3-creatine. Seven weeks later, a spot urine sample was obtained to determine background urinary D3-creatinine enrichment remaining from the first D3-creatine dose. A second D3-creatine dose was then administered, 3 days later, a spot urine sample was obtained to determine the new elevated urinary D3-creatine enrichment, and background enrichment from the first dose was subtracted to yield the difference in urinary D3-creatine enrichment that was used to calculate the creatine pool size of the now 17-week-old rats.
Urine samples collected continuously over the first 3 days after tracer administration confirmed the finding in our first study [5] that any spillage occurs mainly in the first 24 h after the tracer dose, and spillage in the present study was subtracted from the absolute amount of D3-creatine administered on an individual rat basis for calculating creatine pool size. Urinary spillage was ≤1 % of the tracer dose administered (10 weeks of age: 0.53 ± 0.14 %; 17 weeks of age: 0.49 ± 0.04 %).
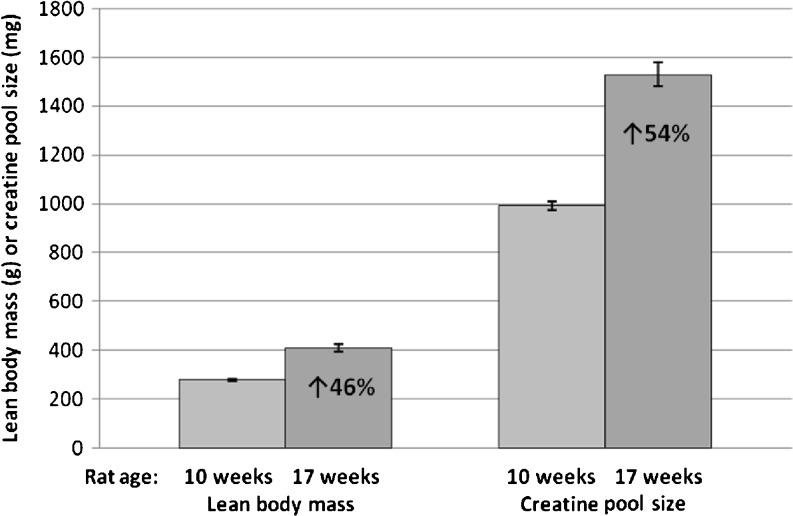
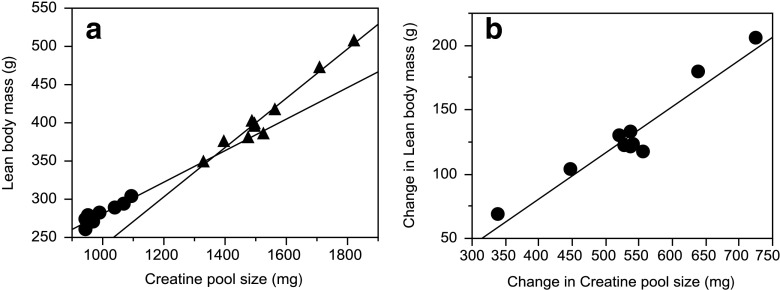
There was close agreement between the increase in body creatine pool size measured by D3-creatine dilution (54 % increase over week 10, P <0.0001) and increase in lean body mass measured by QMR (47 % increase over week 10, P <0.0001) from 10–17 weeks of age (Fig. 3). The within-group Pearson’s coefficient of correlation calculated for the relationship between body creatine pool size and lean body mass (Fig. 4a) was significant at both 10 weeks of age (r = 0.92, P = 0.0002) and 17 weeks of age (r = 0.98, P <0.0001).
Fig. 3.
Significant increase in lean body mass (measured by repeated quantitative magnetic resonance, P <0.0001) and body creatine pool size (measured by the repeated D3-creatine dilution method, P <0.0001) in rats at 17 weeks compared to 10 weeks of age
Fig. 4.
a Within-group Pearson’s coefficient of correlation calculated for the relationship between body creatine pool size (measured by the repeated D3-creatine dilution method) and lean body mass (measured by repeated quantitative magnetic resonance) at 10 and 17 weeks of age (r = 0.9196, P = 0.0002, and r = 0.9786, P <0.0001, respectively). b Within-rat Pearson’s coefficient of correlation for the relationship between change in body creatine pool size and change in lean body mass (r >0.9629; P <0.0001)
We further explored this relationship by examining the within-rat correlation to assess whether a change in body creatine pool size with an individual rat is associated with a change in lean body mass. Fig. 4b shows a significant correlation between change in lean body mass and change in body creatine pool size for individual rats (r >0.96; P <0.0001).
Power calculations were done to compare the smallest detectable difference in body creatine pool size that can be detected in groups of 10 rats with 90 % power (at α = 0.05) by either single application of the creatine dilution method in different age groups in a cross-sectional study or the repeated application of the method in the same rats in the present longitudinal study. In our previous cross-sectional study we had indicated a smallest detectable difference of 242-mg creatine pool size [5]. We used the data from the 9 and 17-week-old rats from that previous study and calculated a smallest detectable difference of 187-mg creatine pool size based only on the 9 and 17-week-old rat data, for a more suitable comparison to the age range used in the present longitudinal study. In contrast, repeated application of the method yielded a smallest detectable difference of 118-mg creatine pool size in a longitudinal study. This translates to the ability to detect in 9–10-week-old rats a minimum of 21 % difference in body creatine pool size in cross-sectional studies vs. a 12 % change in longitudinal studies when the method is applied repeatedly in the same animals, for group sizes of 10 animals.
Discussion
We demonstrated that LC-MS/MS analysis of creatinine enrichment produced results that were, essentially, identical to analysis by IRMS [5] and that the D3-creatine dilution method may be used for longitudinal assessment of changes in skeletal muscle mass. LC-MS/MS analysis to measure urinary D3-creatinine enrichment was developed and validated against IRMS-based analyses of the same samples. The data from this study show that the decrease in urinary D3-creatinine enrichment over several weeks follow a slow, linear decline, enabling background subtraction from subsequent applications of the D3-creatine dilution method, producing an accurate and precise repeat measure of muscle mass.
This method takes advantage of a number of well-known aspects of creatine biology which support the assumption of a single pool model in calculating creatine pool size. Up to 98 % of body creatine is estimated to reside in skeletal muscle [2]. However, because skeletal muscle has no creatine synthesis mechanism, it is actively transported against a large concentration gradient after absorption from dietary sources or synthesis in the kidney and liver (reviewed in [4]). It is, therefore, possible to deliver an oral tracer dose of creatine that will be transported into skeletal muscle and, thus, the dilution of the tracer can provide an assessment of creatine pool size and skeletal muscle mass [7, 8]. Turnover of skeletal muscle creatine occurs as a result of a non-enzymatic, irreversible conversion of a small proportion of creatine to creatinine, and creatinine is rapidly and fully excreted in the urine. Thus, measurement of the enrichment of urine D3-creatinine provides in principle the opportunity to sample the intramyocellular enrichment of creatine and accurately estimate total-body creatine pool size [5].
We demonstrated that the decrease in urinary D3-creatinine enrichment is slow and linear. Using the method of Hoberman et al. [6], we calculated the creatine turnover rate constant in rats from the slope of the decrease in natural log of urinary D3-creatinine enrichment over time, as 2.73 %/day. Thus, within any given 2–3-day period after achievement of isotopic steady state, urinary D3-creatinine enrichment is essentially stable, permitting its subtraction as background enrichment in repeated applications of the D3-creatine dilution method for body creatine pool size determination. The creatine turnover rate constant calculated in this study agrees well with past studies in rats, but is higher than that reported in humans. This difference is very likely due to an increase in rat muscle creatine that occurs with rat growth that has not been taken into account in past studies of rat creatine turnover. The true creatine turnover rate constant is the rate at which the creatine-phophocreatine pool is lost by the non-enzymatic, irreversible cyclization to creatinine, which is then excreted via urine without reentering muscle, and must be replaced by uptake into muscle of creatine endogenously synthesized in other tissues or absorbed from the diet [4]. Bloch et al. [9] reported in rats an average creatine turnover per day of ∼2 %, and using the Bloch et al. raw data and the same method as outlined by Hoberman et al. [6], Borsook and Dubnoff [10] calculated the creatine turnover rate constant as 2.7 %/day, in agreement with our data. The creatine turnover rate constant for humans is most often reported as 1.7 %/day [3, 4], based on the work of Fitch et al. [11] as an average of four subjects with no muscle disease, and studies of two normal adult male subjects by Hoberman, et al. [6, 12], who found values of 1.43 %/day [12] and 1.64 %/day [6]. We hypothesize that the higher creatine turnover rate constant in rats compared to humans is due to more rapid dilution of the tracer by steady addition of endogenous creatine to skeletal muscle during this rapid growth period of rats, when creatine is entering muscle not only to replace what is lost to creatinine conversion, but also to support new muscle growth. As noted above, this contribution of growth was not taken into account in past studies in rats. It is possible to model this growth effect on creatinine dilution over time by calculating the rate constant of increase in creatine pool size. Based on the slope of the natural log of creatine pool size vs. time, the value for the group was as 0.94 ± 0.04 %/day. Since MPE and creatine pool size are inversely related, subtracting this growth effect of 0.94 %/day from the 2.73 %/day yields a true creatine turnover rate constant in rats of 1.79 %/day, more in line with reported human values, which were determined in adults subjects with stable muscle mass. Because the conversion of creatine to creatinine is non-enzymatic, it is reasonable that this rate constant might be similar across species with different metabolic body size.
These creatine turnover calculations further suggest a secondary approach for estimating change in creatine pool size in the situation of muscle accrual. The increase in the apparent creatine turnover rate constant during isotopic die-away after a single dose of D3-creatine, minus the estimated true creatine turnover rate constant (1.7 %/day), might represent a metric creatine pool size change. This approach would not require a second tracer dose of D3-creatinine in a longitudinal study, for as long a period of time as the enrichment after the single D3-creatine tracer dose remained detectable. We modeled this effect in the current study by calculating the apparent creatine turnover constant minus the average “true” creatine turnover constant (1.7 %/day) for each rat and extrapolating to 17 weeks to calculate the increase in creatine pool size, and comparing this to the increase in pool size determined using the second application of the D3-creatine dilution method reported in the current study. The results were essentially identical (the 17 week creatine pool size determined by creatine turnover rate method and second D3-creatine dilution method are 1,572 ± 57 and 1,530 ± 45 mg, respectively), and the creatine pool size for individual rats determined by the creatine turnover rate method and the repeated D3-creatine dilution method are highly correlated (r = 0.95, P <0.0001). While this alternative approach is potentially simple and precise (it would entail only a single D3-creatine tracer dose) for determination of creatine pool size increase in a longitudinal study, it involves correction for a non-measured parameter (estimated true creatine turnover rate constant) and assumes a stable creatine turnover rate in all subjects. This approach, though promising, will require confirmation in clinical studies and in larger numbers of subjects, particularly in disease states.
Tracer dosing based on kilogram body weight used in this study performed similarly to the fixed absolute amount of tracer in every animal used in our previous work [5], indicating some level of flexibility in dose selection. As with past cross-sectional studies, there was a significant correlation between creatine pool size and lean body mass within the group at both the 10 and 17-week-old time points. In this study, our last study [5], and unpublished observations, we have consistently noted that as the rats grow and individual body mass variability naturally increases, there is a trend to stronger within group correlations between creatine pool size and lean body mass, and even more tolerance to a range of acceptable tracer doses.
Most importantly, body creatine pool size measured by repeat application of the D3-creatine dilution method in rats growing from 10 to 17 weeks of age agreed well with an independent assessment of lean body mass by quantitative magnetic resonance. There was a strong correlation between change in lean body mass and change in body creatine pool size within individual. We demonstrate that repeated use of this method is accurate as long as the baseline urine D3-creatinine enrichment is measured and accounted for after dosing with D3-creatine and achievement of urinary isotopic steady state. Power calculations indicate the smallest detectable change in creatine pool size by repeated application of the method is nearly half the smallest change detectable in cross-sectional studies of different rats. The new LC-MS/MS analysis of urinary D3-creatinine enrichment and demonstration of the feasibility of repeated measurements of body creatine pool size in the longitudinal study reported here provide additional data supporting the utility of the D3-creatine dilution method for monitoring the time course and amount of muscle wasting and effects of potential anabolic therapies. Clinical trials are currently underway to validate this method in healthy subjects and patients experiencing muscle wasting. Replication of the result of this longitudinal study in humans will be critical to a broad application of this D3-creatine dilution method in humans.
Acknowledgments
The authors wish to acknowledge Mark Snead for practical contributions, and Alan Russell and Robin O’Connor-Semmes for valuable discussions. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle, 2010;1:7–8 (von Haehling S, Morley JE, Coats AJ and Anker SD).
Conflict of interest
Elements of study design and IRMS analyses at KineMed, Inc. were done with funds provided by GlaxoSmithKline. Marc K. Hellerstein is Chief of the Scientific Advisory Board and owns stock in KineMed, Inc.
References
- 1.Heymsfield SB, Arteaga C, McManus BS, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-h urinary creatinine method. Am J Clin Nutr. 1983;37:478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 2.Hunter A. The biological distribution of creatine and creatinine. Creatine and creatinine. London: Longmans, Green and Co. LTD; 1928. [Google Scholar]
- 3.Walker JB. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol. 1979;50:177–242. doi: 10.1002/9780470122952.ch4. [DOI] [PubMed] [Google Scholar]
- 4.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 5.Stimpson SA, Turner SM, Clifton LG, Poole JC, Mohammed HA, Shearer TW, et al. Total-body creatine pool size and skeletal muscle mass determination by creatine-(methyl -d 3) dilution in rats. J Appl Physiol. 2012;112:1940–1948. doi: 10.1152/japplphysiol.00122.2012. [DOI] [PubMed] [Google Scholar]
- 6.Hoberman HD, Sims EAH, Peters JH. Creatine and creatinine metabolism in the normal male adult studied with the aid of isotopic nitrogen. J Biol Chem. 1948;172:45–58. [PubMed] [Google Scholar]
- 7.Kreisberg RA, Bowdoin B, Meador C. Measurement of muscle mass in humans by isotopic dilution of creatine- 14 C. J Appl Physiol. 1970;28:264–267. doi: 10.1152/jappl.1970.28.3.264. [DOI] [PubMed] [Google Scholar]
- 8.Meador C, Kreisberg RA, Friday JP, Bowdoin B, Coan P, Armstrong J, et al. Muscle mass determination by isotopic dilution of creatine- 14 C. Metabolism. 1968;17:1104–1108. doi: 10.1016/0026-0495(68)90089-9. [DOI] [PubMed] [Google Scholar]
- 9.Bloch K, Schoenheimer R, Rittenberg D. Rate of formation and disappearance of body creatine in normal animals. J Biol Chem. 1941;138:155–166. [Google Scholar]
- 10.Borsook H, Dubnoff JW. The hydrolysis of phophocreatine and the origin of urinary creatinine. J Biol Chem. 1947;168:493–510. [PubMed] [Google Scholar]
- 11.Fitch CD, Lucy DD, Bornhofen JH, Dalrymple GV. Creatine metabolism in skeletal muscle. II. Creatine kinetics in man. Neurology. 1968;18:32–42. doi: 10.1212/WNL.18.1_Part_1.32. [DOI] [PubMed] [Google Scholar]
- 12.Hoberman HD, Sims EAH, Engstrom WW. The effect of methyltestosterone on the rate of synthesis of creatine. J Biol Chem. 1948;173:111–116. [PubMed] [Google Scholar]