Abstract
Alcohol/ethanol has been reported to derived necrosis and apoptosis with an oxidative stress in gastric mucosal cells. However the clear evidence for reactive oxygen species (ROS) generation by alcohol in gastric cells in vitro is none. In this study, we elucidated ethanol is an oxidative stress inducer on rat gastric epithelial cells by electron paramagnetic resonance measurement in living cells. We also confirmed whether ethanol-induced cellular ROS was derived from mitochondria or not. The results of cellular ROS determination showed that an increment of cellular ROS was shown for 15 min from exposing 1% (v/v) ethanol. Lipid peroxidation in cellular membrane also induced by 1% ethanol and the tendency is same in the results of cellular ROS determination. JC-1 stained showed the decrement of mitochondrial membrane potential. Additionally the localization of cellular ROS coincided with mitochondria. These results indicated that ethanol is not merely a necrotizing factor for gastric epithelial cells, but also an oxidative stress inducer via injured mitochondria.
Keywords: alcohol, reactive oxygen species, mitochondria, stomach cells, Electron Paramagnetic Resonance
Introduction
Alcohol/ethanol is an aggressive factor for gastrointestinal tract. The favorite alcohol like a beer or wine is contained 4–20% ethanol. World Health Organization (WHO) reported that alcohol is a casual factor in 60 types of both diseases and injuries and component causes in 200 others. Almost 4% of all deaths worldwide attributed to alcohol, which was about 2.25 million in 2004. Global distribution of deaths (%) related in gastrointestinal tract were 7.0% of esophagus cancer, 3.4% of mouth/oropharynx cancers, and 0.8% of colon/rectum cancers in 2004.(1) Commonly, alcohol consumers are suffering from a bout of heartburn and nausea. Reflux of caustic gastric contents, reactive oxygen species (ROS) such as superoxide radical and hydroxyl radical and release of lysosomal enzymes, is known to directly or indirectly cause symptoms such as heartburn and nausea.(2,3) These symptoms suggested that alcohol is probably an oxidative stress. In liver, toxic effects of alcohol have long been studying because excessive consumption of alcohol relates with alcohol hepatitis.(4,5) Oxidative stress is important factor for liver injury especially alcoholic liver diseases.(5,6) In fact, ethanol-exposed cells generated ROS.(7) Esophageal diseases also relate with oxidative stress.(8) Bile acids and/or gastric acids are known to induce oxidative stress and alter signaling pathways, such as mitogen-activated protein kinase (MAPK), nuclear factor-kappa B (NF-κB) and Signal Tranducer and Activator of Transcription 3 (STAT3).(9–11) Acidic environment by bile acids induced oxidative stress, DNA damage, and mitochondrial damage.(12)
Gastric acid has also been one of the most important aggressive factors for gastric mucosa since Schwartz(13) said a famous dictum ”No acid No ulcer” in 1910. The acid has been regarded as a merely necrotizing factor. However, we have recently reported that a moderate acidic condition exposure involved gastric epithelial injuries via mitochondrial superoxide production.(14) In another words, gastric acid is not only a necrotizing factor but also an oxidative stress inducer. Since acidic environments inhibited mitochondrial electron transport to generate superoxide anion, alcohol may also inhibit it to involve ROS generation. However the direct evidence for ROS generation by alcohol in gastric cells in vitro is none, while the alcohol causes gastric injuries.(15,16)
Electron paramagnetic resonance (EPR) is unique beyond comparison to analyze ROS directly. Ikeda et al.(17) developed the compounds for evaluation of nuclear oxidative stress in living cells by EPR. In addition, Kamibayashi et al.(18) synthesized a spin trap agent CYPMPO which can consummate the analysis of superoxide. We tried blending EPR measurement in living cells with CYPMPO. As a consequence of the combination, mitochondrial ROS such as superoxide anion can be directly detected in living cells by EPR with CYPMPO.(19)
In this study, we elucidate whether alcohol is an oxidative stress inducer or not with a rat gastric mucosal cell line, RGM-1.(20) For this purpose, we measured living gastric epithelial cells’ ROS spectra with an EPR apparatus. Moreover, to clear whether the ethanol-induced ROS is derived from mitochondria, we also investigated the microscopic observation with fluorescent probes detecting both mitochondrial electron potential and ROS.
Materials and Methods
Materials
Aminophenyl Fluorescein (APF) (SEKISUI MEDICAL CO., LTD., Tokyo, Japan), 2-[5,5-Dimethyl-2-oxo-2λ5-(1,3,2)dioxaphosphinan-2-yl]-2-methyl-3,4-dihydro-2H-pyrrole 1-oxide (CYPMPO) (Radical Research Inc., Tokyo, Japan), β-Nicotinamide adenine dinucleotide (NADH) (SIGMA), D-Glutamic acid (SIGMA), Malic Acid (Wako Pure Chem. Ind. Ltd., Osaka, Japan), Succinic acid (SIGMA-ALDRICH), Diphenyl-1pyrenylphosphine (DPPP) (DOJINDO, Kumamoto, Japan), Cell counting kit-8 (DOJINDO), MitoRed (DOJINDO) and Ethanol (Wako) were purchased. Alcohol-contained culture medium was prepared by mixing alcohol, and the culture medium was used after filter-sterilized (Millex 0.22 µm, Millipore Co., Billerica, State).
Cell culture
RGM-1 was cultured in DMEM/F12 (Gibco). This culture medium contained 10% inactivated FBS and 1% penicillin/streptomycin. Cells were cultured in 5% CO2 cell culture incubator at 37°C.
Cell viability test by WST assay
Cell viability test was examined with cell counting kit-8 according to the manufacturer’s instructions. RGM-1 was dispersed in the 96-well dish at 10,000 cells/well and it was incubated for overnight. The medium was replaced to the alcohol-contained culture medium which contained ethanol of 0, 0.1, 0.2, 0.5, 1, 2, 5, 10, 15, 20% (v/v) and it was incubated for 0, 3, and 24 h. After incubation, medium was replaced to the medium contained 10%-cell counting kit-8 of 100 µL and cells were incubated for 1 h. The absorbance of 450 nm was measured by Varioskan plate reader (Thermo Fisher Scientific K. K., Kanagawa, Japan).
Lipid peroxide determination by DPPP
The lipid peroxidation was measured by DPPP as follows; Cells were dispersed at the concentration of 31,250 cells/cm2, and cells were incubated for 24 h. The culture medium was thereafter replaced to the culture medium contained 10 µM DPPP. After incubated for 15 min, cells were washed twice with cold PBS. The fluorescence intensities at Ex. 352 nm and Em. 380 nm of DPPP were measured by the plate reader.
Intracellular ROS determination by APF
Free radicals (hydroxyl radical and peroxynitrite) were detected by APF as follows; APF was diluted with PBS and it exposed to the cells at the concentration of 1 µM for 30 min. After incubation, cells were washed using a cold PBS twice. The intensities of APF-fluorescent were measured by Varioskan at Ex. 490 nm and Em. 515 nm. Cellular fluorescent images were observed with a chilled CCD camera (AxioCam color, ZEEISS, Germany)-mounted epi-fluorescence microscope (Axiovert 135M, ZEISS) connected to an image analyzing system (AxioVision, ZEISS).
The determination of the area at ROS-production in the cell
Cells were cultured on the 35 mmφ glass-bottom dish at the density of 15,000 cells/cm2. Cells were exposed to 0, 1 and 5% ethanol/medium for 1 h. Medium was replaced to PBS with 10 µM APF and 200 nM MitoRed. Cells were incubated for 30 min. After washed with cold PBS twice, cells were taken pictures using confocal microscopy (LSM700, ZEISS).
Electron paramagnetic resonance (EPR) measurement
The methods of EPR measurement were consulted previous reports and so on.(21,22) Cells were cultured on the slide glass until confluent. The slide glass was immersed into different alcohol-contained medium (0, 0.1, 0.2, 0.5, 1, 2, 5, 10, 15 and 20% ethanol) for 0, 15, 30 and 60 min in the 5% CO2 incubator at 37°C. After the incubation, the slide glass was put on the tissue glass (Radical Research Inc., Tokyo, Japan). 100 µL of the solution for EPR measurement, which was prepared that the respiratory substrates (5 mM Succinic acid, 5 mM Malic acid, 5 mM D-Glutamic acid, 5 mM NADH) and 10 mM CYPMPO was dissolved in phosphate buffer saline, was poured in the tissue glass. And then the EPR spectra were recorded by using a JEOL-TE X-band spectrometer (JEOL, Tokyo, Japan). All EPR spectra were obtained under the following conditions: 10 mW incident microwave power, 0.1 mT modulation width, 8 min sweep time, 7.5 mT sweep width, 0.1 s time contrast, 333.5 mT center field, and 15 mT scan range. Spectral computer simulation was performed using a Win-Rad Radical Analyzer System (Radical Research).
Static analysis
Significant static value (p value) was calculated using ANOVA followed by Turkey HSD.
Results
Ethanol induced the cell death
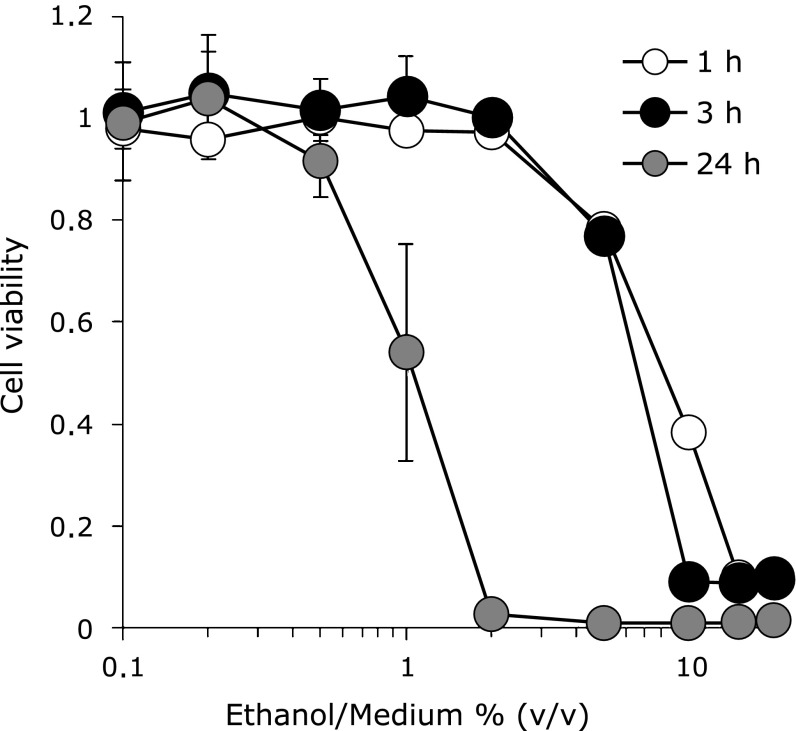
The cell viability after exposing ethanol was determined by cell viability test in comparison with the normal rat gastric mucosa cells (RGM-1). Fig. 1 showed 1% ethanol had cytotoxicity for 24 h. RGM-1 died completely in the medium contained one hour exposure of more 15% (v/v) ethanol, and we decided that necrosis was involved on these cells. On the other hand, the cells survived environments under less than 10% ethanol for several hours suggested that another kind of death was derived on these cells.
Fig. 1.
Cell viability after ethanol exposure. Cell viability was evaluated by WST assay. The different ethanol concentration medium was made by adding ethanol to culture medium. The absorbance at 450 nm was measured by plate reader. Cell viabilities of negative controls for 1, 3 and 24 h (Ethanol/Medium = 0% (v/v)) were 1 ± 0.05, 1 ± 0.01 and 1 ± 0.10, respectively. Cell; RGM-1, n = 6, Error bar; SD.
Ethanol induced cellular ROS
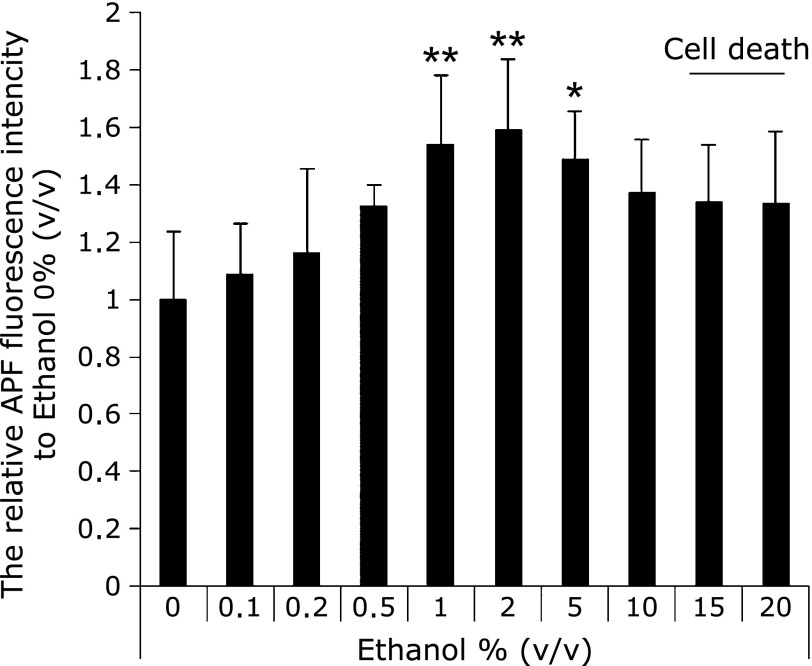
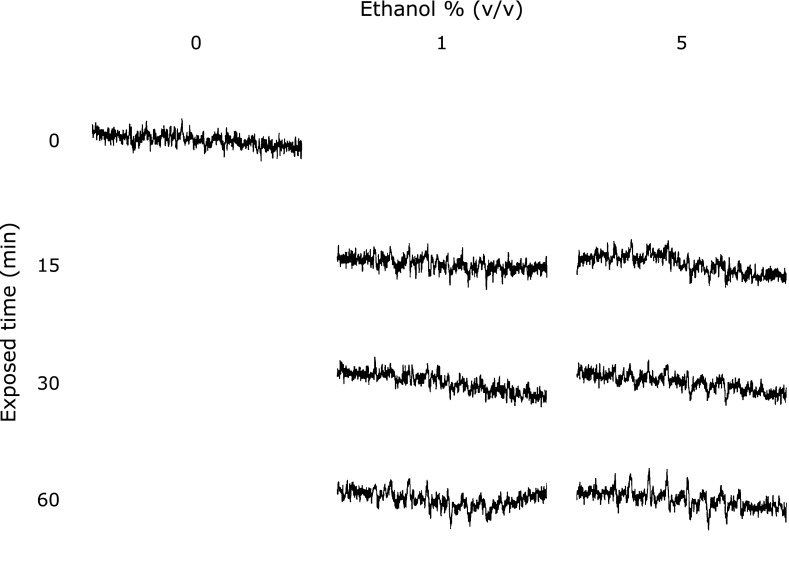
The ROS concentration from the cells was determined by both the AFP study and the EPR measurement using spin-trapping agents (CYPMPO). Fig. 2 showed the results of cellular ROS determination by APF. Cellular ROS was increased with ethanol concentration. The amounts of cellular ROS in 1–5% ethanol exposing cells was significantly higher than that in the control cells. Fig. 3 showed EPR signals intensities in RGM-1 exposed different concentrations of ethanol. These results showed the 15 min exposure of 1% ethanol induced ROS from the RGM-1.
Fig. 2.
The determination of intracellular ROS by APF. Intracellular ROS was significantly increased under the condition of 1–5% (v/v) ethanol after incubation for 1 h. 15–20% ethanol-exposed cells were completely death. n = 5, Error bar; SD. *p<0.05, **p<0.01.
Fig. 3.
The EPR spectra from RGM-1 after ethanol exposure. The intensity of EPR signals was strong after exposed-ethanol. This phenomena was begun after incubated for 15 min. Spin-trapping agent; CYPMPO.
Ethanol induced lipid peroxidation
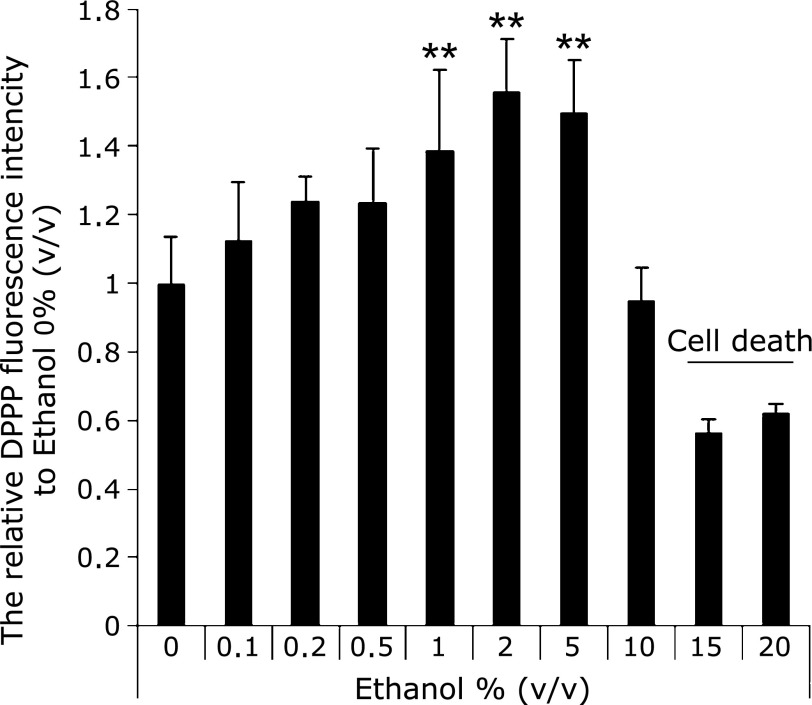
Fig. 4 showed the amounts of lipid peroxidation in cellular membrane after one hour exposure with ethanol. The graph shows the intensity of DPPP fluorescence. These results showed same tendency of the results in APF measurements (Fig. 2).
Fig. 4.
The evaluation of oxidative stress based on lipid peroxide. Lipid peroxidation was significantly increased under the condition of 1–5% (v/v) ethanol after incubation for 1 h. 15–20% ethanol-exposed cells were completely death. n = 6, Error bar; SD. **p<0.01.
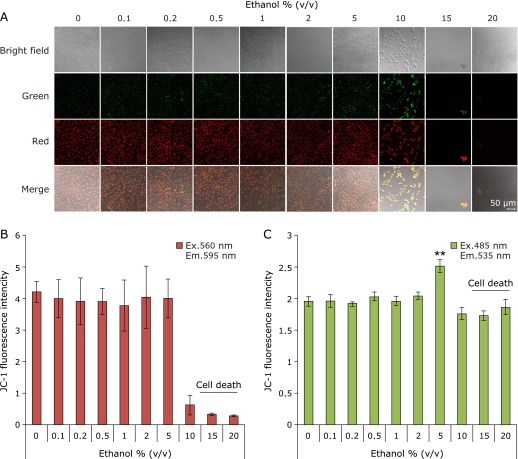
Ethanol injured mitochondrion
We confirmed that ethanol injured mitochondria. Fluorescence characteristics of JC-1 were changed in accordance with mitochondrial membrane potential dependence. Green fluorescence and Red fluorescence of JC-1 means injured mitochondria (decreasing membrane potential) and healthy mitochondria (normal membrane potential), respectively. Fig. 5 shows the results of JC-1 stained. Injured mitochondria were showed in 5% ethanol exposed cells (Fig. 5A and C). These results indicated that ethanol injured mitochondrion.
Fig. 5.
Mitochondrial injury by exposing ethanol. Mitochondrial injury was measured by JC-1 after exposed-ethanol for 1 h. Green fluorescence (Ex. 485 nm, Em. 535 nm) and red fluorescence (Ex. 560 nm, Em. 595 nm) show mitochondrial injury and healthy mitochondria, respectively. Muted mitochondria were significantly increased under the condition of 5% (v/v) ethanol. (A) Confocal microscopic fluorescent images, (B) Red fluorescence intensity of JC-1, (C) Green fluorescence intensity of JC-1. The fluorescence intensity was measured by plate reader. n = 4, Error bar; SD.
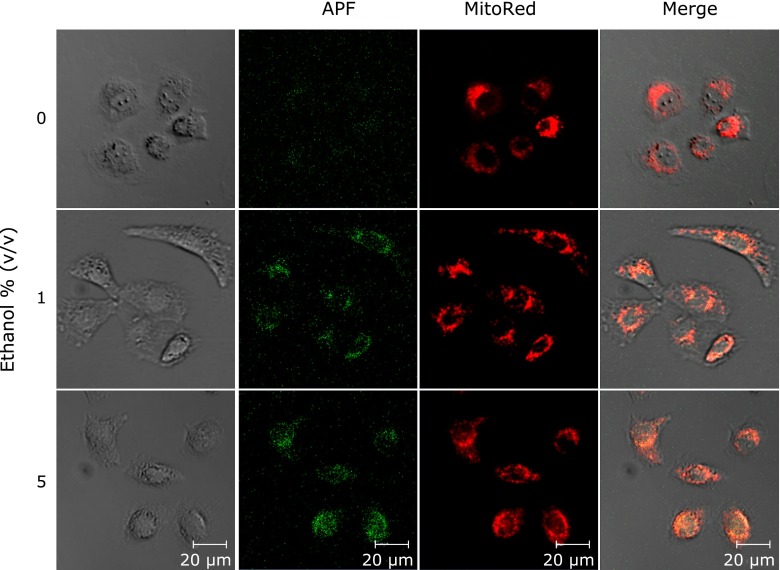
Mitochondria produced ROS by exposing ethanol
We confirmed that the localization of ROS and mitochondria due to clarify the ROS production site. Fig. 6 is pictures of stained-cells with APF and MitoRed. The cells were exposed with 0, 1 and 5% ethanol for 1 h. The MitoRed fluorescence coincided with the APF fluorescence. This result indicated that mitochondria were the ROS production organelle after the ethanol exposure.
Fig. 6.
Co-localization of APF and mitochondria. Cells were exposed to 0, 1 and 5% (v/v) ethanol/medium for 1 h. After incubation, cells were stained by APF and MitoRed. Scale bar; 20 µm.
Discussion
In this study, we demonstrated for the first time that ethanol treatments involved reactive ROS production, in particular superoxide anion, in gastric epithelial cells.
We performed this study under the condition from 0 to 20%, which is popular alcohol’s ethanol concentration. More than 15% ethanol exposure derived immediate cell death within 1 h, while cells were survived for a few hours under less than 10% ethanol condition although cellular ROS production was involved. We thus proposed that high concentration of ethanol more than 15% was a necrotizing factor, while moderate concentration of ethanol was an oxidative stress. Suzuki et al.(23) have demonstrated that the effects of ethanol may be associated with a disturbance in the balance between gastric mucosal protective and aggressive factors. In fact, administration of a low dose ethanol have been reported to protect the gastric mucosa from gastric lesions,(24–26) however, it lead to apoptotic cell death in vitro.(27) Gastrointestinal tracts including the stomach are called the first-pass metabolism of alcohol. In the metabolism, microsomal ethanol oxidizing system (MEOS) requires CYP2E1 (cytochrome P450 family) for generating oxidized NADPH,(28,29) which used to localize in cytoplasms. CYP2E1 accelerates the expression of cyclooxygenase-2 (COX-2) in liver.(30) COX-2 produces prostaglandins, and it should protect gastric lesions in vivo. However, there are few reports investigating the relations between ethanol-induced ROS and mitochondrion.
We have demonstrated that NSAIDs and bisphosphonate(14,21,31) involved superoxide anion production by EPR measurement using separated mitochondria. EPR measurement with living cells also proved that gastric acid is a mitochondrial superoxide anion inducer, whereas it has been generally accepted as a necrotizing factor.(10) The pictures of microscopic observation for ethanol-exposing cells showed the decrement of mitochondrial membrane potential (Fig. 5) and co-localization of mitochondria and APF-stained cellular ROS (Fig. 6). Thus we concluded that ethanol inhibited a mitochondrial electron transfer system to involve superoxide anion production. Mitochondrial ROS was likely to play a role to derive the cellular injury. Mitochondrial ROS has been reported to be related with many diseases. As one example, several reports indicates that mitochondrial ROS production enhances tumor specific properties.(32) Mitochondrial ROS also indicates the relation with the expression of oncogene expression.(33)
In our life, alcohol is diluted with the contents in the stomach. Additionally, almost alcohol contains some antioxidant like polyphenols, resveratrol and flavonoid. These antioxidants were expected that it has health effects for healthy and affected individual.(34–38) They should play a part of defense against alcohol-toxicity. In fact, resveratrol in red wine suppressed ethanol-induced cytotoxicity comparison with white wine and beer.(27) Recently, new chemicals with the capacity to scavenge ROS were synthesized. (39–41) We expected to contribute to develop the prevention of alcoholic harmful effects.
In conclusion, ethanol is not merely a necrotizing factor for gastric epithelial cells, but also an oxidative stress inducer. ROS after ethanol treatment were involved from mitochondria. Now we are undergoing the study to clear the relations between ethanol-induced ROS and carcinogenesis in gastric epithelial cells.
Acknowledgments
This work was partially supported by the Japan Society for the Promotion of Science (JSPS) and Grant-in-Aid for Scientific Research (KAKENHI) #24106503, #70272200.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.World Health Organization. Global Status Report on Alcohol 2004. World Health Organization; 2004. pp. 35–58. [Google Scholar]
- 2.Li Y, Wo JM, Ellis S, Ray MB, Jones W, Martin RC. A novel external esophageal perfusion model for reflux esophageal injury. Dig Dis Sci. 2006;51:527–532. doi: 10.1007/s10620-006-3165-4. [DOI] [PubMed] [Google Scholar]
- 3.Oh TY, Lee JS, Ahn BO, et al. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic Biol Med. 2001;30:905–915. doi: 10.1016/s0891-5849(01)00472-5. [DOI] [PubMed] [Google Scholar]
- 4.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012;13:133–142. doi: 10.1111/j.1751-2980.2011.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matharu Z, Enomoto J, Revzin A. Miniature enzyme-based electrodes for detection of hydrogen peroxide release from alcohol-injured hepatocytes. Anal Chem. 2013;85:932–939. doi: 10.1021/ac3025619. [DOI] [PubMed] [Google Scholar]
- 8.Goldman A, Chen HD, Roesly HB, et al. Characterization of squamous esophageal cells resistant to bile acids at acidic pH: implication for Barrett’s esophagus pathogenesis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G292–G302. doi: 10.1152/ajpgi.00461.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorak K, Chavarria M, Payne CM, et al. Activation of the interleukin-6/STAT3 antiapoptotic pathway in esophageal cells by bile acids and low pH: relevance to barrett’s esophagus. Clin Cancer Res. 2007;13:5305–5313. doi: 10.1158/1078-0432.CCR-07-0483. [DOI] [PubMed] [Google Scholar]
- 10.Souza RF, Shewmake K, Terada LS, Spechler SJ. Acid exposure activates the mitogen-activated protein kinase pathways in Barrett’s esophagus. Gastroenterology. 2002;122:299–307. doi: 10.1053/gast.2002.30993. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Latif MM, O’Riordan J, Windle HJ, et al. NF-κB activation in esophageal adenocarcinoma: relationship to Barrett’s metaplasia, survival, and response to neoadjuvant chemoradiotherapy. Ann Surg. 2004;239:491–500. doi: 10.1097/01.sla.0000118751.95179.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz K. Uber penetrierende Magen- und Jejunalgeschwure. Beitrage zurKlinischen Chirurgie. 1910;67:96–128. [Google Scholar]
- 14.Nagano Y, Matsui H, Tamura M, et al. NSAIDs and acidic environment induce gastric mucosal cellular mitochondrial dysfunction. Digestion. 2012;85:131–135. doi: 10.1159/000334685. [DOI] [PubMed] [Google Scholar]
- 15.Salaspuro M. Acetaldehyde and gastric cancer. J Dig Dis. 2011;12:51–59. doi: 10.1111/j.1751-2980.2011.00480.x. [DOI] [PubMed] [Google Scholar]
- 16.Hajrezaie M, Golbabapour S, Hassandarvish P, et al. Acute toxicity and gastroprotection studies of a new schiff base derived copper (II) complex against ethanol-induced acute gastric lesions in rats. PLoS One 2012; 7: e51537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Ikeda M, Nakagawa H, Ban S, Tsumoto H, Suzuki T, Miyata N. Development of a DNA-binding TEMPO derivative for evaluation of nuclear oxidative stress and its application in living cells. Free Radic Biol Med. 2010;49:1792–1797. doi: 10.1016/j.freeradbiomed.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Kamibayashi M, Oowada S, Kameda H, et al. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) Free Radic Res. 2006;40:1166–1172. doi: 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 19.Tamura M, Matsui H, Nagano Y, et al. Salt is an oxidative stressor for gastric epithelial cells. J Physiol Pharmacol. 2013;64:89–94. [PubMed] [Google Scholar]
- 20.Kobayashi I, Kawano S, Tsuji S, et al. RGM1, a cell line derived from normal gastric mucosa of rat. In Vitro Cell Dev Biol Anim. 1996;32:259–261. doi: 10.1007/BF02723056. [DOI] [PubMed] [Google Scholar]
- 21.Rai K, Matsui H, Kaneko T, et al. Lansoprazole inhibits mitochondrial superoxide production and cellular lipid peroxidation induced by indomethacin in RGM1 cells. J Clin Biochem Nutr. 2011;49:25–30. doi: 10.3164/jcbn.10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukohda M, Ueno S, Kamibayashi M, Okada M, Yamawaki H, Hara Y. Influences of organic solvents on CYPMPO-electron spin resonance spectra in in vitro radical generating systems. J Vet Med Sci. 2010;72:1547–1550. doi: 10.1292/jvms.10-0232. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 2012;50:35–39. doi: 10.3164/jcbn.11-115SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka K, Nishimoto K, Tomisato W, et al. Adaptive cytoprotection induced by pretreatment with ethanol protects against gastric cell damage by NSAIDs. Dig Dis Sci. 2004;49:210–217. doi: 10.1023/b:ddas.0000017440.46863.56. [DOI] [PubMed] [Google Scholar]
- 25.Kopic S, Corradini S, Sidani S, et al. Ethanol inhibits gastric acid secretion in rats through increased AMP-kinase activity. Cell Physiol Biochem. 2010;25:195–202. doi: 10.1159/000276553. [DOI] [PubMed] [Google Scholar]
- 26.Robert A, Nezamis JE, Lancaster C, Davis JP, Field SO, Hanchar AJ. Mild irritants prevent gastric necrosis through ”adaptive cytoprotection” mediated by prostaglandins. Am J Physiol. 1983;245:G113–G121. doi: 10.1152/ajpgi.1983.245.1.G113. [DOI] [PubMed] [Google Scholar]
- 27.Loguercio C, Tuccillo C, Federico A, Fogliano V, Del Vacchio Blanco C, Romano M. Alcoholic beverages and gastric epithelial cell viability: effect on oxidative stress-induced damage. J Physiol Pharmacol. 2009;60 (Suppl 7):87–92. [PubMed] [Google Scholar]
- 28.Julkunen RJ, Di Padova C, Lieber CS. First pass metabolism of ethanol—a gastrointestinal barrier against the systemic toxicity of ethanol. Life Sci. 1985;37:567–573. doi: 10.1016/0024-3205(85)90470-9. [DOI] [PubMed] [Google Scholar]
- 29.Pronko P, Bardina L, Satanovskaya V, Kuzmich A, Zimatkin S. Effect of chronic alcohol consumption on the ethanol- and acetaldehyde-metabolizing systems in the rat gastrointestinal tract. Alcohol Alcohol. 2002;37:229–235. doi: 10.1093/alcalc/37.3.229. [DOI] [PubMed] [Google Scholar]
- 30.Uzma N, Kumar BS, Priyadarsini KI. Hepatoprotective, immunomodulatory, and anti-inflammatory activities of selenocystine in experimental liver injury of rats. Biol Trace Elem Res. 2011;142:723–734. doi: 10.1007/s12011-010-8807-x. [DOI] [PubMed] [Google Scholar]
- 31.Matsui H, Nagano Y, Shimokawa O, et al. Gastric acid induces mitochondrial superoxide production and lipid peroxidation in gastric epithelial cells. J Gastroenterol. 2011;46:1167–1176. doi: 10.1007/s00535-011-0434-6. [DOI] [PubMed] [Google Scholar]
- 32.Friday E, Oliver R, 3rd, Welbourne T, Turturro F. Glutaminolysis and glycolysis regulation by troglitazone in breast cancer cells: relationship to mitochondrial membrane potential. J Cell Physiol. 2011;226:511–519. doi: 10.1002/jcp.22360. [DOI] [PubMed] [Google Scholar]
- 33.Ralph SJ, Rodríguez-Enríquez S, Neuzil J, Saavedra E, Moreno-Sánchez R. The causes of cancer revisited: ”mitochondrial malignancy” and ROS-induced oncogenic transformation—why mitochondria are targets for cancer therapy. Mol Aspects Med. 2010;31:145–170. doi: 10.1016/j.mam.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Hsia SM, Wang KL, Wang PS. Effects of resveratrol, a grape polyphenol, on uterine contraction and Ca2+ mobilization in rats in vivo and in vitro. Endocrinology. 2011;152:2090–2099. doi: 10.1210/en.2010-1223. [DOI] [PubMed] [Google Scholar]
- 35.Cui X, Jin Y, Hofseth AB, et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res (Phila) 2010;3:549–559. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao CV, Vijayakumar M. Effect of quercetin, flavonoids and α-tocopherol, an antioxidant vitamin, on experimental reflux oesophagitis in rats. Eur J Pharmacol. 2008;589:233–238. doi: 10.1016/j.ejphar.2008.04.062. [DOI] [PubMed] [Google Scholar]
- 37.Del Rio D, Borges G, Crozier A. Berry flavonoids and phenolics: bioavailability and evidence of protective effects. Br J Nutr. 2010;104 (Suppl 3):S67–S90. doi: 10.1017/S0007114510003958. [DOI] [PubMed] [Google Scholar]
- 38.Gross G, Jacobs DM, Peters S, et al. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J Agric Food Chem. 2010;58:10236–10246. doi: 10.1021/jf101475m. [DOI] [PubMed] [Google Scholar]
- 39.Yoshitomi T, Suzuki R, Mamiya T, Matsui H, Hirayama A, Nagasaki Y. pH-sensitive radical-containing-nanoparticle (RNP) for the L-band-EPR imaging of low pH circumstances. Bioconjug Chem. 2009;20:1792–1798. doi: 10.1021/bc900214f. [DOI] [PubMed] [Google Scholar]
- 40.Vong LB, Tomita T, Yoshitomi T, Matsui H, Nagasaki Y. An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology. 2012;143:1027–1036.e3. doi: 10.1053/j.gastro.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 41.Liu M, Li H, Luo G, Liu Q, Wang Y. Pharmacokinetics and biodistribution of surface modification polymeric nanoparticles. Arch Pharm Res. 2008;31:547–554. doi: 10.1007/s12272-001-1191-8. [DOI] [PubMed] [Google Scholar]