Abstract
Background
Geriatric cachexia is distinct from other age-related muscle wasting syndromes; however, detection and therefore treatment is challenging without the availability of valid instruments suitable for application in the clinical setting. This study assessed the sensitivity and specificity of a newly developed screening instrument utilising portable assessments against previously defined and commonly accepted diagnostic criteria for detection of geriatric cachexia.
Methods
Cross-sectional analyses from 71 older adults’ post-surgical fixation for hip fracture were performed. The diagnostic criteria required measures of appendicular skeletal muscle index derived from dual-energy X-ray absorptiometry and anorexia assessed by ≤70 % of estimated energy requirements. These assessments were replaced with mid-upper arm muscle circumference and the Simplified Nutritional Appetite Questionnaire, respectively, to create a field instrument suitable for screening geriatric cachexia. Sensitivity, specificity and positive and negative predictive values were calculated.
Results
The current diagnostic algorithm identified few patients as cachectic (4/71; 5.6 %). The sensitivity and specificity of the geriatric cachexia screening tool was 75 and 97 %, respectively. The screening tool had a positive predictive value of 60 % and a negative predictive value of 99 %.
Conclusions
Given the unexpected prevalence of cachexia in such a vulnerable group, these results may suggest problems in operationalising of the consensus definition and diagnostic criteria. Although the application of a newly developed screening tool using portable field measures looks promising, the authors recommend additional research to identify the prevalence of geriatric cachexia, which captures all diagnostic criteria from the consensus definition. Future investigation may then be positioned to explore the predictive validity of screening tools using portable field measures, which potentially achieve higher sensitivity.
Keywords: Cachexia, Older adults, Hip fracture, Validity, Reliability
Introduction
Age-related diseases associated with skeletal muscle atrophy have become one of the most extensively developed topics of clinical investigation. Despite much research, the aetiology of geriatric syndromes associated with skeletal muscle wastage remains unclear [1, 2]. Despite progressive losses in skeletal muscle mass (SMM) and function, i.e. sarcopenia being a common characteristic of ageing [1, 3], muscle wasting is not specific to age-related sarcopenia. Physical inactivity [4], starvation [5, 6] and frailty [7, 8] are all associated with skeletal muscle atrophy and therefore should be distinguished from other forms of muscle wasting syndromes such as cachexia.
Older adults with recent hip fracture are an important clinical group at increased risk of muscular disuse, immobilisation, progressive disability, institutionalisation and subsequent mortality post-surgery [9, 10]. Low SMM is often present upon hospital admission among hip fracture patients [11, 12], which often worsens throughout the admission [13]. Appropriate therapy is therefore paramount to facilitate improved health outcomes and increased participation in rehabilitation in this cohort [14]. The effectiveness of a more traditional nutritional approach such as energy and protein supplementation in this patient group, however, is not convincing [11, 13]. Current evidence suggests that nutritional supplementation alone may not prevent skeletal muscle wasting in this group secondary to the pathophysiology of cachexia [15, 16]. Therefore, the provision of appropriate therapy depends on the accurate identification of cachexia.
In an older adult population, cachexia is infrequently diagnosed and therefore rarely treated. A recent secondary analysis from a sample of older adults aged ≥65 years participating in ambulatory rehabilitation showed that one in five participants (37/187) were defined as cachectic [17]. At the present time, cachexia lacks a universally accepted definition, which represents a key issue for identification and the provision of treatment. Independent of this, however, it is generally accepted that cachexia is a multi-factorial syndrome associated with underlying illness characterised by ongoing loss of body weight and skeletal muscle (with or without fat mass), anorexia and systemic inflammation [2, 15, 16, 18]. The most commonly accepted consensus definition and set of diagnostic criteria published by the Society of Sarcopenia, Cachexia and Wasting Disorders (SCWD) allows clinicians and researchers to make a definitive diagnosis of cachexia [18]. For a clear diagnosis of cachexia, however, one of the challenges for clinicians is the application of easy, non-invasive portable field assessments. While there are multiple clinical tools used for the assessment of cancer cachexia [19–22], this is yet to be explored nor validated in post-surgical older adults with limited mobility.
The purpose of this cross-sectional analysis was to present preliminary evidence for the assessment of construct validity of a newly developed screening tool utilising portable field methods against the previously defined diagnostic criteria for detection of geriatric cachexia in community-dwelling older adults’ post-surgical fixation for hip fracture.
Methods
Patients and recruitment
This was a cross-sectional analysis performed as part of the INTERACTIVE trial (ACTRN 12607000017426), a prospective randomised controlled trial of a nutrition and exercise programme in community-dwelling older adults’ post-surgical fixation for hip fracture [23]. Participants were recruited from three acute care settings including Flinders Medical Centre, Adelaide, SA, Flinders Private Hospital, Adelaide, SA and Hornsby Ku-ring-gai Hospital, Sydney, NSW [23]. Data contributing to the final analyses were from baseline measures, routinely performed within 14 days post-surgery.
Participants aged ≥ 65years were eligible for the study if they were admitted with a diagnosis of hip fracture confirmed by radiology report, had a Mini-Mental State Examination score of ≥18, had a body mass index (BMI) between 18.5 and 35 kg/m2, and were community dwelling within existing local service boundaries. Exclusion criteria included a pathological fracture or malignancy, those residing in residential care, non-English speaking, limited to stand transfers only post-surgery or non-ambulatory pre-fracture, unable to provide informed consent or deemed medically unstable within 14 days post-surgery. This study was conducted according to the guidelines described in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Flinders Clinical Research Ethics Committee (Protocol 110/067). Written informed consent was obtained from all subjects.
Measurements and procedures
Cachexia diagnostic algorithm
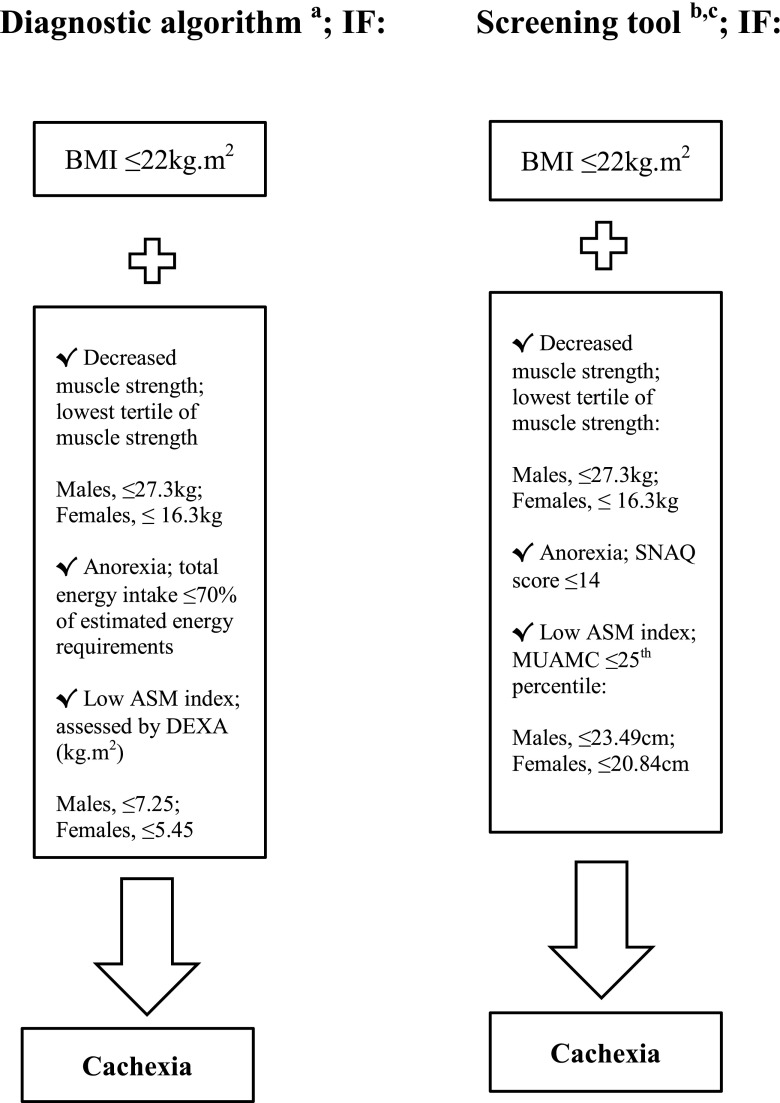
In 2008, the SCWD published a commonly accepted consensus definition in addition to a set of diagnostic criteria for a diagnosis of cachexia [18]. Weight loss (e.g. BMI ≤20kg m2, or loss of body weight ≥5 % within the previous 12 months or less) is the cornerstone of this consensus definition [18]. Furthermore, the presence of at least three of the following criteria, i.e. decreased muscle strength, fatigue, anorexia, low appendicular skeletal muscle (ASM) index and abnormal biochemical parameters (inflammation, anaemia or low albumin), is required for a diagnosis of cachexia [18]. The diagnostic algorithm and diagnostic cut-offs established by the SCWD are shown in Fig. 1. Measures of ASM index (male, ≤7.25kg; female, ≤5.45kg), recorded dietary intake ≤70 % of estimated energy requirements and isometric hand-grip strength (lowest tertile for grip strength; male, ≤27.3 kg; female, ≤16.3 kg) coupled with a BMI ≤22 kg m2 were used as the diagnostic algorithm for the detection of cachexia in the present study (Fig. 1). A BMI ≤ 22kg m2, rather than ≤20 kg m2 was used as the critical level of BMI for older adults in the present study [24–26].
Fig. 1.
Diagnostic algorithm for cachexia established by the Society of Sarcopenia, Cachexia and Wasting Disorders (SCWD) and the proposed new screening tool for detection of geriatric cachexia in hip fracture patients. In the present study, the newly developed screening tool was assessed against three diagnostic measures from the diagnostic algorithm for cachexia. BMI body mass index, FFM fat-free mass, ASM appendicular skeletal muscle, DEXA dual energy X-ray absorptiometry, SNAQ Simplified Nutritional Appetite Questionnaire, MUAMC mid-upper arm muscle circumference. a Diagnostic cutoffs from the proposed diagnostic algorithm for cachexia were derived from Evans et al. [18]. b Screening tool diagnostic criteria cut-offs for isometric hand-grip strength and MUAMC were obtained from wave 1 of the Australian Longitudinal Study of Ageing (ALSA) [35]. c A score ≤14 for SNAQ identifies persons with anorexia at risk of significant weight loss of at least 5 % within 6 months [38]
Weight and height
Body weight was recorded to the nearest 0.1 kg using calibrated digital scales (Tanita, BF-681 Scale and Body Fat Monitor, Tokyo, Japan) with participants wearing light clothing and without footwear. Participants unable to mobilise were weighed using a calibrated weigh chair. Knee height was measured on the non-injured leg with participants wearing no footwear with the participant in a supine or seated position. Height was estimated from knee height using validated age and gender specific equations [27, 28]. BMI was calculated as weight (kilogram) divided by the square of height (metre).
Dual-energy X-ray absorptiometry
Whole-body and regional body composition was estimated using dual-energy X-ray absorptiometry (DEXA) (Lunar Prodigy, GE Healthcare, UK) with the automated reporting GE EnCORE bone densitometry software (version 10.51.006). The system software provides estimates of FFM, lean soft tissue, fat mass and bone mineral density for total body and body segments including both arms, both legs and the trunk. ASM was calculated as the sum of lean soft tissue mass in both arms and legs [29] with the ASM index calculated using the formula, ASM/height2 (kg/m2) [30]. Prior to all DEXA scans, all participants underwent a DEXA screening checklist to ensure safety and validity of the technique. Participants were excluded from the DEXA scan if they reported a history of nuclear scans or other X-ray examinations in the previous 0–14 days or had a recorded body weight ≥130 kg. Prior to the scan, all participants were asked to remove all metal accessories, were asked to identify any medications taken in the previous 24 h (including calcium or iron supplements) and were asked to identify any history of previous fracture and/or metal implants. The software recognises metal in the body, such as artificial joints, allowing exclusion from calculations prior to analysis. Participants were dressed in hospital gowns and positioned in a supine position on the table top with their feet in a neutral position with hands flat by their sides. All DEXA scans were performed by a licenced technician.
Dietary intake and analyses
Dietary intake was assessed using a single multiple-pass 24-h dietary recall. Hospital ready-reckoners were used to estimate energy and protein intake. Individual estimated energy requirements were calculated using gender-specific Harris–Benedict equations [31] to estimate resting metabolic rate, with adjustments made for physical activity (1.2), trauma (1.35) and weight gain (0.25 kg/week) [32]. All dietary analyses were performed by an accredited practising dietitian.
Isometric hand-grip strength
Isometric hand-grip strength was used as a reliable and valid surrogate measure of muscle strength [33, 34] for the diagnostic algorithm and in the development of the screening tool. Grip-strength was measured in the dominant hand with a calibrated Smedley Hand Dynamometer (Tokyo, Japan). All measures were performed on three separate occasions with ~60-s rest intervals between each measure with the mean of the three measures used for analyses. All measures were recorded to the nearest 0.1 kg.
Development of cachexia screening tool
In keeping with the cachexia diagnostic algorithm, BMI ≤22 kg m2 and isometric hand-grip strength were maintained in the newly developed screening tool. Measures of ASM index derived from DEXA and anorexia assessed by 24-h dietary recall in the diagnostic algorithm were replaced with portable assessments of body composition and anorexia including mid-upper arm muscle circumference (MUAMC) and the Simplified Nutritional Appetite Questionnaire (SNAQ), respectively. Diagnostic cut-off criteria for isometric hand-grip strength (lowest tertile for grip strength; male, ≤27.3 kg; female, ≤16.3 kg) and MUAMC (≤25th percentile; male, ≤23.49 cm; female, ≤20.84 cm) were derived from age and gender specific data obtained from wave 1 of the Australian Longitudinal Study on Ageing [35]. The newly developed screening tool and specific diagnostic cut-offs are shown in Fig. 1.
Upper-arm anthropometry
MUAMC is derived from two upper-arm anthropometric techniques, MUAC and triceps skinfold thickness (TSF). MUAC was measured to the nearest 0.1 cm using a flexible steel measuring tape (KDS Corporation, Kyoto, Japan), and TSF was measured to the nearest 0.2 mm using a calibrated Harpenden skinfold calliper (Baty, International Sussex, UK). All anthropometric measures were performed by trained dietitians and/or physiotherapists, with each measure performed on three separate occasions with the mean of the three measures used for analyses. Unless affected by injury, all anthropometric measures were taken on the right-hand side of the body. MUAMC was estimated from TSF (mm) thickness and MUAC (cm) using the formula: MUAMC = MUAC(cm) − 0.3142 × TSF(mm) [36, 37].
Appetite
Appetite was assessed using the SNAQ; a quick and feasible four-item derivative of the eight-item Council on Nutrition Appetite Questionnaire developed by the Council for Nutritional Strategies in Long-Term Care in institutionalised and community-dwelling older adults [38]. The SNAQ takes approximately ~1 min to complete and was administered either by trained dietitians and/or physiotherapists. Participants were asked to respond to the four-item questionnaire, which included a self-rating of appetite, satiation, gustation and the frequency of meals eaten throughout the day. The total SNAQ score is the sum of scores from the four items, with lower scores indicating deterioration in appetite. Possible scores range from 4 (worst) to 20 (best). A score of ≤14 for the four-item questionnaire identifies persons with anorexia at risk of significant weight loss of at least 5 % within 6 months [38].
Statistical analyses
Analyses were performed using SPSS for Windows 17.0 software (SPSS Inc, Chicago, IL, USA). Contingency tables were used to determine the specificity, sensitivity and predictive values for the screening tool as a function of its ability to predict geriatric cachexia against the commonly accepted diagnostic algorithm. For continuous data, mean (SD) were reported with frequencies and percentages reported for categorical data. Independent samples t tests were used to assess gender differences for each diagnostic parameter of cachexia. Significance was set at P < 0.05.
Results
A sample of 71 older adults (female, n = 52; male, n = 19) recruited from the INTERACTIVE trial with DEXA results available were included in the final analyses. The mean ± SD age of participants at baseline was 82.2 ± 5.8 years. The primary measures of interest in the present study are shown in Table 1. Significant gender differences were observed with women having greater TSF thickness (P = 0.001) and BMI (P = 0.03); men, however, had significantly greater isometric hand-grip strength (P < 0.001) relative to women.
Table 1.
Characteristics for each diagnostic parameter of cachexia at baseline post-surgical fixation for hip fracture in male and female participants (all such values reported as mean ± SD)
| Characteristics | Mean | SD |
|---|---|---|
| Male, n = 19 | ||
| Weight (kg) | 69.7 | 12.7 |
| BMI (kg m2)a | 23.9 | 2.9 |
| MUAC (cm) | 26.7 | 3.3 |
| TSF (mm)a | 11.5 | 4.8 |
| MUAMC (cm) | 23.2 | 2.5 |
| ASM Index (kg m2) | 6.7 | 0.9 |
| SNAQ score | 13.6 | 2.9 |
| Isometric hand-grip strength (kg)a | 23.9 | 7.6 |
| Female, n = 52 | ||
| Weight (kg) | 66.4 | 12.9 |
| BMI (kg m2)a | 25.9 | 3.8 |
| MUAC (cm) | 27.1 | 3.9 |
| TSF (mm)a | 16.4 | 5.4 |
| MUAMC (cm) | 21.9 | 3.1 |
| ASM Index (kg m2) | 6.4 | 0.9 |
| SNAQ score | 13.1 | 2.2 |
| Isometric hand-grip strength (kg)a | 15.9 | 4.7 |
BMI body mass index, MUAC mid-upper arm circumference, TSF triceps skinfold thickness, MUAMC mid-upper arm muscle circumference, ASM appendicular skeletal muscle, SNAQ Simplified Nutritional Appetite Questionnaire
aSignificant differences between men and women by independent samples t test (P < 0.05)
The newly developed screening tool identified ~7 % of participants as cachectic (5/71) compared with ~5.5 % (4/71) by the consensus definition. By comparison with the consensus definition, the screening tool correctly identified three participants as cachectic (i.e. true positives) and classified 65 participants as non-cachectic (i.e. true negatives). Two participants were falsely classified as cachectic by the screening tool. The sensitivity and specificity of the screening tool was 75 and 97 %, respectively. The screening tool had a positive predictive value of 60 % and a negative predictive value of 99 %. The number of participants scoring positively (i.e. below the diagnostic parameters) on cachexia features is shown in Table 2.
Table 2.
Frequency (percentage) of hip fracture patients (n = 71) presenting above or below each diagnostic parameter for geriatric cachexia
| Diagnostic parameters | Above diagnostic criteria (n, %) | Below diagnostic criteria (n, %) |
|---|---|---|
| BMI ≤22 kg m2 | 61 (85.9 %) | 10 (14.1 %) |
| ASM index (kg m2): ≤7.25 men; ≤5.45 womena | 49 (69 %) | 22 (31 %) |
| Isometric grip-strength: ≤27.3kg men; ≤16.3 kg womenb | 31 (43.7 %) | 40 (56.3 %) |
| Dietary energy intake ≤70 % estimated energy requirementsa | 42 (59.2 %) | 29 (40.8 %) |
| SNAQ score ≤14c | 21 (29.6 %) | 50 (70.4 %) |
| MUAMC: ≤23.49 cm men; ≤20.84 womenb (n = 142) | 39 (54.9 %) | 32 (45.1 %) |
BMI body mass index, ASM appendicular skeletal muscle, SNAQ Simplified Nutritional Appetite Questionnaire, MUAMC mid-upper arm muscle circumference
aDiagnostic cut-offs from the proposed diagnostic algorithm for cachexia were derived from Evans et al. [18]
bScreening tool diagnostic criteria cut-offs for isometric hand-grip strength and MUAMC were obtained from wave 1 of the Australian Longitudinal Study of Ageing (ALSA) [35]
cA score ≤14 for SNAQ identifies persons with anorexia at risk of significant weight loss of at least 5 % within 6 months [38]
Discussion
This study assessed the sensitivity and specificity of a newly developed screening tool in older adults’ post-surgical fixation for hip fracture. These preliminary findings demonstrate that the screening instrument displays sufficient levels of agreement when applied against the commonly accepted diagnostic algorithm with a sensitivity and specificity of 75 and 97 %, respectively. However, unexpectedly, only 1 in 20 older adults with hip fracture were identified as cachectic when the diagnostic algorithm was applied.
Age-related diseases such as cachexia are of high interest to clinicians at present, but there are gaps in operationalising the approach; a key gap at the present time in the application of the consensus definition is a thorough investigation of its clinical usefulness and effectiveness in a variety of populations [39]. The prevalence of cachexia in chronic conditions such as cancer, chronic heart failure, chronic kidney disease, rheumatoid arthritis and chronic obstructive pulmonary disease has previously been reported [39]. However, little is known about the prevalence of cachexia in an ageing population. Yaxley et al. [17] recently identified one in five older adults participating in ambulatory rehabilitation as cachectic. In contrast to the present study, however, Yaxley et al. [17] used different diagnostic measures to those that were used in the present study [18].
To our knowledge, this is the first study to assess the construct validity of a newly developed screening tool utilising portable field methods against the commonly accepted consensus definition for the detection of geriatric cachexia. Previously due to the lack of a formal cachexia screening instrument, malnutrition screening tools such as the Mini Nutritional Assessment have been proposed for a timely diagnosis [40, 41]. However, using nutrition assessment tools alone for a diagnosis of geriatric cachexia is inappropriate if they are not validated against the diagnostic algorithm of the consensus definition. Impaired regulation of appetite and decreased energy intake with ageing is a common phenomenon among older adults, often described as the anorexia of ageing [6, 16, 42]. Consequently, a non-specific diagnosis of malnutrition, particularly in an older orthopaedic population, may result in the mis-diagnosis of severe wasting disease such as cachexia and therefore not conducive to the provision of timely and appropriate therapy. Using the same consensus framework used in the present study, a recent exploratory analysis in patients with stage III non-small-cell lung cancer, cachexia was present in 11 of 40 (28 %) patients [43]. These investigators also reported that ~50 % of non-cachectic patients scored positively on cachexia features including moderate weight loss, systemic inflammation, anorexia and reduced handgrip strength [43]. In the present study, we reported similar findings for diagnostic parameters including anorexia, isometric grip strength and MUAMC. Furthermore, the previous study by van der Meij et al. [43] also reported issues in the operationalising of the consensus definition whereby cancer patients presenting with ≥5 % weight loss in combination with two positive characteristics of cachexia were classified as non-cachectic by the consensus framework.
While the prevalence of cachexia in this group was somewhat unexpected, there are some important considerations that should be applied in the interpretation of these results. One important consideration was the potential for selection bias at study entry (i.e. BMI between 18.5 and 35 kg/m2, community dwelling, medically stable and ambulatory pre-fracture) resulting in potential cachectic patients being excluded. In the present study, we used BMI of ≤22 kg m2 as the critical level of BMI; given the mean BMI of this cohort (male, 23.9 kg m2; female, 25.9 kg m2) and a lack of access to weight loss history of participants pre-surgery, potential cachectic patients may have been misdiagnosed as non-cachectic. It is widely recognised that BMI is an indirect and imperfect measure of assessing body composition. This may be particularly true in an older adult population where lean and fat mass both act as important nutritional preserves during times of illness [25]. Moreover, there is evidence that current BMI classifications may be overly restrictive in older adults, suggesting that BMI thresholds should be re-evaluated further [25, 44]. Specifically, results from the present study revealed few patients with BMI ≤22 kg m2, yet almost three quarters of the study population reported SNAQ scores ≤14, suggesting increased risk of significant weight loss within 6 months [38]. Despite this, it may be argued that the timing of administering the SNAQ and the subsequent SNAQ score in postoperative older adults does not accurately reflect chronic anorexia given that it is a measure of recent dietary intake.
Of clinical and diagnostic importance, abnormal biochemical parameters including markers of inflammation, a key clinical characteristic of cachexia [15, 16, 18], were not included in our screening instrument. Although blood analyses are not necessarily a practical field measure, their exclusion in the present study may be an important factor contributing to the underestimation of geriatric cachexia in this sample of hip fracture patients. However, despite a wealth of literature suggesting that systemic inflammation plays a role in the pathogenesis of cachexia and ageing [2, 16, 45, 46], in the present study, there is potential for overlap between post-operative transient inflammation and systemic inflammation resulting from other aetiologies.
Irrespective of the potential limitations, we report an acceptable sensitivity (75 %) and high specificity (97 %) for the application of a screening tool utilising portable field methods against the previously defined diagnostic criteria in the acute care setting. The application of validated portable field instruments coupled with diagnostic measures including weight loss history (i.e. ≥5 % in 12 months or less) and markers of inflammation may be of clinical importance for a diagnosis of cachexia in an older orthopaedic population. Although DEXA is commonly referred to as the reference technique for assessing body composition, its high cost, lack of access and the difficulty of positioning post-surgical patients in the correct position for measurement secondary to pain and immobility make this method not always practical for older adults recovering from orthopaedic surgery [12, 47]. Alternatively, upper arm anthropometry is a quick, inexpensive and portable method of assessing body composition and nutritional status in older adults [48–50]. Furthermore, an advantage of the SNAQ when compared against other geriatric assessment tools or dietary recall is its feasibility in the clinical environment. The SNAQ has previously been validated for application in older adults for the identification of anorexia and as a predictor of significant weight loss of at least 5 % within 6 months [38, 51].
In conclusion, this study provides preliminary evidence of the difficulty in identifying cachexia in an older adult population using current diagnostic criteria. Validation of geriatric cachexia screening instruments remains a necessity, however, a “gold standard” is still lacking. Further prospective research in a heterogeneous group of hip fracture patients, which captures all diagnostic criteria from the consensus definition to identify the prevalence of cachexia that is closer to the expected, would be of benefit. Future investigation may then be positioned to explore the predictive validity of screening tools that use non-invasive, portable field measures for the identification of geriatric cachexia, which potentially achieve higher sensitivity.
Acknowledgments
This research was supported by the National Health and Medical Research Council (NHMRC), Australia. None of the authors has any conflict of interest to state. M.C, I.D.C., C.W., S.K., and M.D.M were responsible for designing the study, analysing and interpreting the data and preparing the manuscript. A.M.V. contributed to data collection, analysing and interpreting the data and preparing the manuscript. The authors confirm that they comply with the principles of ethical publishing in the Journal of Cachexia, Sarcopenia, and Muscle 2010;1:7–8 (von Haehling S, Morley JE, Coats AJ and Anker SD).
References
- 1.Muscaritoli M, Lucia S, Molfino A, Cederholm T, Rossi Fanelli F. Muscle atrophy in aging and chronic diseases: is it sarcopenia or cachexia? Intern Emerg Med. 2013. doi:10.1007/s11739-012-0807-8:1-8. [DOI] [PubMed]
- 2.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer J, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–9. [DOI] [PubMed]
- 3.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Manini TM, Everhart JE, Anton SD, Schoeller DA, Cummings SR, Mackey DC, et al. Activity energy expenditure and change in body composition in late life. Am J Clin Nutr. 2009;90:1336–1342. doi: 10.3945/ajcn.2009.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. JAMD. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morley JE. Undernutrition in older adults. Fam Pract. 2012;29:89–93. doi: 10.1093/fampra/cmr054. [DOI] [PubMed] [Google Scholar]
- 7.Morley JE. Frailty: diagnosis and management. J Nutr Health Aging. 2011;15:667–670. doi: 10.1007/s12603-011-0338-4. [DOI] [PubMed] [Google Scholar]
- 8.Evans WJ, Paolisso G, Abbatecola AM, Corsonello A, Bustacchini S, Strollo F, et al. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology. 2010;11:527–536. doi: 10.1007/s10522-010-9297-0. [DOI] [PubMed] [Google Scholar]
- 9.Singh NA, Quine S, Clemson LM, Williams EJ, Williamson DA, Stavrinos TM, et al. Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. JAMDA. 2012;13:24–30. doi: 10.1016/j.jamda.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Outcome after surgery for the treatment of hip fracture in the extremely elderly. J Bone Joint Surg. 2008;90:1899–1905. doi: 10.2106/JBJS.G.00883. [DOI] [PubMed] [Google Scholar]
- 11.Avenell A, Handoll HHG. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. 2010;1:1–68. doi: 10.1002/14651858.CD001880.pub5. [DOI] [PubMed] [Google Scholar]
- 12.Villani AM, Miller MD, Cameron I, Kurrle S, Whitehead C, Crotty M. Body composition in older community-dwelling adults with hip fracture: portable field methods validated by dual-energy X-ray absorptiometry. Br J Nutr. 2012;109:1219–29. [DOI] [PubMed]
- 13.Miller MD, Crotty M, Whitehead C, Bannerman E, Daniels LA. Nutritional supplementation and resistance training in nutritionally at risk older adults following lower limb fracture: a randomized controlled trial. Clin Rehabil. 2006;20:311–323. doi: 10.1191/0269215506cr942oa. [DOI] [PubMed] [Google Scholar]
- 14.Al-Ani AN, Flodin L, Söderqvist A, Ackermann P, Samnegård E, Dalén N, et al. Does rehabilitation matter in patients with femoral neck fracture and cognitive impairment? A prospective study of 246 patients. Archives Phys Med Rehab. 2010;91:51–57. doi: 10.1016/j.apmr.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 16.Morley JE, Anker SD, Evans W. Cachexia and aging: an update based on the Fourth International Cachexia Meeting. J Nutr Health Aging. 2009;13:47–55. [DOI] [PubMed]
- 17.Yaxley A, Miller MD, Fraser RJ, Cobiac L, Crotty M. The complexity of treating wasting in ambulatory rehabilitation: is it starvation, sarcopenia, cachexia or a combination of these conditions? Asia Pacific J Clin Nutr. 2012;21:386. [PubMed] [Google Scholar]
- 18.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Fox KM, Brooks JM, Gandra SR, Markus R, Chiou C-F. Estimation of cachexia among cancer patients based on four definitions. J Oncol. 2009;2009:693458. [DOI] [PMC free article] [PubMed]
- 20.Bozzetti F, Mariani L. Defining and classifying cancer cachexia: a proposal by the SCRINIO working group. J Parenter Enteral Nutr. 2009;33:361–367. doi: 10.1177/0148607108325076. [DOI] [PubMed] [Google Scholar]
- 21.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncology. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 22.Huhmann MB, August DA. Review of American society for parenteral and enteral nutrition (A.S.P.E.N.) clinical guidelines for nutrition support in cancer patients: nutrition screening and assessment. Nutr Clinical Practice. 2008;23:182–188. doi: 10.1177/0884533608314530. [DOI] [PubMed] [Google Scholar]
- 23.Thomas S, Humphreys K, Miller M, Cameron I, Whitehead C, Kurrle S, et al. Individual nutrition therapy and exercise regime: a controlled trial of injured, vulnerable elderly (INTERACTIVE trial). BMC Geriatr. 2008;8:4. [DOI] [PMC free article] [PubMed]
- 24.Sergi G, Perissinotto E, Pisent C, Buja A, Maggi S, Coin A, et al. An adequate threshold for body mass index to detect underweight condition in elderly persons: the Italian longitudinal study on aging (ILSA) J Gerontol Series A: Biol Sci Med Sci. 2005;60:866–871. doi: 10.1093/gerona/60.7.866. [DOI] [PubMed] [Google Scholar]
- 25.Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity. 2007;15:1827–1840. doi: 10.1038/oby.2007.217. [DOI] [PubMed] [Google Scholar]
- 26.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Archives Intern Med. 2001;161:1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 27.Chumlea WC, Roche AF, Steinbaugh ML. Estimating stature from knee height for persons 60 to 90 years of age. J Am Geriatr Soc. 1985;33:116–120. doi: 10.1111/j.1532-5415.1985.tb02276.x. [DOI] [PubMed] [Google Scholar]
- 28.Pini R, Tonon E, Cavallini MC, Bencini F, Di Bari M, Masotti G, et al. Accuracy of equations for predicting stature from knee height and assessment of statural loss in an older Italian population. J Gerontol Series A: Biol Sci Med Sci. 2001;56:3–7. doi: 10.1093/gerona/56.1.B3. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 30.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 31.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long CL, Schaffel N, Geiger JW, Schiller WR, Blakemore WS. Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. J Parenter Enteral Nutr. 1979;3:452–456. doi: 10.1177/0148607179003006452. [DOI] [PubMed] [Google Scholar]
- 33.Schaubert KL, Bohannon RW. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. J Strength Conditioning Research. 2005;19:717–720. doi: 10.1519/R-15954.1. [DOI] [PubMed] [Google Scholar]
- 34.Norman K, Stobäus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011;30:135–142. doi: 10.1016/j.clnu.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead CH, Giles LC, Andrews GR, Finucane P. Anthropometric and laboratory markers of nutritional status in a large sample of older Australians: the ALSA study. Australasian J Ageing. 2000;19:85–90. doi: 10.1111/j.1741-6612.2000.tb00150.x. [DOI] [Google Scholar]
- 36.Heymsfield SB, McManus C, Smith J, Stevens V, Nixon DW. Anthropometric measurement of muscle mass: revised equations for calculating bone-free arm muscle area. Am J Clin Nutr. 1982;36:680–690. doi: 10.1093/ajcn/36.4.680. [DOI] [PubMed] [Google Scholar]
- 37.Heymsfield SB, McManus C, Stevens V, Smith J. Muscle mass: reliable indicator of protein–energy malnutrition severity and outcome. Am J Clin Nutr. 1982;35:1192–1199. doi: 10.1093/ajcn/35.5.1192. [DOI] [PubMed] [Google Scholar]
- 38.Wilson MMG, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005;82:1074–1081. doi: 10.1093/ajcn/82.5.1074. [DOI] [PubMed] [Google Scholar]
- 39.von Haehling SD, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. [DOI] [PMC free article] [PubMed]
- 40.Blum D, Strasser F. Cachexia assessment tools. Current Opinion Supportive Palliative Care. 2011;5:350–355. doi: 10.1097/SPC.0b013e32834c4a05. [DOI] [PubMed] [Google Scholar]
- 41.Gioulbasanis I, Georgoulias P, Vlachostergios PJ, Baracos V, Ghosh S, Giannousi Z, et al. Mini Nutritional Assessment (MNA) and biochemical markers of cachexia in metastatic lung cancer patients: interrelations and associations with prognosis. Lung Cancer. 2011;74:516–520. doi: 10.1016/j.lungcan.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Morley JE. Anorexia of aging: a true geriatric syndrome. J Nutr Health Aging. 2012;16:422–425. doi: 10.1007/s12603-012-0061-9. [DOI] [PubMed] [Google Scholar]
- 43.van der Meij BS, Schoonbeek CP, Smit EF, Muscaritoli M, van Leeuwen PAM, Langius JAE. Pre-cachexia and cachexia at diagnosis of stage III non-small-cell lung carcinoma: an exploratory study comparing two consensus-based frameworks. BJN. 2012;1:1–9. doi: 10.1017/S0007114512004527. [DOI] [PubMed] [Google Scholar]
- 44.van Uffelen JGZ, Berecki-Gisolf J, Brown WJ, Dobson AJ. What Is a healthy body mass index for women in their seventies? Results from the Australian longitudinal study on women's health. J Gerontol Series A: Biol Sci Med Sci. 2010;65A:847–853. doi: 10.1093/gerona/glq058. [DOI] [PubMed] [Google Scholar]
- 45.Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–1350. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 46.Morley JE, Thomas DR, Wilson M-MG. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 47.Plank LD. Dual-energy X-ray absorptiometry and body composition. Current Opinion Clinical Nutr Metabol Care. 2005;8:305–309. doi: 10.1097/01.mco.0000165010.31826.3d. [DOI] [PubMed] [Google Scholar]
- 48.Enoki H, Kuzuya M, Masuda Y, Hirakawa Y, Iwata M, Hasegawa J, et al. Anthropometric measurements of mid-upper arm as a mortality predictor for community-dwelling Japanese elderly: The Nagoya Longitudinal Study of Frail Elderly (NLS-FE) Clin Nutr. 2007;26:597–604. doi: 10.1016/j.clnu.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Powell-Tuck J, Hennessy EM. A comparison of mid upper arm circumference, body mass index and weight loss as indices of undernutrition in acutely hospitalized patients. Clin Nutr. 2003;22:307–312. doi: 10.1016/S0261-5614(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 50.Miller MD, Crotty M, Giles LC, Bannerman E, Whitehead C, Cobiac L, et al. Corrected arm muscle area: an independent predictor of long-term mortality in community-dwelling older adults? J Am Geriatr Soc. 2002;50:1272–1277. doi: 10.1046/j.1532-5415.2002.50316.x. [DOI] [PubMed] [Google Scholar]
- 51.Morley JE. Assessment of malnutrition in older persons: a focus on the Mini Nutritional Assessment. J Nutr Health Aging. 2011;15:87–90. doi: 10.1007/s12603-011-0018-4. [DOI] [PubMed] [Google Scholar]