Abstract
Scope
Epidemiological studies on the association between pregnancy outcomes and use of periconceptional folic acid are often based on maternal reported intake. Use of folic acid during pregnancy is associated with a higher socioeconomic status known to have an impact on diet quality. We have studied plasma B vitamin status according to reported use of folic acid supplements during the periconceptional period in Norwegian women.
Methods and results
Plasma levels of folate, cobalamin, pyridoxal 5′-phosphate (vitamin B6), riboflavin and the metabolic markers total homocysteine, methylmalonic acid and 3-hydroxykynurenine were measured in pregnancy week 18 and related to reported intake of folic acid from 4 weeks prior to conception throughout week 18 in 2911 women from the Norwegian Mother and Child Cohort Study (MoBa) conducted by the Norwegian Institute of Public Health.
Being a folic acid user during the periconceptional period was associated with a better socioeconomic status, and a higher intake of several micronutrients, including vitamins, trace-metals and omega 3 fatty acids. Folic acid users had a significantly better plasma B vitamin status.
Conclusions
Epidemiological data based on maternal reported intake of folic acid supplements during pregnancy, should take into account the numerous nutritional implications, in addition to higher blood folate levels, of being a folic acid user.
Keywords: Folic acid, periconceptional, B vitamin status, pregnancy, micronutrients
INTRODUCTION
Randomized, controlled, intervention trials with 0.8 and 4.0 mg folic acid alone or in combination with other vitamins have demonstrated a significant reduction in both occurrence [1] and recurrence [2] rate of neural tube defects (NTDs). The only intervention trial with 0.4 mg folic acid alone was a large study in China, where the NTD rate was reduced by 41–79% in different regions [3]. These results are consistent with those obtained from epidemiological studies demonstrating an association between periconceptional intake of multivitamins containing folic acid and a reduced rate of NTDs [4, 5].
Based on the randomized, controlled trials, women planning or capable of pregnancy have been recommended to take a daily dose of 0.4 to 0.8 mg of folic acid during the periconceptional period, defined as one month before conception throughout the first 8–12 weeks of pregnancy [6]. This recommendation, with small modifications, has been implemented in many countries, but despite this there has not been a concomitant reduction in the prevalence of NTDs in countries that have not implemented folic acid fortification [7]. As the neural tube closes early, 28 days after conception, folic acid supplementation has to be established during the early periconceptional period [8]. Use of folic acid supplementation among fertile women is generally low and many pregnancies are not planned [9, 10]. Only 10% of Norwegian women used folic acid supplement as recommended, and even among women who planned their pregnancy or conceived by in vitro fertilization, the use of folic acid during the preconceptional period was only 16 to 32% [11].
Observational data drawn from cohort, case-control studies and meta-analyses are mainly based on questionnaires collecting data on maternal use of folic acid supplements in pregnancy. A female folic acid user is likely to be a non-smoker, older, married and have higher education, higher income and lower parity [11], indicators of a better socioeconomic status. As socioeconomic position is considered to have a substantial impact on diet quality [14], the implication of being a folic user on nutritional status, apart from improved folate levels, should be taken into account, when evaluating possible effects of folic acid user status on pregnancy outcome.
Since 1998 Norwegian official guidelines have recommended a daily intake of 0.4 mg of folic acid from one month prior to conception throughout the first 8–12 pregnancy weeks [9] We studied plasma B vitamin status in 2911 pregnant women from the Norwegian Mother and Child Cohort Study (MoBa) according to their reported use of folic acid and other micronutrient supplements during the periconceptional period until pregnancy week 18. Maternal plasma levels of folate and other B vitamins and metabolic markers of B vitamin status [12, 13], obtained in pregnancy week 18, were measured and related to reported intake of micronutrient supplements. The purpose of our investigation was to describe differences in relevant characteristics and B-vitamin status, between women who take and women who do not take folic acid supplements.
MATERIALS AND METHODS
Study population
This study is based on a subsample of 3000 women included in the Norwegian Mother and Child Cohort Study, a long-term, prospective study conducted by the Norwegian Institute of Public Health and including more than 100 000 Norwegian pregnant women and their infants during 1999–2008 [14, 15]. The women who were included gave birth between July 2002 and December 2003, and donated a blood sample in pregnancy week 18, returned a baseline questionnaire and were registered in the Medical birth Registry of Norway. Twin pregnancies were excluded, leaving 2941 singleton pregnancies available for analyses. A detailed description of the substudy population and the sampling procedures has been published [16]. Written informed consent was obtained from each participant, and the study was approved by the Regional Committee for Medical Research Ethics (permission number 2009/2593a) and the Norwegian Data Inspectorate.
Blood sampling and laboratory analyses
Maternal nonfasting blood samples were collected in EDTA tubes in median pregnancy week 18. The samples were centrifuged within 30 minutes after collection and stored at 4°C until shipped overnight to the Biobank of MoBa at the Norwegian Institute of Public Health in Oslo. On the day of receipt (usually within 1–2 days), plasma was aliquoted into polypropylene microtiter plates and stored at −80°C until analyses.
Plasma folate was determined by a Lactobacillus casei microbiological assay [17] and plasma cobalamin (vitamin B12) by a Lactobacillus leichmannii microbiological assay [18]. Concurrent intake of antibiotics may interfere with microbiological assays and falsely reduce plasma levels of the vitamins [19]. In order to control for this, we excluded samples with plasma folate levels <2.33 nmol/L (n=30, i.e. the lower 1 percentile), leaving a total of 2911 samples to be included in the study.
Plasma levels of total homocysteine (tHcy), a marker of folate and cobalamin status, and methylmalonic acid (MMA), a marker of cobalamin status, were assayed using a GC-MS method based on methylchloroformate derivatization [20], whereas plasma levels of riboflavin (vitamin B2), pyridoxal 5′-phosphate (PLP) (vitamin B6) and 3-hydroxykynurenine (HK), a marker of vitamin B6 status, were analyzed using a liquid chromatography-tandem mass spectrometry assay [21].
Covariates
Information on use of micronutrient supplements, including cod liver oil and omega-3, was obtained from the baseline cohort questionnaire, administered around week 18 of pregnancy. Use of micronutrient supplements was reported in 4-week intervals, from 4 weeks before conception up to pregnancy week 18.
Use of folic acid supplement was coded as a dichotomous variable, with any intake reported from 4 weeks prior to conception up to pregnancy week 18 (folic acid user) versus no intake of folic acid (folic acid non-user). The subjects were further subcategorized according to use of folic acid supplement alone, use of folic acid together with other supplements, use of supplements other than folic acid alone and use of no supplements.
Data on maternal age at delivery, marital status and parity were obtained from the Medical Birth Registry of Norway. Data on maternal education, body mass index (BMI, kg/m2) before pregnancy and in pregnancy week 18 and current smoking habits were obtained from the baseline cohort questionnaire.
Statistical analyses
Values are presented as medians with the interquartile range. Differences in medians were examined by the Mann-Whitney U test and the Kruskal Wallis test. Differences in categorical variables were assessed with the chi-square test. Multiple-linear regression models were used to assess the relation of being a folic acid user, maternal daily smoking, parity and age with plasma B vitamin status obtained around pregnancy week 18 (median).
Graphical illustration of the dose-response relationship between folate and tHcy, cobalamin and MMA, PLP and HK, were obtained by generalized additive models (GAM).
GAMs were computed using the package mgcv (version 1.4–1) in R (The R Foundation for Statistical Computing, version 2.8.1) [22], and the SPSS/PASW statistical program version 18 was used for the remaining statistical analyses. Two-sided p-values <0.05 were considered statistically significant.
RESULTS
Characteristics of the study population according to reported use of folic acid supplements
Seventy-one percent (2067/2911) of the mothers reported use of folic acid any time during this period. The remaining women (844/2911) had not taken folic acid, but 262/2911 (9%) reported taking other micronutrient supplements, while 582/2911 (20%) reported no use of micronutrients. Characteristics according to folic acid user status, i.e. use of folic acid alone or in combination with other micronutrients, including cod liver oil/Omega-3 fatty acids, any time during the period ranging from 4 weeks prior to conception to pregnancy week 18, are presented in Table 1. Folic acid users were more likely to be primipara, married, non-smokers, have a higher education and a lower BMI (Table 1).
Table 1.
Maternal characteristics according to folic acid user status, n=2911
| Folic acid user | Folic acid non-usera | P values | |
|---|---|---|---|
| Number | N=2067 | N=844 | |
| Age, years, vn | 29.9 (4) | 29.5 (5) | 0.04b |
| Current BMI, mean (SD) | 25.4 (8.3) | 26.7 (11.9) | 0.004b |
| Current weight increase, kg, mean (SD) | 2.7 (4.0) | 3.0 (3.8) | 0.04b |
| Marital status | |||
| Married, n (%) | 2016 (98%) | 793 (94%) | <0.001c |
| Education | |||
| Primary school, n (%) | 120 (6%) | 131 (16%) | <0.001c |
| Secondary school, n (%) | 606 (31%) | 326 (41%) | |
| University or college, n (%) | 1237 (63%) | 340 (43%) | |
| Parity | |||
| Primipara, n (%) | 938 (45%) | 278 (33%) | <0.001c |
| Number of former children, mean (SD) | 1.4 (0.7) | 1.6 (0.8) | <0.001b |
| Smoking | |||
| Daily smoking, n (%) | 131 (6%) | 115 (14%) | <0.001c |
| Number of cigarettes/day, mean (SD) | 7.2 (8.0) | 9.75 (10.6) | 0.18b |
| Plasma cotinin level in daily smokers, μmol/L, mean (SD) | 489 (268–769) | 504 (307–737) | 0.63d |
No supplements, n=582, + Other supplements, n=262
Student’s t-test
Chi-Square test
Kruskal-Wallis test
Intake of other micronutrient supplements according to folic acid user status
Among the 2067 folic acid users, 406 (20%) took only folic acid supplements. The remaining 1661 (80%) reported taking additional micronutrient supplements during the period from 4 weeks prior to conception to pregnancy week 18 (Table 2). Intake of any other B vitamins or vitamin C were reported by 36.4%, intake of any fat soluble vitamins (A, D and E) by 40.8%, intake of any trace metals by 27.8% and intake of cod liver oil and/or Omega-3 fatty acids by 33.7% of the women. Among the 844 folic acid non-users, intake of these micronutrients were less common (0.5–13.2 %) and the most frequent supplements reported used were iron and cod liver oil (12.9–13.2%) (Table 2).
Table 2.
Reported intake of micronutrient supplements according to folic acid user status
| Micronutrient supplement | Folic acid user N=2067 |
Folic acid non-user N=844a |
||
|---|---|---|---|---|
| Number | % | Number | % | |
| Vitamin B1; Thiamin | 815 | 39.4% | 28 | 3.3% |
| Vitamin B2; Riboflavin | 819 | 39.6% | 29 | 3.4% |
| Vitamin B3; Niacin | 440 | 21.3% | 13 | 1.5% |
| Vitamin B5; Panthotenic acid | 618 | 29.9% | 18 | 2.1% |
| Vitamin B6; Pyridoxin | 824 | 39.9% | 30 | 3.6% |
| Vitamin B7; Biotin | 219 | 10.6% | 4 | 0.5% |
| Vitamin B12; Cobalamin | 724 | 35.0% | 18 | 2.1% |
| Vitamin A | 804 | 38.9% | 32 | 3.8% |
| Vitamin C | 867 | 41.9% | 59 | 7.0% |
| Vitamin D | 850 | 41.1% | 35 | 4.1% |
| Vitamin E | 878 | 42.5% | 35 | 4.1% |
| Calcium | 305 | 14.8% | 13 | 1.5% |
| Chromium | 601 | 29.1% | 12 | 1.4% |
| Copper | 536 | 25.9% | 8 | 0.9% |
| Iodine | 520 | 25.2% | 8 | 0.9% |
| Iron | 924 | 44.7% | 111 | 13.2% |
| Magnesium | 473 | 22.9% | 12 | 1.4% |
| Selenium | 603 | 29.2% | 10 | 1.2% |
| Zink | 633 | 30.6% | 15 | 1.8% |
| Cod liver oil | 684 | 33.1% | 109 | 12.9% |
| Omega 3 fatty acids | 710 | 34.3% | 63 | 7.5% |
For comparison of intake of micronutrients between user and non-users, all P-values < 0.001 by Chi-Square test
No supplements, n=582, + Other supplements, n=262
Plasma B vitamins according to folic acid user status
Being a folic acid user was associated with an overall better plasma B vitamin status (Table 3), with significantly higher levels of plasma folate, cobalamin, PLP and riboflavin and concomitantly lower levels of the metabolic markers plasma tHcy (marker of folate and cobalamin status) and HK (marker of vitamin B6 status) than in non-users. No difference was observed for plasma MMA levels (marker of cobalamin status) between folic acid users and non-users (Table 3).
Table 3.
Maternal plasma levels of vitamins and metabolic markers in pregnancy week 18 according to folic acid user status
| Parameters, median (interquartile range) | Folic acid user | Folic acid non-usera | P valuesb |
|---|---|---|---|
| Number | N=2067 | N=844 | |
| Serum Folate, nmol/L | 10.6 (7.4–17.7) | 5.6 (4.3–7.7) | <0.001 |
| Serum Cobalamin, pmol/L | 309 (249–378) | 291 (233–366) | <0.001 |
| Plasma PLP, nmol/L | 29.3 (21.9–42.0) | 24.1 (18.6–30.4) | <0.001 |
| Plasma Riboflavin, nmol/L | 8.4 (5.7–14.1) | 6.9 (4.8–10.9) | <0.001 |
| Plasma tHcy, μmol/L | 4.75 (4.14–5.54) | 5.53 (4.77–6.58) | <0.001 |
| Plasma MMA, μmol/L | 0.13 (0.10–0.16) | 0.13 (0.11–0.16) | 0.11 |
| Plasma 3-OH-kynurenine, μmol/L | 23.6 (18.0–30.0) | 24.3 (18.9–31.8) | 0.005 |
No supplements, n=582, + Other supplements, n=262
Mann Whitney test
A minority, 238 (11.5%), of the folic acid users reported taking folic acid every week during this 22 week period. There was a decreasing trend in plasma B vitamin status according to use every week, infrequent use or no use during the period (Table 4).
Table 4.
Maternal plasma levels of vitamins and metabolic markers in pregnancy week 18 according to the degree of folic acid supplement use
| Parameters, median (interquartile range) | Folic acid use every week from week −4 to +17 | Folic acid use sometimes from week −4 to +17 | Folic acid non usera | P valuesb |
|---|---|---|---|---|
| Number | N=238 | N=1829 | N=844 | |
| Serum Folate, nmol/L | 15.7 (9.4–23.1) | 10.2 (7.3–16.6) | 5.7 (4.3–7.7) | <0.001 |
| Serum Cobalamin, pmol/L | 319 (260–395) | 307 (248–375) | 291 (233–366) | <0.001 |
| Plasma PLP, nmol/L | 36.9 (25.5–53.4) | 28.3 (21.6–40.6) | 24.1 (18.6–30.4) | <0.001 |
| Plasma Riboflavin, nmol/L | 10.2 (6.9–16.6) | 8.2 (5.6–13.7) | 6.9 (4.8–10.9) | <0.001 |
| Plasma tHcy, μmol/L | 4.42 (3.99–5.08) | 4.79 (4.21–5.56) | 5.51 (4.76–6.52) | <0.001 |
| Plasma MMA, μmol/L | 0.12 (0.10–0.16) | 0.13 (0.10–0.16) | 0.13 (0.11–0.16) | 0.20 |
| Plasma 3-OH-kynurenine, μmol/L | 22.3 (17.0–28.1) | 23.7 (18.1–30.3) | 24.2 (18.9–31.8) | 0.001 |
No supplements, n=584 + other supplements, n=263
Kruskal Wallis test
Plasma B vitamin status according to use of folic acid and other micronutrient supplements
The highest plasma B vitamin levels were seen in women who reported intake of folic acid together with other micronutrient supplements (Table 5). Even the median plasma folate level was significantly higher in these women, compared to women who took folic acid alone (p<0.001). Women who took folic acid alone, had a significantly better folate status compared to no-supplement users (p<0.001), but no differences were observed for other B vitamins levels (p>0.10) (Table 5).
Table 5.
Maternal plasma levels of vitamins and metabolic markers in pregnancy week 18 according to maternal use of folic acid and other micronutrients
| Parameters, median (interquartile range) | Folic acid only user | Folic acid and other supplements user | Other supplement only user | No supplements user | P valuesa |
|---|---|---|---|---|---|
| Number | N=406 | N=1661 | N=262 | N=582 | |
| Serum Folate, nmol/L | 8.8 (6.6–13.5) | 11.2 (7.7–18.7) | 5.6 (4.3–7.3) | 5.8 (4.2–7.7) | <0.001 |
| Serum Cobalamin, pmol/L | 289 (240–358) | 313 (251–382) | 294 (234–368) | 287 (233–364) | <0.001 |
| Plasma PLP, nmol/L | 24.7 (19.4–31.0) | 31.3 (22.8–44.8) | 25.6 (19.4–34.3) | 23.6 (18.3–29.5) | <0.001 |
| Plasma Riboflavin, nmol/L | 6.9 (5.1–10.5) | 8.8 (5.9–14.8) | 7.3 (5.0–11.3) | 6.8 (4.8–10.8) | <0.001 |
| Plasma tHcy, μmol/L | 4.87 (4.31–5.64) | 4.70 (4.11–5.49) | 5.51 (4.77–6.50) | 5.53 (4.75–6.57) | <0.001 |
| Plasma MMA, μmol/L | 0.13 (0.10–0.16) | 0.13 (0.10–0.16) | 0.13 (0.11–0.17) | 0.13 (0.10–0.16) | 0.15 |
| Plasma 3-OH-Kynurenine, μmol/L | 23.9 (18.7–30.5) | 23.5 (17.8–29.9) | 23.7 (18.7–31.2) | 24.6 (19.0–32.0) | 0.02 |
Kruskal Wallis test
Maternal factors as determinants of plasma B vitamin status in pregnancy week 18
In a multiple linear regression model, both folic acid user status, current daily smoking, parity and age were significant determinants of plasma B vitamin status in pregnancy week 18 (Table 6). However, between these four maternal factors, folic acid user status was the most consistent and strongest determinant, with significant influence on plasma folate, cobalamin, PLP, riboflavin, tHcy and HK.
Table 6.
Determinants of maternal plasma B vitamin status in pregnancy week 18 by multiple linear regression (n=2871)
| Independent variables | Serum Folate, nmol/L | Serum Cobalamin, pmol/L | Plasma PLP, nmol/L | Plasma Riboflavin, nmol/L | Plasma tHcy, μmol/L | Plasma MMA, μmol/L | Plasma 3-OH-kynurenine, μmol/L | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | p | B | p | B | p | B | p | B | p | B | p | B | p | |
| Use of folic acida | 6.0 | <0.001 | 14 | 0.003 | 7.2 | <0.001 | 1.9 | 0.001 | −0.81 | <0.001 | −0.003 | 0.16 | −1.5 | 0.001 |
| Current daily smoking, 0–1 | −1.0 | <0.001 | −10 | 0.008 | −3.2 | <0.001 | −0.7 | 0.12 | 0.30 | 0.001 | −0.003 | 0.07 | −1.3 | <0.001 |
| Parityb | −1.1 | <0.001 | −4 | 0.11 | −1.6 | 0.004 | −1.0 | 0.001 | −0.03 | 0.62 | −0.002 | 0.14 | 0.2 | 0.53 |
| Agec | 2.3 | <0.001 | 13 | 0.004 | 2.7 | 0.005 | 0.3 | 0.58 | 0.07 | 0.53 | 0.005 | 0.02 | 0.3 | 0.46 |
Use of folic acid supplement any time from 4 weeks before pregnancy up to pregnancy week 17
Parity, categorized; 0, 1, 2, 3+
Age, categorized; <25 y, 25–29 y, 30–34 y, >34 y
Plasma B vitamin status
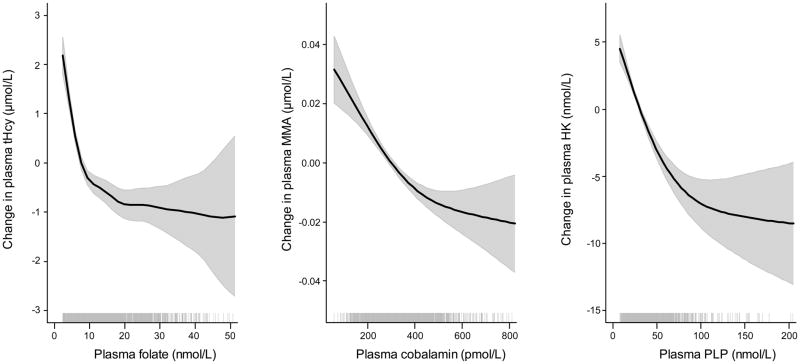
Inverse Spearman correlations were observed between the vitamins and their respective metabolic markers; folate vs tHcy (r=−0.48, p<0.001), cobalamin vs tHcy (r=−0.24, p<0.001), cobalamin vs MMA (r=−0.16, p<0.001) and PLP vs HK (r=−0.24, p<0.001). The dose-response curves were non-linear with steepest slopes at low levels of B vitamins (Figure 1). Plasma folate was positively correlated to the other B vitamins; cobalamin (r=0.13, p<0.001), PLP (r=0.35, p<0.001) and riboflavin (r=0.26, p<0.001)..
Figure 1.
Dose-response relationship between folate and tHcy, cobalamin and MMA, PLP and HK by Generalized additive models (GAM)
DISCUSSION
Folic acid user status during the periconceptional period to pregnancy week 18 was associated with an overall better plasma B vitamin status. Folic acid users were more likely than non-users to report intake of other vitamins, trace-minerals and cod liver oil/omega-3 fatty acids during the same period.
Limitations
Compared to the total Norwegian pregnant population, women who participated in the MoBa study are older and more frequent vitamin users, non-smoking and primiparous, making this a more homogenous population and not completely representative of the general pregnant population in Norway [23].
Blood samples and questionnaire data were recorded in median pregnancy week 18. An accurate recall of the use of various micronutrients from 4 weeks before through 18 weeks of pregnancy may be difficult; however, we observed a significant correlation between reported intake of folic acid and plasma folate levels, indicating an accurate data collection.
The folate level in EDTA plasma samples tends to degrade somewhat in room temperature for the first two days [24, 25]. As the blood samples were collected in various places in Norway and were in transit for usually 1–2 days, this might have reduced the median plasma folate level. Yet, the expected inverse association between folate and tHcy was maintained, and we observed a positive association between reported intake of folic acid and plasma folate levels (Table 5) which suggests integrity of the data set.
Characteristics of a folic acid user
Being a folic acid user in this cohort of Norwegian mothers was associated with several indicators of a better socioeconomic status (Table 1), which is also observed in other populations [26–28] and in a larger cohort of the MoBa study [11].
Socioeconomic status is an important modifier of diet, health and pregnancy outcome and has been associated with a number of congenital anomalies, stillbirth, preterm birth, birthweight and infant death [29–31].
B vitamin status of a folic acid user
Folic acid users were more likely to take additional micronutrient supplements (Table 2) and only a minority (20%) followed the Norwegian recommendation and took folic acid alone (Table 5). As most multivitamin supplements on the Norwegian market contain folic acid (0.2 mg), vitamin B12 (1–2 μg), vitamin B6 (1.5–2 mg) and vitamin B2 (1.6–2 mg), this pattern was reflected in maternal plasma B vitamin levels. As a group, folic acid users had higher levels of plasma folate, cobalamin, PLP and riboflavin and conversely lower levels of their metabolic markers tHcy (folate and cobalamin) and HK (PLP), indicating a better functional B vitamin status (Table 3).
The blood folate concentration sufficient for prevention of NTDs is not known, but incidence of NTDs has been shown to be inversely associated with folate status [32]. An increased intake of food folate and of folic acid in particular, is known to increase blood folate levels [33, 34]. Similar relations according to intake of food and supplements are observed for other B vitamins [35]. Accordingly, we observed significantly higher plasma B vitamin levels in women who reported taking folic acid every week from 4 weeks prior to conception through pregnancy week 18, compared to those who used folic acid for shorter intervals and non-users (Table 4).
In a multiple linear regression model, the strongest predictor of plasma B vitamin status in pregnancy week 18 was folic acid user status, followed by current daily smoking, parity and age (Table 6). This observation agrees with the reported effect of smoking [36], parity and maternal age [37] on B vitamin status.
Biochemical considerations
Pregnancy induced physiological changes like hemodilution, altered renal function, hormonal status and binding-protein concentrations, which are known to affect plasma B vitamin levels and the relation to their specific metabolic markers [38, 39]. We observed an inverse correlation between folate and tHcy as expected, and weak, but still significant, correlations between cobalamin and tHcy/MMA, and between PLP and HK (Figure 1). MMA is considered a sensitive marker of functional cobalamin status, but the reliability during pregnancy is questioned [38, 40]. We found a moderate, inverse relation between cobalamin and MMA also in early pregnancy (Figure 1). Data on the relation between PLP and HK in pregnant women has not been previously published, but we observed a similar relation in pregnant women (Figure 1) as has been previously observed in cardiovascular patients [41].
CONCLUDING REMARKS
Being a folic acid user during the periconceptional period is associated not only with a better socioeconomic status, but also with a higher intake of several micronutrients and a significantly better plasma B vitamin status. The interpretation of epidemiological data based on maternal reported intake of folic acid supplements during pregnancy, should take into account the numerous nutritional implications, additional to higher blood folate levels, of being a folic acid user.
Acknowledgments
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no. NO-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no. 151918/S10). We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
Abbreviations
- MoBa
the Norwegian Mother and Child Cohort
- tHcy
total homocysteine
- MMA
methylmalonic acid
- HK
3-hydroxykynurenine
- PLP
pyridoxal 5′-phosphate
Footnotes
Conflict of Interest Declaration: No potential conflict of interest relevant to this article was reported
References
- 1.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 2.Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 3.Berry RJ, Li Z, Erickson JD, Li S, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341:1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 4.Shaw GM, Schaffer D, Velie EM, Morland K, Harris JA. Periconceptional vitamin use, dietary folate, and the occurrence of neural tube defects. Epidemiology. 1995;6:219–226. doi: 10.1097/00001648-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. Jama. 1993;269:1257–1261. [PubMed] [Google Scholar]
- 6.Folic acid for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626–631. doi: 10.7326/0003-4819-150-9-200905050-00009. [DOI] [PubMed] [Google Scholar]
- 7.Botto LD, Lisi A, Robert-Gnansia E, Erickson JD, et al. International retrospective cohort study of neural tube defects in relation to folic acid recommendations: are the recommendations working? BMJ. 2005;330:571. doi: 10.1136/bmj.38336.664352.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhaulakhandi DB, Rohilla S, Rattan KN. Neural tube defects: review of experimental evidence on stem cell therapy and newer treatment options. Fetal Diagn Ther. 2010;28:72–78. doi: 10.1159/000318201. [DOI] [PubMed] [Google Scholar]
- 9.Daltveit AK, Vollset SE, Lande B, Oien H. Changes in knowledge and attitudes of folate, and use of dietary supplements among women of reproductive age in Norway 1998–2000. Scand J Public Health. 2004;32:264–271. doi: 10.1080/14034940310019515. [DOI] [PubMed] [Google Scholar]
- 10.Trends in folic acid supplement intake among women of reproductive age - California, 2002–2006. MMWR Morb Mortal Wkly Rep. 2007;2007/56:1106–1109. [PubMed] [Google Scholar]
- 11.Nilsen RM, Vollset SE, Gjessing HK, Magnus P, et al. Patterns and predictors of folic acid supplement use among pregnant women: the Norwegian Mother and Child Cohort Study. Am J Clin Nutr. 2006;84:1134–1141. doi: 10.1093/ajcn/84.5.1134. [DOI] [PubMed] [Google Scholar]
- 12.Savage DG, Lindenbaum J, Stabler SP, Allen RH. Serum methylmalonic acid and total homocysteine in the diagnosis of deficiencies of cobalamin and folate. Am J Med. 1994;96:239–246. doi: 10.1016/0002-9343(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 13.Midttun O, Ulvik A, Ringdal Pedersen E, Ebbing M, et al. Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J Nutr. 2010;141:611–617. doi: 10.3945/jn.110.133082. [DOI] [PubMed] [Google Scholar]
- 14.Magnus P, Irgens LM, Haug K, Nystad W, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 15.Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, et al. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol. 2006;21:619–625. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsen RM, Vollset SE, Monsen AL, Ulvik A, et al. Infant birth size is not associated with maternal intake and status of folate during the second trimester in Norwegian pregnant women. J Nutr. 2010;140:572–579. doi: 10.3945/jn.109.118158. [DOI] [PubMed] [Google Scholar]
- 17.O’Broin S, Kelleher B. Microbiological assay on microtitre plates of folate in serum and red cells. J Clin Pathol. 1992;45:344–347. doi: 10.1136/jcp.45.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelleher BP, Broin SD. Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J Clin Pathol. 1991;44:592–595. doi: 10.1136/jcp.44.7.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baril L, Carmel R. Comparison of radioassay and microbiological assay for serum folate, with clinical assessment of discrepant results. Clin Chem. 1978;24:2192–2196. [PubMed] [Google Scholar]
- 20.Windelberg A, Arseth O, Kvalheim G, Ueland PM. Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography-mass spectrometry. Clin Chem. 2005;51:2103–2109. doi: 10.1373/clinchem.2005.053835. [DOI] [PubMed] [Google Scholar]
- 21.Midttun O, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:1371–1379. doi: 10.1002/rcm.4013. [DOI] [PubMed] [Google Scholar]
- 22.R Development Core Team. R a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 23.Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 24.O’Broin JD, Temperley IJ, Scott JM. Erythrocyte, plasma, and serum folate: specimen stability before microbiological assay. Clin Chem. 1980;26:522–524. [PubMed] [Google Scholar]
- 25.Hannisdal R, Ueland PM, Eussen SJ, Svardal A, Hustad S. Analytical recovery of folate degradation products formed in human serum and plasma at room temperature. J Nutr. 2009;139:1415–1418. doi: 10.3945/jn.109.105635. [DOI] [PubMed] [Google Scholar]
- 26.McDonnell R, Johnson Z, Doyle A, Sayers G. Determinants of folic acid knowledge and use among antenatal women. J Public Health Med. 1999;21:145–149. doi: 10.1093/pubmed/21.2.145. [DOI] [PubMed] [Google Scholar]
- 27.Carmichael SL, Shaw GM, Yang W, Laurent C, et al. Correlates of intake of folic acid-containing supplements among pregnant women. Am J Obstet Gynecol. 2006;194:203–210. doi: 10.1016/j.ajog.2005.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong-Van den Berg LT, Hernandez-Diaz S, Werler MM, Louik C, Mitchell AA. Trends and predictors of folic acid awareness and periconceptional use in pregnant women. Am J Obstet Gynecol. 2005;192:121–128. doi: 10.1016/j.ajog.2004.05.085. [DOI] [PubMed] [Google Scholar]
- 29.James WP, Nelson M, Ralph A, Leather S. Socioeconomic determinants of health. The contribution of nutrition to inequalities in health. BMJ. 1997;314:1545–1549. doi: 10.1136/bmj.314.7093.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. 2010;39:263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Mortensen LH, Helweg-Larsen K, Andersen AM. Socioeconomic differences in perinatal health and disease. Scand J Public Health. 2011;39:110–114. doi: 10.1177/1403494811405096. [DOI] [PubMed] [Google Scholar]
- 32.Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995;274:1698–1702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- 33.Cuskelly GJ, McNulty H, Scott JM. Effect of increasing dietary folate on red-cell folate: implications for prevention of neural tube defects. Lancet. 1996;347:657–659. doi: 10.1016/s0140-6736(96)91205-2. [DOI] [PubMed] [Google Scholar]
- 34.McNulty B, Pentieva K, Marshall B, Ward M, et al. Women’s compliance with current folic acid recommendations and achievement of optimal vitamin status for preventing neural tube defects. Hum Reprod. 2011;26:1530–1536. doi: 10.1093/humrep/der078. [DOI] [PubMed] [Google Scholar]
- 35.van Guldener C, Stehouwer CD. Homocysteine-lowering treatment: an overview. Expert Opin Pharmacother. 2001;2:1449–1460. doi: 10.1517/14656566.2.9.1449. [DOI] [PubMed] [Google Scholar]
- 36.Ulvik A, Ebbing M, Hustad S, Midttun O, et al. Long- and short-term effects of tobacco smoking on circulating concentrations of B vitamins. Clin Chem. 2010;56:755–763. doi: 10.1373/clinchem.2009.137513. [DOI] [PubMed] [Google Scholar]
- 37.Goedhart G, van der Wal MF, van Eijsden M, Bonsel GJ. Maternal vitamin B-12 and folate status during pregnancy and excessive infant crying. Early Hum Dev. 2010;87:309–314. doi: 10.1016/j.earlhumdev.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 38.Metz J, McGrath K, Bennett M, Hyland K, Bottiglieri T. Biochemical indices of vitamin B12 nutrition in pregnant patients with subnormal serum vitamin B12 levels. Am J Hematol. 1995;48:251–255. doi: 10.1002/ajh.2830480409. [DOI] [PubMed] [Google Scholar]
- 39.Bruinse HW, van den Berg H. Changes of some vitamin levels during and after normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 1995;61:31–37. doi: 10.1016/0028-2243(95)02150-q. [DOI] [PubMed] [Google Scholar]
- 40.Murphy MM, Molloy AM, Ueland PM, Fernandez-Ballart JD, et al. Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. J Nutr. 2007;137:1863–1867. doi: 10.1093/jn/137.8.1863. [DOI] [PubMed] [Google Scholar]
- 41.Midttun O, Ulvik A, Ringdal Pedersen E, Ebbing M, et al. Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J Nutr. 2011;141:611–617. doi: 10.3945/jn.110.133082. [DOI] [PubMed] [Google Scholar]