Abstract
Background
Skeletal muscle mass declines after the age of 50. Loss of skeletal muscle mass is associated with increased morbidity and mortality.
Objective
This study aims to identify predictors of low skeletal muscle mass in older adults toward development of a practical clinical assessment tool for use by clinicians to identify patients requiring dual-energy X-ray absorptiometry (DXA) screening for muscle mass.
Methods
Data were drawn from the National Health and Nutrition Examination Surveys (NHANES) from 1999 to 2004. Appendicular skeletal mass (ASM) was calculated based on DXA scans. Skeletal muscle mass index (SMI) was defined as the ratio of ASM divided by height in square centimeters. Elderly participants were classified as having low muscle mass if the SMI was 1 standard deviation (SD) below the mean SMI of young adults (20–40 years old). Logistic regression was conducted separately in males and females age ≥65 years of age to examine the relationship between patients identified as having low muscle mass and health behavior characteristics, adjusting for comorbid conditions. The model was validated on a separate sample of 200 patients.
Results
Among the NHANES study population, 551 (39.7 %) males and 374 (27.5 %) females had a SMI below the 1 SD cutoff point. NHANES study subjects with a low SMI were older (mean age, 76.2 vs. 72.7 for male; 76.0 vs. 73.7 for female; and both p < 0.0001) and had a lower body mass index (mean BMI, 24.1 vs. 29.4 for male; 22.9 vs. 29.7 for female; p < 0.0001). In adjusted logistic regression analyses, age (for males) and BMI (for both males and females) remained statistically significant. A parsimonious logistic regression model adjusting for age and BMI only had a C statistic of 0.89 for both males and females. The discriminatory power of the parsimonious model increased to 0.93 for males and 0.95 for females when the cutoff defining low SMI was set to 2 SD below the SMI of young adults. In the validation sample, the sensitivity was 81.6 % for males and 90.6 % for females. The specificity was 66.2 % for males and females.
Conclusions
BMI was strongly associated with a low SMI and may be an informative predictor in the primary care setting. The predictive model worked well in a validation sample.
Keywords: Sarcopenia, Muscle loss, Predictive model, Screening, Clinical practice
Introduction
Age-related decline in muscle mass has significant implications for patient quality of life and subsequent morbidity [1]. Although this loss of muscle mass with age has historically been termed sarcopenia, literally “the poverty of flesh,” more recent definitions of sarcopenia are not systematic and include loss of muscle strength and function. Beginning in the fourth decade of life, on average, adults lose 3 to 5 % of muscle mass per decade [2], a rate of decline that increases to 1 to 2 %/year after age 50 [3]. Sarcopenia is differentiated from other disorders associated with skeletal muscle loss, including cachexia and wasting. The term wasting was originally used to describe the rapid, unintentional loss of both muscle and adipose tissue in persons with HIV disease. Wasting results primarily from anorexia and inadequate dietary intake, with subsequent negative protein and calorie balance. Cachexia is defined as the accelerated loss of muscle mass seen with chronic inflammation caused by acute disease. It is a slower process than wasting, and its pathogenesis includes metabolic alterations resulting from a disease-mediated increase in cytokine production. Sarcopenia has a more insidious course than cachexia. There are numerous underlying mechanisms behind sarcopenia, which collectively result in decreased anabolism leading to net muscle loss over time. Unlike cachexia and wasting, sarcopenia occurs in persons who are otherwise free of obvious disease, although few older adults with sarcopenia are free of other disease. In 2000, the direct health care costs attributed to sarcopenia were estimated to be $18.5 billion, which is slightly more than the estimate of $16.3 billion for osteoporosis [1].
This cost is reflected in the growing number of clinical trials seeking to improve the lives of those with sarcopenia. At present, 107 trials are listed in www.clinicaltrials.gov for sarcopenia. These trials are testing nutrition, resistance training, insulin, testerone, dietary supplements, electrostimulation, and an experimental drug. Given the increasing search for a therapy, cost-effective screening strategies will be important. The goal of this paper is to develop a two-step strategy that would avoid unnecessary dual-energy X-ray absorptiometry (DXA) or bioelectrical impedance. The goal is to rapidly and efficiently identify those likely to benefit and those unlikely to benefit from diagnostic screening.
There are currently no established practice guidelines for clinicians pertaining to screening, diagnosis, treatment, or management [4]. In epidemiologic studies, sarcopenia is defined in terms of low muscle mass; specifically, a skeletal muscle mass index (SMI) less than 2 standard deviations (SD) below the mean SMI for young adults. Based on this, epidemiologic studies estimate the prevalence of sarcopenia to be as high as 50 % in people 80 years and older [5–7]. A 2002 study by Iannuzzi-Suchich et.al. assessed prevalence and predictors of sarcopenia in a large cohort of community-based men and women 64–92 years old [7]. Body composition was measured using DXA scans in 337 individuals and body mass index (BMI) was found to be a strong predictor of skeletal muscle mass in women and men. The Rancho Bernardo Study conducted among community-dwelling men and women aged 55–98 years examined prevalence of sarcopenia and risk factors associated with sarcopenia [8]. The study identified lack of physical activity and current smoking as reversible risk factors for sarcopenia. A recently published study by Foley et al. found a strong association between class I sarcopenia, defined as muscle mass 1 SD below the mean of young adults as assessed by bioimpedance analysis, and deficits in kidney function among participants of National Health and Nutrition Examination Survey (NHANES III) [9].
The goal of the current study is to construct and validate a risk of low muscle mass model using the latest (1999–2004) nationally representative NHANES DXA scan data to construct the model and a sample of patients in a large clinic system to validate the predictive potential of the model. The first objective of the study is to identify patients with low muscle mass and to examine the association of baseline characteristics, comorbid conditions, and health behaviors with low muscle mass. These characteristics can be organized into a practical screening tool for identifying patients that are likely to have sarcopenia in clinical practice. The second objective of the study is to evaluate the screening tool on a sample of patients in the University of Utah clinic system.
Methods
This project used a two-step process to develop a model to identify people with sarcopenia. First, we fit a multivariate model using data from NHANES. The model was then validated using a sample of patients from the University of Utah Health Care System half of whom were predicted to have sarcopenia and half who were predicted to have normal muscle mass. Validation was a comparison of the prediction to the DXA scan results. The two data sources are described below. Three study populations are defined: a reference population aged 20–40, a prediction model study population aged 65+, and a validation study population aged 65+. The first two populations are those individuals in the NHANES population having a DXA scan. The validation population was recruited from the University of Utah Health system.
Prediction model
Data source
The NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the USA. The survey is a unique resource that combines interviews and physical examinations. NHANES is a major program of the National Center for Health Statistics (NCHS), part of the Centers for Disease Control and Prevention (CDC) that has the responsibility for producing vital and health statistics. The NHANES interview includes demographic, socioeconomic, dietary, and health-related questions. The examination component consists of medical, dental, and physiological measurements, as well as laboratory tests administered by highly trained medical personnel.
In each NHANES survey year, a nationally representative sample of the US civilian non-institutionalized population is selected using a complex, stratified, multistage probability sampling design. Approximately 5,300 individuals of all ages are interviewed in their homes; of these, approximately 5,000 complete the health examination component of the survey [10]. DXA scans were administered to eligible survey participants 8 years of age and older in the NHANES mobile examination centers.
Study population
Inclusion criteria
Subjects included NHANES participants with DXA scans within the study time-frame of 1999 to 2004 who were age 20–40 years or 65+ with available true DXA scores and height measurements. In the 1999–2004 NHANES data, DXA data were missing more often than other variables. To provide a complete data file, the CDC used multiple imputations to provide complete DXA information. For this study, however, only true DXA readings were used.
Low muscle mass definition
Muscle mass was defined using appendicular skeletal mass (ASM) [11]. ASM was determined by combining the lean tissue mass of the regions of arms and legs, excluding all other body regions from analysis. SMI was calculated by adjusting ASM for height by dividing ASM by height in square centimeters (SMI = ASM/height2) [11]. Low muscle mass was then defined as a SMI of 1.0 SD below the mean SMI of the reference population (adults aged 20–40) calculated separately for males and females. Foley, in an earlier study using NHANES III, labeled this “class I sarcopenia” [9]. The reference age range was chosen based on the distribution of SMI by age group stratified by sex. The age- and sex-adjusted SMI was relatively stable for ages 20 to 40 while the SMI began to drop at age ≥50. In NHANES, using the thresholds of 1.0, 1.5, and 2.0 SD from the reference populations, the prevalence of low muscle mass for males was 30.9, 24.2, and 14.2 % and for females 6.7, 4.4, and 0.8 %. The final threshold of 1.0 SD below the mean was chosen so that the observed prevalence was consistent with the prevalence found in prior work [5, 7–9]. This class I threshold for low muscle mass is different from other work because the NHANES population over samples African-American and Hispanic populations. African-Americans have lower rates of sarcopenia [12], which lowered our prevalence.
Variables
For prediction model study patients (NHANES age 65+) with DXA scans, demographic, health behavior, body fat, and comorbidities were used. Demographics included age, sex, race, education, income, marital status, and health insurance status. Health behavior variables included alcohol use, smoking, and tobacco use. Comorbidities included weight history, coronary heart disease, dyslipidemias, peripheral vascular disease, stroke, cancer, emphysema, diabetes mellitus, thyroid disease, anemia, and arthritis. An index of body fat was created analogously to the definition of skeletal muscle mass using the sum of body fat on the extremities divided by height squared.
Statistical analysis
All statistical analyses took into account the sampling weights in the NHANES methodology as spelled out on the NCHS website. Descriptive statistics were calculated to test for differences in demographic and comorbid conditions between patients with and without low muscle mass for males and females separately. For continuous variables differences were evaluated using a t-test. For categorical variables differences were evaluated using a chi-square test.
Multivariate logistic regression models were developed to examine the association between low muscle mass and baseline characteristics and health behaviors in the prediction model population. Multivariate logistic regression is subject to over-fitting, producing overly optimistic fit estimates in other samples. We used bootstrap sampling with replacement to estimate our prediction model. Four models were fit: a fully saturated model and a parsimonious model for each sex. These models were estimated using the STATA bootstrap procedure with 500 replications [13]. Model fit was assessed using the pseudo-R2 and the C statistic. There are many versions of the pseudo-R2. We used the likelihood method of Cox and Snell, which like other methods does not reach a maximum of 1.0 [14, 15]. The C statistic ranges from 50 to 100 %. It is equivalent to the area under the curve for a receiver operating characteristic curve.
The predicted probability of having low muscle mass, based on the model coefficients, was used to develop a clinical screening grid for age and BMI. The clinical screening grid is intended to be useful both as a tool and a measure of the face validity of the model. If the pattern in the grid is not diagonal, with the probabilities increasing from the upper right to the lower left, the models do not accurately fit the known information about low muscle mass.
Further assessment of the models was conducted by evaluating the definition of low muscle mass against six physical function variables in NHANES: difficulty walking a quarter mile; difficulty walking ten steps; difficulty stooping, crouching, or kneeling; difficulty lifting or carrying; difficulty standing from an armless chair; and the time to walk 20 ft. Many of the physical function variables have missing values, so analysis of the association between physical function and low muscle mass was performed using the multiple imputation procedures in SAS [15].
Validation
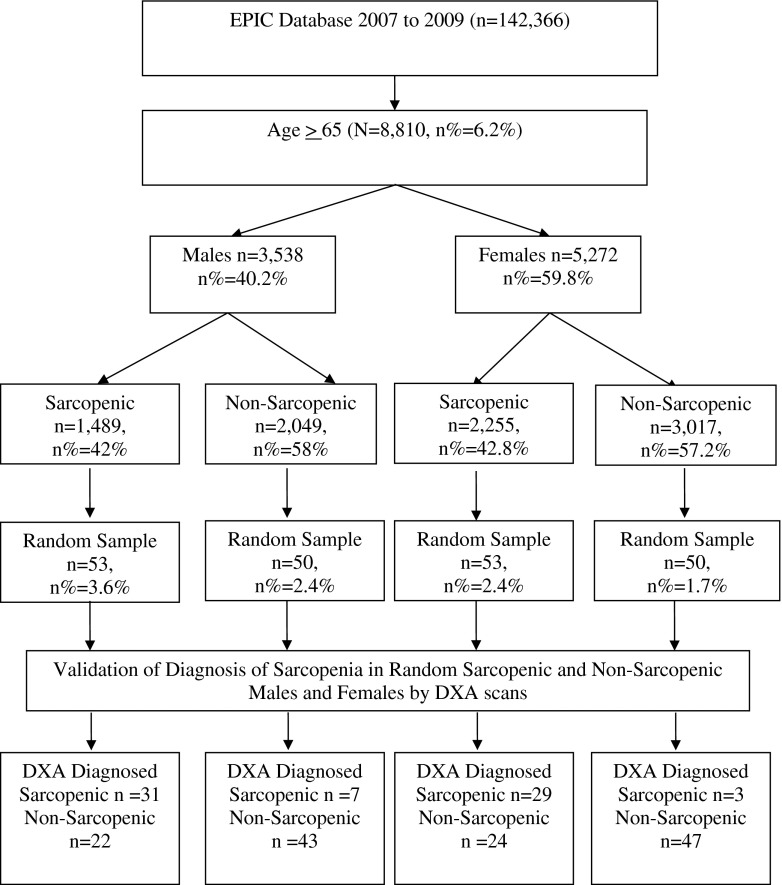
The model was validated using patients (validation study population) in the University of Utah Health Care System. Using the EPIC electronic health record, patients aged 65 years and over with recorded visits between July 2007 and June 2009 and a BMI value were recruited. Patients were excluded if they had any procedure requiring radiographic contrast material in the prior 3 days or weighed more than 300 lb (the limit of the DXA machine), were more than 6.5 in. tall (limit of machine) or could not walk. The NHANES-derived model was applied to the eligible patients. Using the results of the NHANES model, patients were assigned to either sarcopenic or non-sarcopenic using predictive thresholds of 30 % for males and 20 % for females. A computer-generated random number (between 0 and 1) was assigned to the sarcopenic and non-sarcopenic males and females. This computer-generated random number was then sorted in ascending order, and consecutive batches of 50 subjects from each group (total 200) were identified. Opt-out letters that included information about the study and opt-out information were sent to a batch of 200 subjects at a time. Subjects not interested in participating in the study called the study coordinator and opted out within five business days of receiving the letter. Subjects that did not opt out were contacted by a research assistant 10 days after receiving the letter. Subjects were contacted no more than three times by phone. When reached by phone, subjects were briefed about the study, confirmed their eligibility, and were consented to participate in the study. Participants in the study were scheduled for a DXA scan at the University of Utah Diabetes Center. A research assistant met subjects and administered the informed consent for study participation.
Patients who consented to participate were scanned using a DXA. Individuals were classified as having low muscle mass using the same algorithm as used in the NHANES data. To validate the risk of sarcopenia model, we calculated the sensitivity, specificity, positive predicted value, negative predicted value, and positive likelihood ratio comparing those predicted to have low muscle mass by the NHANES-derived model with those identified as having low muscle mass using DXA and the NHANES algorithm as the gold standard. Figure 1 provides the detailed flowchart of patient selection for the validation study.
Fig. 1.
Flowchart of patient selection for validation study
The prediction model project was approved on 13 Feb 2009, and the validation study was approved on 16 Sept 2009 by the University of Utah Institutional Review Board.
Results
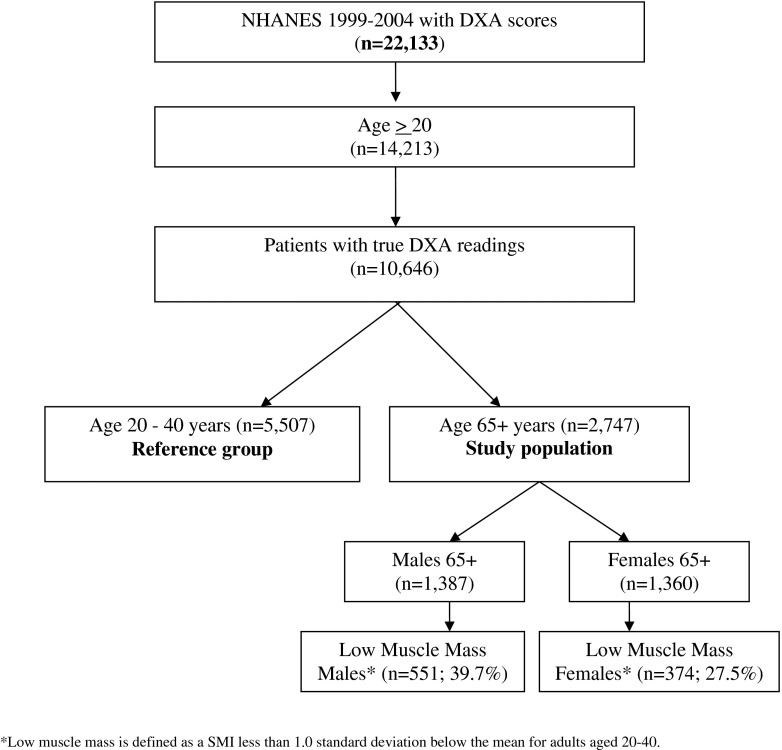
A total of 22,133 NHANES participants had DXA scans (Fig. 2). Participants ≥20 years of age with true DXA scores and height measurements (n = 10,646) were eligible for inclusion in the study. The final reference population included 5,507 people. The prediction model study population included 1,387 males and 1,360 females age 65 years and greater. Of the prediction model study population, the proportion of males with low muscle mass was 39.7 % (n = 551). The proportion of females with low muscle mass was 27.5 % (n = 374). Table 1 presents the descriptive statistics for all variables comparing males and females with and without low muscle mass. The mean age of the male patients with low muscle mass was 76.2 compared with 72.7 for males without low muscle mass. In females, the mean age was 76.0 and 73.7 in patients with and without low muscle mass, respectively. Statistically significant differences are shown in the right-hand columns of the descriptive table for females and males, respectively.
Fig. 2.
Flowchart of patient selection for predictive modeling study. Asterisk, a SMI less than 1.0 SD below the mean for adults aged 20–40
Table 1.
Descriptive statistics for males and females
| Independent variables | Females = 1,360 | Males = 1,387 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females without low muscle mass (n = 986) | Females with low muscle mass (n = 374) | P value | Males without low muscle mass (n = 836) | Males with low muscle mass (n = 551) | P value | |||||
| N | % or SD | N | % or SD | N | % or SD | N | % or SD | |||
| Age at screening (mean, SD) | 73.7 | 6.3 | 76 | 6.71 | <0.001 | 72.7 | 5.91 | 76.2 | 6.19 | <0.001 |
| Race/ethnicity | ||||||||||
| Non-Hispanic White | 473 | 48 % | 220 | 59 % | 0.001 | 449 | 54 % | 280 | 51 % | 0.650 |
| Non-Hispanic Black | 182 | 18 % | 48 | 13 % | 135 | 16 % | 91 | 17 % | ||
| Hispanics | 308 | 31 % | 94 | 25 % | 228 | 27 % | 166 | 30 % | ||
| Other | 23 | 2 % | 12 | 3 % | 24 | 3 % | 14 | 3 % | ||
| Married or living with partner | 572 | 58 % | 232 | 62 % | 0.178 | 198 | 24 % | 168 | 30 % | 0.005 |
| Education level | ||||||||||
| 9th grade or 9–11th grade | 393 | 40 % | 148 | 40 % | 0.151 | 338 | 40 % | 217 | 39 % | 0.659 |
| High school graduate/GED or equivalent | 246 | 25 % | 77 | 21 % | 193 | 23 % | 120 | 22 % | ||
| College graduate or above | 347 | 35 % | 149 | 40 % | 305 | 36 % | 214 | 39 % | ||
| Annual household income | ||||||||||
| 15,000 | 373 | 38 % | 147 | 39 % | 0.438 | 230 | 28 % | 179 | 32 % | 0.020 |
| $15,000 to $34,999 | 317 | 32 % | 119 | 32 % | 280 | 33 % | 201 | 36 % | ||
| $35,000 to $54,999 | 155 | 16 % | 47 | 13 % | 158 | 19 % | 88 | 16 % | ||
| >$55,000 | 141 | 14 % | 61 | 16 % | 168 | 20 % | 83 | 15 % | ||
| Covered by health insurance | 946 | 96 % | 362 | 97 % | 0.466 | 802 | 96 % | 533 | 97 % | 0.443 |
| Insurance type | ||||||||||
| Private insurance | 444 | 45 % | 161 | 43 % | 0.511 | 417 | 50 % | 254 | 46 % | 0.168 |
| Medicare | 830 | 84 % | 329 | 88 % | 0.079 | 674 | 81 % | 471 | 85 % | 0.020 |
| Medicaid/CHIP | 92 | 9 % | 32 | 9 % | 0.658 | 54 | 6 % | 43 | 8 % | 0.337 |
| Other | 15 | 2 % | 3 | 1 % | 0.300 | 17 | 2 % | 8 | 1 % | 0.426 |
| Body mass index (mean, SD) | 29.7 | 5.12 | 22.9 | 2.94 | <0.000 | 29.4 | 3.93 | 24.1 | 2.84 | <0.000 |
| <25 | 161 | 16 % | 291 | 78 % | <0.000 | 85 | 10 % | 337 | 61 % | <0.000 |
| ≥25 to 30 | 408 | 41 % | 82 | 22 % | 447 | 53 % | 204 | 37 % | ||
| ≥30 | 417 | 42 % | 1 | 0 % | 304 | 36 % | 10 | 2 % | ||
| Systolic blood pressure (mean, SD) | 144 | 22.8 | 145 | 25.2 | 0.493 | 137 | 20.4 | 139 | 23 | 0.094 |
| Diastolic blood pressure (mean, SD) | 66.3 | 15.5 | 64.7 | 17.1 | 0.099 | 68.4 | 16.7 | 65.4 | 17.3 | 0.002 |
| Total cholesterol (mean, SD) | 218 | 40.8 | 222 | 40 | 0.053 | 198 | 40.8 | 195 | 40 | 0.204 |
| 200 mg/dl | 367 | 37 % | 125 | 33 % | 0.193 | 458 | 55 % | 327 | 59 % | 0.094 |
| ≥200 mg/dl | 619 | 63 % | 249 | 67 % | 378 | 45 % | 224 | 41 % | ||
| HDL cholesterol (mean, SD) | 57.3 | 16.2 | 65.5 | 17.7 | <0.000 | 46.4 | 12.2 | 50.2 | 15.5 | <0.000 |
| 40 mg/dl | 149 | 15 % | 28 | 7 % | 0.000 | 292 | 35 % | 163 | 30 % | 0.038 |
| ≥40 mg/dl | 837 | 85 % | 346 | 93 % | 544 | 65 % | 388 | 70 % | ||
| Current smoker | 53 | 5 % | 23 | 6 % | 0.579 | 59 | 7 % | 85 | 15 % | <0.000 |
| Alcohol use | 410 | 42 % | 150 | 40 % | 0.622 | 640 | 77 % | 391 | 71 % | 0.020 |
| Average level of physical activity each day | ||||||||||
| Low | 295 | 30 % | 91 | 24 % | 0.125 | 211 | 25 % | 166 | 30 % | 0.084 |
| Moderate | 681 | 69 % | 279 | 75 % | 596 | 71 % | 372 | 68 % | ||
| Heavy | 10 | 1 % | 4 | 1 % | 29 | 3 % | 13 | 2 % | ||
| Comorbid conditions | ||||||||||
| Fracture | 115 | 12 % | 49 | 13 % | 0.467 | 95 | 11 % | 58 | 11 % | 0.626 |
| History of falls | 386 | 39 % | 155 | 41 % | 0.440 | 216 | 26 % | 181 | 33 % | 0.005 |
| Osteoporosis | 199 | 20 % | 105 | 28 % | 0.002 | 17 | 2 % | 17 | 3 % | 0.215 |
| Cancer | 155 | 16 % | 81 | 22 % | 0.010 | 167 | 20 % | 146 | 26 % | 0.005 |
| Stroke | 77 | 8 % | 24 | 6 % | 0.382 | 67 | 8 % | 57 | 10 % | 0.137 |
| CHD | 79 | 8 % | 26 | 7 % | 0.513 | 119 | 14 % | 79 | 14 % | |
| Diabetes mellitus type II | 215 | 22 % | 37 | 10 % | <0.000 | 158 | 19 % | 91 | 17 % | 0.258 |
| Arthritis | 573 | 58 % | 178 | 48 % | 0.001 | 332 | 40 % | 197 | 36 % | 0.137 |
| Emphysema | 28 | 3 % | 19 | 5 % | 0.043 | 27 | 3 % | 50 | 9 % | <.000 |
| Bronchitis | 81 | 8 % | 46 | 12 % | 0.021 | 40 | 5 % | 31 | 6 % | 0.487 |
| Thyroid disease | 57 | 6 % | 16 | 4 % | 0.272 | 9 | 1 % | 8 | 1 % | 0.534 |
| Hypertension | 605 | 61 % | 181 | 48 % | <.000 | 438 | 52 % | 250 | 45 % | 0.011 |
| Anemia | 28 | 4 % | 7 | 3 % | 0.359 | 8 | 1 % | 14 | 4 % | 0.018 |
Table 2 shows the comparison of demographic, clinical, and comorbid conditions between those with and without low muscle mass using unadjusted and adjusted odds ratios. Adjusted odds ratios take into account the other variables in Table 1. The mean age of low muscle mass males (76.2; SD 6.19) was significantly higher (p < 0.001) than the mean age of males without low muscle mass (72.7; SD 5.91). Statistically significant unadjusted odds ratios between men with and without low muscle mass were also found for marital status, income, BMI, diastolic blood pressure, cholesterol, smoking, alcohol, history of falls, osteoporosis, cancer, coronary heart disease, emphysema, hypertension, anemia, and body fat. The adjusted odds ratios were significant for age, BMI, cholesterol, smoking, diabetes emphysema, bronchitis, and body fat.
Table 2.
Unadjusted and adjusted odds ratio for low muscle mass in males and females
| Males | Males | |||
|---|---|---|---|---|
| Unadjusted odds ratio | Adjusted odds ratio | Unadjusted odds ratio | Adjusted odds ratio | |
| Age at screening (95 % CI) | 1.097 (1.08–1.12) | 1.078 (1.05–1.11) | 1.056 (1.04–1.08) | 1.002 (0.97–1.03) |
| Race/ethnicity (reference = other) | ||||
| Non-Hispanic White (95 % CI) | 1.081 (0.80–1.47) | 1.429 (0.89–2.29) | 0.567 (0.40–0.81) | 0.836 (0.50–1.39) |
| Non-Hispanic Black (95 % CI) | 1.168 (0.91–1.50) | 1.343 (0.92–1.97) | 0.656 (0.50–0.87) | 0.592 (0.40–0.88) |
| Hispanics (95 % CI) | 0.935 (0.48–1.84) | 0.760 (0.28–2.05) | 1.122 (0.55–2.30) | 1.616 (0.55–4.73) |
| Married or living with partner (reference = no) | ||||
| Yes (95 % CI) | 0.708 (0.56–0.90) | 1.243 (0.83–1.85) | 0.831 (0.66–1.08) | 1.002 (0.69–1.45) |
| Education level (reference = <12 grade) | ||||
| High school graduate/GED or equivalent (95 % CI) | 0.968 (0.73–1.29) | 0.780 (0.50–1.21) | 1.140 (0.61–1.14) | 0.853 (0.54–1.35) |
| College graduate or above (95 % CI) | 1.093 (0.86–1.40) | 0.854 (0.58–1.25) | 0.946 (0.87–1.49) | 1.083 (0.74–1.59) |
| Annual household income (reference = <$35,000) | ||||
| ≥$35,000 (95 % CI) | 0.704 (0.56–0.88) | 0.768 (0.54–1.10) | 1.275 (0.73–1.23) | 1.044 (0.71–1.54) |
| Covered by health insurance (reference = no) | ||||
| Yes (95 % CI) | 1.255 (0.70–2.25) | 1.375 (0.53–3.59) | 0.625 (0.66–2.46) | 0.641 (0.24–1.73) |
| BMI* (mean) | 0.584 (0.56-0.61) | 0.325 (0.28–0.37) | 0.619 (0.59-0.65) | 0.436 (0.39–0.49) |
| BMI (reference = <25) | ||||
| ≥25 to <30 (95 % CI) | 0.582 (0.55–0.62) | 0.111 (0.59–0.66) | ||
| ≥30 (95 % CI) | 0.115 (0.09–0.15) | 0.001 (0.08–0.15) | ||
| SBP† (mean; 95 % CI) | 1.004 (1.00–1.01) | 0.994 (1.00–1.01) | ||
| DBP‡ (mean; 95 % CI) | 0.990 (0.98-1.00) | 0.992 (0.71–1.39) | 0.994 (0.99-1.00) | 1.223 (0.83–1.80) |
| Total cholesterol (mean) | 0.995 (0.99-1.00) | 1.002 (1.00-1.00) | ||
| Total cholesterol (reference < 200 mg/dl) | ||||
| ≥200 mg/dl (95 % CI) | 0.83 (0.67–1.03) | 1.028 (0.92–1.52) | ||
| HDL (mean; 95 % CI) | 1.020 (1.01–1.03) | 0.977 (0.97–0.99) | 2.200 (1.02–1.04) | 0.992 (0.98–1.00) |
| HDL (reference < 40 mg/dl) | ||||
| ≥40 mg/dl (95 % CI) | 1.278 (1.01–1.61) | 1.154 (1.44–3.36) | ||
| Current smoker (reference = no) | ||||
| Yes (95 % CI) | 2.402 (1.69–3.41) | 1.876 (1.06–3.32) | 0.941 (0.70–1.91) | 0.729 (0.34–1.58) |
| Alcohol use (reference = no) | ||||
| Yes (95 % CI) | 0.748 (0.59–0.96) | 1.332 (0.90–1.98) | 0.714 (0.50–1.02) | 1.328 (0.74–1.20) |
| Average level of physical activity each day (reference = low) | ||||
| Moderate (95 % CI) | 0.793 (0.62–1.01) | 0.789 (0.54–1.17) | 1.297 (1.01–1.75) | 1.021 (0.67–1.55) |
| Heavy (95 % CI) | 0.570 (0.29–1.13) | 1.130 (0.41–3.11) | 1.142 (0.40–4.23) | 0.871 (0.14–5.31) |
| Fracture (95 % CI) | 0.918 (0.65–1.30) | 0.890 (0.52–1.52) | 1.100 (0.80–1.63) | 0.887 (0.54–1.47) |
| History of falls (95 % CI) | 1.404 (1.11–1.78) | 1.025 (0.70–1.50) | 1.544 (0.86–1.40) | 1.355 (0.94–1.95) |
| Osteoporosis (95 % CI) | 1.534 (0.78–3.03) | 0.509 (0.17–1.49) | 1.482 (1.17–2.03) | 0.865 (0.58–1.30) |
| Cancer (95 % CI) | 1.444 (1.12–1.86) | 1.044 (0.70–1.57) | 0.810 (1.10–2.00) | 1.226 (0.80–1.89) |
| Stroke (95 % CI) | 1.324 (0.91–1.92) | 1.166 (0.65–2.08) | 0.858 (0.50–1.30) | 0.869 (0.43–1.76) |
| CHD‡ (95 % CI) | 1.008 (0.74–1.37) | 1.035 (0.63–1.71) | 0.394 (0.54–1.36) | 1.146 (0.59–2.22) |
| Diabetes mellitus type II (95 % CI) | 0.849 (0.64–1.13) | 1.992 (1.26–3.15) | 0.655 (0.27–0.57) | 0.791 (0.46–1.36) |
| Arthritis (95 % CI) | 0.845 (0.68–1.06) | 1.108 (0.78–1.58) | 1.832 (0.52–0.83) | 0.688 (0.48–0.98) |
| Emphysema (95 % CI) | 2.990 (1.85–4.84) | 2.632 (1.17–5.92) | 1.567 (1.01–3.32) | 2.415 (0.95–6.13) |
| Bronchitis (95 % CI) | 1.186 (0.73–1.92) | 3.144 (1.27–7.76) | 0.729 (1.07–2.30) | 2.093 (1.15–3.82) |
| Thyroid disease (95 % CI) | 1.354 (0.52–3.53) | 0.591 (0.41–1.29) | ||
| Hypertension (95 % CI) | 0.755 (0.61–0.94) | 1.279 (0.90–1.82) | 0.676 (0.47–0.75) | 1.110 (0.78–1.58) |
| Fat index (95 % CI) | 0.999 (1.00–1.00) | 1.002 (1.00–1.00) | 0.999 (1.00-1.00) | 1.001 (1.00–1.00) |
*BMI body mass index
†SBP systolic blood pressure
‡DBP diastolic blood pressure
†HDL high-density lipoprotein
‡CHD coronary heart disease
For women, statistically significant unadjusted odds ratios (p < 0.05) were found for age, race, BMI, cholesterol, osteoporosis, cancer, diabetes, arthritis, emphysema, bronchitis, and hypertension. When adjusted for other covariates, statistical significance was found for race, BMI, alcohol use, diabetes, bronchitis, and body fat.
Bootstrapped logistic regression models were used to develop predictive models for low muscle mass. All of the models fit well. The male models had a higher pseudo-R2. The area under the curve (AUC; C statistic) for all four models was above 88 %. The fully saturated models had slightly higher AUCs. However, because the difference was very small, less than 1 %, the AUC indicates that the parsimonious model can be used for screening without a clinically meaningful loss of predictive accuracy.
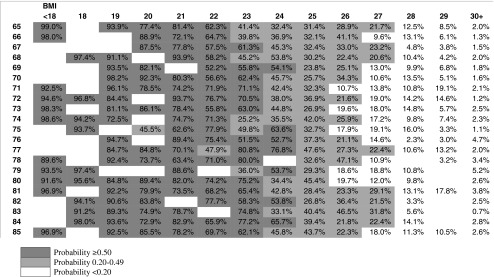
Clinical screening grids are shown in Tables 3 and 4. The darkest cells, to the lower left corner of the table represent lower BMI and higher age with a probability of having low muscle mass greater than or equal to 50 %. The lighter cells show low muscle mass probabilities between 20 and 49 %. The 50 % threshold predicts that 7.3 % of men and 1.8 % of women over 65 years of age will have low muscle mass. An additional 11.5 % of men and 6.5 % of women fall between the 20 and 50 % thresholds. The female pattern is less diagonal because the coefficient for age was not significant in the bootstrapped models.
Table 3.
Predicted probabilities of low muscle mass by age and BMI: male complex model
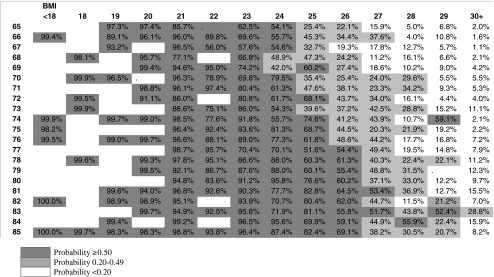
Table 4.
Predicted probabilities of low muscle mass by age and BMI: female complex model
The physical function NHANES validation models included the six function variables and age separately for males and females. For both males and females, age and difficulty lifting or carrying were significant predictors of low muscle mass. Multiple imputations were important as only age was significant in the models that do not use the missing values.
In our 200-person validation sample, the model sensitivity for males was 81.58 %. The model specificity for males was 66.15 %. The positive predictive value, or the probability of a positive result meaning a true positive, was 58.49 %, and the negative predictive value, or the probability of a negative result meaning a true negative, was 86.00 %. The positive likelihood ratio for men was 2.41. For females, the sensitivity was 90.63 % and the specificity was 66.20 %. The positive predictive value was 54.72 %, and the negative predictive value was 94.00 %. The positive likelihood ratio for women was 2.68.
Discussion
The simple prediction model performed well as a screening tool for identifying individuals likely to have low muscle mass and benefit from a DXA scan. The AUC of our models (all above 0.88) is higher than other widely used screening tests in other conditions, including the Women’s Health Initiative prediction model for predicting hip fracture (AUC = 0.80) [16] and the Framingham risk score for predicting coronary heart disease (AUC range 0.5–0.83) [17, 18]. The positive likelihood ratio of 2.41 in men is fairly good, while the LR + of 2.68 in women indicates that the test is just as robust in women. Given the low cost of using the proposed screen, these preliminary results provide good evidence of its utility in identifying patients who would benefit from DXA screening to positively identify low muscle mass without the expense of screening all patients.
Low muscle mass can lead to disability, respiratory distress, falls, and loss of autonomy [19] and can be difficult for physicians to diagnose. There is no specific ICD-9 diagnosis code for this disease, which hinders the ability to identify patients that may benefit from treatment or to conduct outcomes research based on populations identified by ICD-9 code diagnoses. Earlier definitions of sarcopenia were based on low SMI values relative to young adults. This paper provides a predictive model for low muscle mass based on the most recent demographic and clinical characteristics associated with adults in NHANES. The results demonstrate that low BMI is strongly associated with a low SMI among participants in NHANES. Given that a likely future definition of sarcopenia among the elderly may include both muscle mass and measures of functional impairment, the model presented in this paper helps to identify possible sarcopenia primarily in those with low BMI specific to age and sex as a screening tool in clinical practice.
While the diagnosis of low muscle mass can be made with DXA or bioelectrical impedance, these methods are not always clinically feasible for broad population screening. In the case of DXA, the standard software supplied with DXA machines is designed for diagnosis of osteoporosis, so it does not output the measures of SMI with appropriate ranges for use in diagnosis of low muscle mass. In the case of bioelectrical impedance, few physician offices offer this diagnostic tool. Compared with these methods, the most significant variables (BMI and age) modeled in Tables 3 and 4 could be implemented as a screening tool in clinical practice so only patients likely to be diagnosed are subject to referral and screening.
Covariates that were found to be associated with low muscle mass in the adjusted models, such as arthritis and emphysema, are closely associated with limitations in physical activity. Thus, these finding are consistent with the association between physical activity and muscle mass [20, 21]. However, in conditions like arthritis and COPD, limitations in physical activity may in large part be due to joint pain and damage and lung dysfunction while loss of muscle mass may be secondary. Interventions that increase physical activity in individuals who have such capacity but with low BMI or conditions such as arthritis or chronic lung disease could reduce frailty when it is caused by a loss of skeletal muscle mass.
The model developed in this study identifies possible low muscle mass in those with a low BMI. Though frailty and disability in the elderly are often thought to be associated with a decreased BMI, individuals who are characterized as overweight have also been identified to be at a greater risk for developing frailty at a younger age as they suffer more falls [22] and have low levels of physical activity [23] and higher levels of disability than healthy age-matched individuals [24]. Further work is needed to develop models for individuals with high BMI suspected of having low muscle mass.
Limitations
One of the limitations of the validation component of this study is the exclusion of people weighing >300 lb due to DXA scan restraints. Thus, the mean BMI of those tested was not in the obese category, and our results should only be applied to older adults in this BMI range tested (mean BMI of 26.6 ± 4.5). Sarcopenic obesity has been defined in NHANES using quintiles of body fat and relative muscle mass as muscle mass in the upper two quintiles of body fat and the lower two quintiles of muscle mass [25]. Thus, to identify likely sarcopenic obesity would require the addition of a body fat measurement, which we did not include in our definition [26]. Sarcopenic obesity may be more closely related to functional status. Sarcopenic obesity is closely related to frailty [27]. Other work has shown a relationship between sarcopenic obesity and activities of daily living [24]. Ultimately, it is this relationship that makes any prediction model useful. Those identified as having low muscle mass in our study had declines in physical functioning that may preface a loss of autonomy. NHANES only includes community-dwelling adults, so adults with significant disability were not included and functional limitations would be understated. Future research efforts should address our understanding of the functional limitations imposed by sarcopenia in both obese and normal weight populations.
Secondly, we did not account for race is our estimates. Sarcopenia thresholds are known to vary by race [28]. Since our validation population has limited information on race and our goal was create a general screening instrument to determine which patients should receive a DXA scan, this is important, but not fatal. Moreover, for the patients receiving DXA, the thresholds by race for each DXA have not, to our knowledge, been established.
Conclusions
This paper has presented a simple, validated model to improve screening for low muscle mass. The model, used along with a measure of functional impairment, could be easily implemented in physician offices treating elderly populations at risk of sarcopenia.
Acknowledgments
We would like to acknowledge the editorial assistance of Kuan-Ling Kuo and data collection of Hillevi Bauer. Panagiotis Mavros and Shuvayu Sen are employees of Merck. This research was sponsored and funded, in part, by an unrestricted research grant from Merck & Co., Inc. The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle 2010;1:7–8 (von Haehling S, Morley JE, Coats AJ and Anker SD).
References
- 1.Janssen I, et al. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 2.Nair KS. Muscle protein turnover: methodological issues and the effect of aging. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:107–112. doi: 10.1093/gerona/50a.special_issue.107. [DOI] [PubMed] [Google Scholar]
- 3.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–M916. doi: 10.1093/gerona/58.10.M911. [DOI] [PubMed] [Google Scholar]
- 4.Zacker RJ. Health-related implications and management of sarcopenia. JAAPA. 2006;19:24–29. doi: 10.1097/01720610-200610000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner RN, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 6.Melton LJ, Khosla S, Riggs BL. Epidemiology of sarcopenia. Mayo Clin Proc. 2000;75 Suppl:10–12. [PubMed] [Google Scholar]
- 7.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–M777. doi: 10.1093/gerona/57.12.M772. [DOI] [PubMed] [Google Scholar]
- 8.Castillo EM, et al. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med. 2003;25:226–231. doi: 10.1016/S0749-3797(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 9.Foley RN, et al. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279–286. doi: 10.1159/000101827. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey Data. 2010. Available from http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed 19 February 2010
- 11.Heymsfield SB, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 12.Cesari M, Pahor M. Target population for clinical trials on sarcopenia. J Nutr Health Aging. 2008;12:470–478. doi: 10.1007/BF02982708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stata Statistical Software: release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 14.Cox DR, Snell EJ. Monographs on statistics and applied probability. 2. London: Chapman & Hall; 1989. Analysis of binary data. [Google Scholar]
- 15.SAS System for Windows, Version 9.2. Cary, NC: SAS Institute; 2009. [Google Scholar]
- 16.Robbins J, et al. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA. 2007;298:2389. doi: 10.1001/jama.298.20.2389. [DOI] [PubMed] [Google Scholar]
- 17.Tzoulaki I, Liberopoulos G, Ioannidis JP. Assessment of claims of improved prediction beyond the Framingham risk score. JAMA. 2009;302:2345–2352. doi: 10.1001/jama.2009.1757. [DOI] [PubMed] [Google Scholar]
- 18.Wilson PW, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.CIR.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 19.Muscaritoli M, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Chomentowski P, et al. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Gerontol A Biol Sci Med Sci. 2009;64:575–580. doi: 10.1093/gerona/glp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodpaster BH, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24:455–469. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Plotnikoff RC, et al. Factors associated with physical activity in Canadian adults with diabetes. Med Sci Sports Exerc. 2006;38:1526–1534. doi: 10.1249/01.mss.0000228937.86539.95. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner RN, et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 25.Davison KK, et al. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc. 2002;50:1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 26.Stenholm S, et al. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarosz PA, Bellar A. Sarcopenic obesity: an emerging cause of frailty in older adults. Geriatr Nurs. 2009;30:64–70. doi: 10.1016/j.gerinurse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Lee RC, et al. Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000;72:796–803. doi: 10.1093/ajcn/72.3.796. [DOI] [PubMed] [Google Scholar]