Abstract
Urinary sugars excretion has been proposed as a potential biomarker for intake of sugars. In this study we compared two analytical methods [gas chromatography (GC) and enzymatic reactions – UV absorption] for quantifying urinary fructose and sucrose using 24-hour urine samples from a randomized cross-over controlled feeding study. All samples were successfully quantified by the GC method; however 21% and 1.9% of samples were below the detection limit of the enzymatic method for sucrose and fructose, respectively. While the correlation between the two methods was good for fructose (Pearson correlation 0.71), the correlation was weak for sucrose (Pearson correlation 0.27). We favor the GC method due to its better sensitivity, simplicity, and the ability to quantify fructose and sucrose directly in the same run. Of the 106 samples from 53 participants with complete urine collection after two study diets, 24-hour urinary fructose excretion was significantly associated with fructose intake. The sum of 24-hour urinary fructose and sucrose was significantly associated with total sugars consumption. However, variation in intakes of sugars explained only a modest amount of variation in urinary sugars excretion. In the unadjusted models, fructose intake explained 24.3% of urinary fructose excretion; and intake of total sugars 16.3% of the sum of urinary fructose and sucrose. The adjusted models explained 44.3% of urinary fructose excretion and 41.7% of the sum of urinary fructose and sucrose. Therefore, we caution using these biomarkers to predict sugars consumption before other factors that determine urinary sugars excretion are understood.
Keywords: Fructose/urine, Sucrose/urine, Diet, Dietary Sucrose, Gas chromatography, Biomarker, Human
1. Introduction
Consumption of sugars, particularly added sugars, has increased substantially in the US diet in the past several decades [1, 2]. Intake of sugars, as per capita intake of caloric sweeteners, has increased 39% between 1950-59 and 2000 [3]. In 2005, the estimated daily added sugars consumption per person averaged 30 teaspoons [4]. Major sources of added sugars in the American diet are sodas and energy/sports drinks, sugar-sweetened fruit drinks, dairy- and grain-based desserts, and candy [5]. In 2005 - 2008 among US adults ≥ 20 years of age, it is estimated that 50% and 33% of white men and women, 73% and 65% of black men and women, and 76% and 62% of Mexican American men and women, respectively, consumed more than the recommended limit of 36 oz (4.5 8-oz servings) sugar-sweetened beverages per week. Approximately two thirds of white and black men and women and half of all Mexican American men and women consumed more than the recommended limit of 2.5 servings of sweets and bakery desserts per week [6]. In observational studies, higher consumption of sugars has been associated with obesity and a range of chronic diseases such as metabolic syndrome, type 2 diabetes, cardiovascular disease and cancer [7-9]. However, other studies showed no association [10-12]. Such inconsistency may be the result of measurement error associated with commonly used dietary assessment tools such as 24-hour dietary recalls (HDR) and food frequency questionnaires (FFQ). Furthermore, error may also be introduced by the varied terminology that exists to define dietary sugars and the limited information from food composition databases to quantify intake of sugars [13, 14].
It has been shown that the correlation between intakes measured by FFQ and those by a more comprehensive 7-day dietary record is particular poor for socially undesirable foods [15]. Objective biomarkers, such as doubly labeled water and urinary nitrogen, have shown the high prevalence and extent of underreporting of both energy and protein intake [16, 17]. Therefore, it is highly likely that consumption of sugars has also been underreported. Several studies have evaluated the potential of urinary sugars excretion as a biomarker of sugars consumption [18-23]. These studies have observed statistically significant associations between intake of sugars and urinary sucrose and/or fructose. Recently urinary fructose and sucrose, biomarkers calibrated in a feeding study [19], was applied to the Observing Protein and Energy Nutrition (OPEN) study to assess measurement error (ME) in self-reported intake of sugars [24]. The authors concluded that “Both the FFQ and 24HDR were found to be biased; hence, incorporation of the sugars biomarker in calibration studies within the cohorts may be necessary to more reliably estimate associations of sugars and disease.” The authors also pointed out that “the biomarker ME parameters used here were estimated on the basis of only one feeding study with a limited sample size. More such studies, preferably conducted across different populations, are needed to investigate the stability of the parameters used in this analysis.”
In these previous studies, an enzymatic method was used to determine urinary sugars content. We found this method cumbersome and having high inter-batch variation, especially for sucrose. Gas chromatographic analysis of dietary mono- and disaccharides in human blood and urine had been previously described in the literature [25]. We and others routinely use GC to measure probe sugars in urine to test for intestinal permeability [26, 27]. We therefore adapted and modified the GC method as an alternative to quantify urinary fructose and sucrose. In this study, we measured the 24-hour urinary fructose and sucrose content of samples collected on the last day of each feeding period of a randomized cross-over controlled feeding study of low- and high-glycemic load (GL) diets. We compared the results of the two analytical methods and evaluated the relationship between the sum of urinary sucrose & fructose and intake of total sugars as in the OPEN study. We also examined the relationship between urinary fructose and total fructose intake. Our hypotheses were (1) the GC method is better than the enzymatic method in measuring urinary sugars content; and (2) urinary sugars excretion is significantly associated with dietary sugars intake in the Carbohydrates and Related Biomarkers (CARB) study.
2. Methods and materials
2.1. Controlled feeding study design, participants and study diets
Urine specimens for the analytical methods comparison were from a previous study, the Carbohydrates and Related Biomarkers (CARB) Study. The CARB study, conducted between June 2006 and July 2009, was a randomized, cross-over, feeding study testing low- and high-GL diets as described previously [28]. Participants were healthy, nonsmoking men (n = 41) and women (n = 41), aged 18–45 y. They were block-randomized on body mass index (BMI > 18.5 to <25.0 kg/m2 and ≥ 28.0 to 40.0 kg/m2), sex and race/ethnicity (African American, Hispanic, and all others) using a cross-over design where each participant received two isocalorically controlled experimental diets (low GL and high GL) in random order. Participants consumed each diet for 28 days with 28-d washout period in between when they resumed their habitual diet. Participants completed 3-d diet records to estimate their habitual energy intake as part of baseline data collection. These data, together with individually calculated results from the Mifflin equation [29], was used to estimate each person’s energy needs during the feeding study. Within each intervention diet, we created a 7-d menu rotation using ProNutra (version 3.2, Viocare). The two diets were designed to be identical in macronutrient composition (15.0% energy from protein, 30.0% energy from fat, and 55.0% energy from carbohydrate); the diets differed only by GL (125 vs. 250 for the low- and high-GL diets, respectively) and fiber (55 and 28 g/d for the low- and high-GL diets, respectively). Participant weight was monitored thrice weekly and energy adjustments were made (in 200-kcal increments) as needed to maintain baseline weight. The energy intake on the last day of intervention ranged from 1357 to 3643 kcal with a mean of 2549 kcal. Consequently, the absolute amount of sugars intake varied from person to person, although for each participant during each of the 28-d trial diet periods, sugars intake stayed consistent. This study provided a good range of intakes of sugars in a controlled, experimental setting. Sugars content of the diets was calculated using ProNutra®: Metabolic Diet Study Management System (Viocare Technologies, Inc. Princeton, NJ), which uses the USDA Nutrient Database for Standard Reference (version 18), and the Nutrition Data System for Research (version 2005, Nutrition Coordinating Center, University of Minnesota). Study procedures were approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (FHCRC) and all participants provided written, informed consent.
2.2. Urine Collection, Handling, and Storage
Study participants were instructed to collect a 24-hour urine sample on the last day of each of the two feeding periods. The first urine void in the morning was discarded and all urine voids thereafter were collected for the next 24 hours into bottles provided to the participants. Participants were instructed to refrigerate all urine collections without any addition of preservatives. Any incomplete or lost void was recorded by participants. Completed urine collections were brought to the FHCRC clinic the next day. Aliquots of urine samples were stored at −80°C. To determine compliance with 24-hour urine collection, we used the intra-individual variation of body-weight related 24-hour creatinine excretion [30]. Creatinine excretion in urine was measured by a commercial laboratory using a colorimetric and kinetic methodology. The urine collection was considered complete if the coefficient of variation (CV) of intra-individual creatinine excretion was 15% or less [30]. Of the 82 enrolled CARB study participants, 77 had urine collection for both diet periods. One participant’s urine from one diet had extremely high sugar content for unknown reasons. Therefore, this participant was excluded from further data analysis. 18 participants recorded incomplete urine collection for at least one of the two samplings. 13 of these did not pass the creatinine criterion. Overall 53 subjects had complete 24-hour urine collections using the CV of intra-individual creatinine excretion ≤15% criterion.
2.3. Urinary fructose and sucrose analysis using enzymatic method
Sucrose and fructose contents of 24-h urine samples were analyzed with a test kit for enzymatic analysis of sucrose, fructose and glucose (Sucrose/D-Glucose/D-Fructose; Boehringer Mannheim, R-Biopharm, Enzymatic BioAnalysis/Food Analysis) as described previously [19, 23], using a UV reader (Spectro-Max) to measure light absorbance (at 340nm). In an attempt to increase the throughput of the assay, we used 96-well plate format instead of cuvettes. The volume of urine used was 120 μl. The limit of quantitation (LOQ) for both fructose and sucrose was 0.005 mg/ml. A calibration curve was included on each plate, and samples were run in singlet.
2.4. Urinary fructose and sucrose analysis using gas chromatography
Urine samples were thawed, vortexed, and centrifuged to remove any sediment. 125 μl urine or standard mix and 5 μl internal standard (inositol and turanose at 10 mg/ml each) were added to a 2.0 ml eppendorf tube. A 6-level standard curve was constructed with sucrose and fructose at 0.005, 0.01, 0.02, 0.06, 0.19, and 0.56 mg/ml each. Samples were evaporated under nitrogen at 70°C to dryness. 200 μl oxime solution (25 mg/ml hydroxylamine hydrochloride in pyridine) was added to each sample. After incubating at 70°C for 1 hour, samples were centrifuged at 1,090 × g for 5 min. 100 μl supernatant was added to 1.5 ml eppendorf tube containing 100 μl silylating solution [1-(trimethysilyl)imidazole]. Samples were then incubated at 70°C for 30 minutes and evaporated under nitrogen at 70°C. 50 μl of pyridine saturated with hydroxylamine hydrochloride was added to each sample, followed by 200 μl hexane. Samples were vortexed and centrifuged at 1,300 × g for 10 min. 100 μl of the upper phase was transferred to an eppendorf tube and evaporated to dryness under nitrogen at 70°C. Samples were then reconstituted in 100 μl hexane, vortexed and centrifuged again at 1,300 × g for 10 min. 50 μl was transferred for GC analysis. The LOQ for both sugars was 0.001 mg/ml.
GC 7890 and column DB-1701 (30m × 0.250mm × 0.25 μm with 10 m guard column) were from Agilent Technologies (Santa Clara, CA). GC parameters were as follows: injector temperature 250°C, detector temperature 280°C, and initial oven temperature 180 °C, 5 min hold, 10°C/min to 240°C, 5°C/min to 260°C, 3.5°C/min to 274°C, hold 7 min. 2ul injection volume, and 1:50 split ratio. The carrier gas was helium at constant pressure of 20 psi. Hydrogen (35ml/min) and air (350ml/min) were used for the flame ionization detection. Makeup gas was nitrogen at 35 ml/min.
To eliminate the possibility that the GC method was measuring co-eluting unknown factor(s) besides fructose and sucrose, we tested other dietary sugars including glucose, lactose, maltose, galactose, and mannitol. All had different and distinct retention time from fructose and sucrose (data not shown).
2.5. Statistical analyses
Calculation of total sugars in the diet included monosaccharides (galactose, glucose, and fructose) and disaccharides (sucrose, lactose, and maltose). The dietary fructose values included both intrinsic and extrinsic fructose and were calculated as fructose plus half of sucrose. Excretion was measured as fructose and sucrose excretion per 24-hour urine. Scatter plots, Pearson correlation, and paired t-test were used to compare the enzymatic and chromatographic methods for measuring fructose and sucrose in urine. Data were presented as means ± SD.
Urinary sugars excretion values of the 53 participants with complete 24-h urine collection were natural log transformed prior to further analysis to correct the right skew of the data. To account for correlated data due to two measurements from each participant, we used Generalized Estimating Equation (GEE) with robust standard errors to evaluate the relationship between intake of sugars and excretion. During each of the 28-day trial diet periods, intake of sugars for each participant varied little from day to day. Previous study also has shown that sugars appear in urine less than one hour after oral administration [25], we therefore chose last day fructose intake or last day intake of total sugars as predictor variable. In the adjusted models, we controlled for age, gender, and total percent body fat measured by dual-energy X-ray absorptiometry (DEXA). Participants were recruited based on their BMI (18.5 - 25.0 kg/m2 and 28.0 - 40.0 kg/m2), however their percent body fat as measured by DEXA had a relative normal distribution. Therefore percent body fat was adjusted as a continuous variable. β-coefficients, 95% confidence intervals, and p-values were presented. To estimate the contribution of intake of sugars on urinary sugars excretion, R2 from linear regression models was used. For subgroup analysis by diet (high and low GL), and by BMI (normal weight and overweight/obese), linear regression models were used.
All statistical analyses were performed using Stata/SE 12.1 (College Station, TX). All statistical tests are two-sided with significance level set at 0.05.
3. Results
3.1. Comparison of two analytical methods for urinary sugars measurement
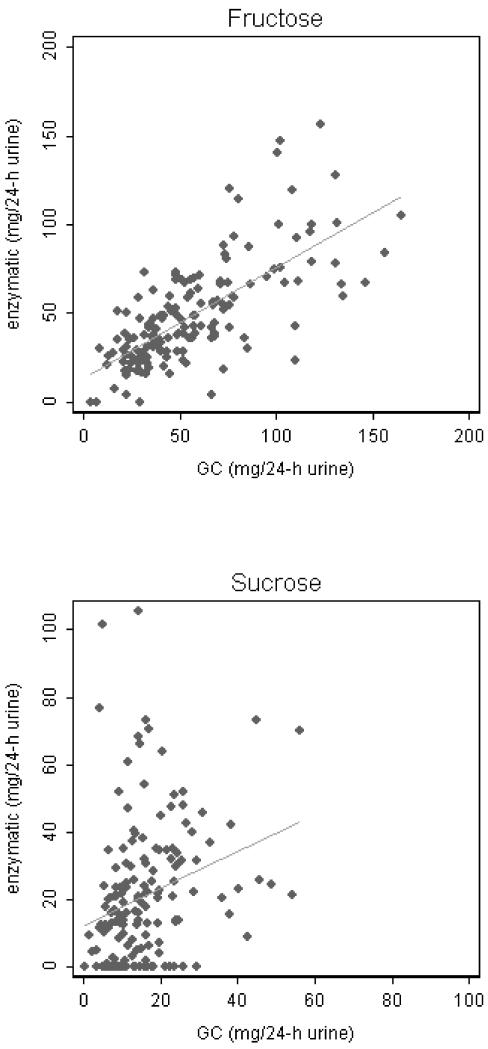
Previous studies exploring the utility of urinary sugars as intake biomarkers used an enzymatic method. Besides the enzymatic method, we also used a GC method to measure urinary fructose and sucrose. In spike-recovery experiments of the GC method, fructose and sucrose standards were spiked into test urine samples at levels similar to what we found in urine. The average recovery was 95 ± 20% and 110 ± 6% for fructose and sucrose, respectively. The two methods were compared using 157 samples from 81 participants, 5 of which had a urine sample from only one of the two diets. Of the 157 study samples, the enzymatic method failed to detect sucrose in 33 and fructose in 3 samples. However, all samples were successfully measured by the GC method. Scatter plots (Figure 1) showed weak correlation of urinary sucrose between the two analytical methods (Pearson correlation 0.27, p < 0.0001); however the correlation for fructose was substantial (Pearson correlation 0.71, p = 0.0007). The Lowess smoother (not shown) flattened after fructose excretion reached about 100 mg per 24-h, indicating possible saturation of the enzymatic method at higher concentrations of fructose. Of the 154 samples with quantifiable urinary fructose levels from both methods, values from the GC method tended to be higher with a mean difference of 7.37 mg per 24-h urine [95% CI: (3.52, 11.23), p = 0.0002; Table 1]. Of the 124 samples with quantifiable urinary sucrose levels from both methods, values from the GC method tended to be lower with a mean difference of 9.91 mg per 24-h urine [95% CI: (6.27, 13.56), p < 0.00005].
Fig. 1.
Scatter plot of 24-h urinary sucrose and fructose contents in 157 samples measured by enzymatic and GC methods. Linear regression lines are shown. Pearson correlation 0.27 for sucrose and 0.71 for fructose.
Table 1.
24-hour urinary fructose and sucrose (mg/24-h) measured by the GC and enzymatic methods
| Urinary sugar |
GC methoda | Enzymatic methoda |
Difference | 95% CI | p-valueb |
|---|---|---|---|---|---|
| Fructosec | 57.19 ± 32.89 | 49.82 ± 28.51 | 7.37 | (3.52, 11.23) | 0.0002 |
| Sucrosed | 16.89 ± 10.75 | 26.80 ± 20.04 | −9.91 | (−6.27, −13.56) | <0.00005 |
Values are means ± SD
paired t-test
n = 154, samples quantifiable by both methods
n = 124, samples quantifiable by both methods
A pooled urine sample as well as a negative control was included with each batch of study samples to monitor assay performance throughout the study. For the GC method, intra assay CV was 2.21% and 1.98% for fructose and sucrose respectively; and inter assay CV 7.32% and 3.20%. The enzymatic method had intra-assay CVs of 5.33% and 6.65% for fructose and sucrose respectively. It had an inter-assay CV of 4.58% for fructose; however, sucrose was below the limit of quantitation in the quality control urine. Our data suggest that the GC method is preferable for the quantification of urinary sucrose and fructose. Subsequent analyses are therefore based on the results from the GC method only.
3.2. The relationship between urinary fructose excretion and fructose intake
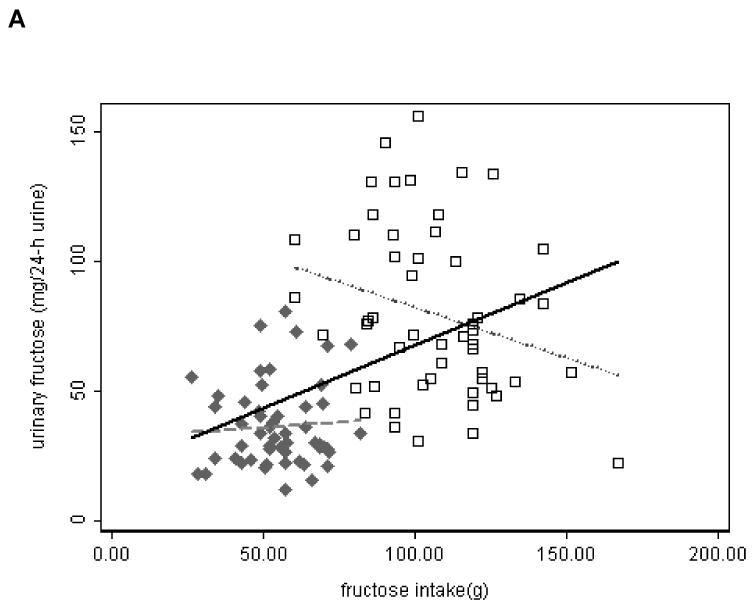
Table 2 summarizes the characteristics of the 53 participants who had complete 24-hour urine collection on the last day of each of the two study diets. Last day total fructose intake, which was calculated as the sum of fructose and half of sucrose, ranged from 26 g to 167 g with a mean of 80 g (Table 3). A total of 106 data points from the GC method were available for the analysis. In the unadjusted regression model, the natural log transformed 24-h urinary fructose was significantly associated with last day total fructose intake [β = 0.0110, 95% CI: (0.0079, 0.0140), p < 0.0005; table 4]. Therefore, if we compared two groups of people with a 10g difference in daily fructose intake, we would expect 11.62% higher median 24-h urinary fructose in the higher intake group compared to the lower intake group.
Table 2.
Characteristics of 53 study participants who had complete 24-hour urine collection on the last day of each of the two study diets
| Normal weight participantsa BMI (18.5-24.9 kg/m2) |
Overweight/obese participantsb BMI (28.0-40.0 kg/m2) |
|
|---|---|---|
| Age (yr)c | 28.8 ± 6.5 | 34.7 ± 8.8 |
| Male sex [n (%)] | 14 (53.8) | 13 (48.1) |
| Race/ethnicity [n (%)] | ||
| White | 12 (46.1) | 17 (63.0) |
| African American | 2 (7.7) | 5 (18.5) |
| Asian | 6 (23.1) | 0 (0) |
| Other | 6 (23.1) | 5 (18.5) |
| Weight (kg)c | 66.1 ± 7.8 | 96.6 ± 19.3 |
| Body mass index | ||
| (kg/m2)c | 22.2 ± 1.7 | 32.1 ± 3.7 |
| Body fat (%)c, female | 31.4 ± 5.4 | 46.9 ± 5.4 |
| Body fat (%)c, male | 19.4 ± 5.1 | 33.4 ± 5.0 |
n = 26
n = 27
Values are means ± SD
Table 3.
Sugars intake of 53 study participants who had complete urine collections from both study diets
| Total sugars intake (g)a | Fructose intake (g)b | Sucrose intake (g) | |
|---|---|---|---|
| High GL diet | 146 ± 30 (82 - 213) | 55 ± 13 (26 - 82) | 65 ± 15 (27 - 110) |
| Low GL diet | 205 ± 40 (119 - 295) | 106 ± 22 (60 - 167) | 65 ± 13 (28 - 94) |
| Overall | 176 ± 46 (82 - 295) | 80 ± 31 (26 - 167) | 65 ± 14 (27 - 110) |
Values are means ± SD (range)
Total sugars intake was calculated as the sum of dietary monosaccharides (galactose, glucose, and fructose) and disaccharides (sucrose, lactose, and maltose) on the last day of the study diet corresponding to the day of urine collection
Fructose intake included both intrinsic and extrinsic fructose and was calculated as fructose plus half of sucrose on the last day of the study diet corresponding to the day of urine collection
Table 4.
Association between 24-h urinary fructose excretion (natural log transformed) and dietary fructose intake in 53 participants after 2 different diets
| GC method |
||||
|---|---|---|---|---|
| Parameter | β | 95% CI | p | |
| Unadjusted model |
||||
| Fructose intake |
0.0110 | (0.0079, 0.0140) | <0.0005 | |
| Adjusted model |
||||
| Fructose intake |
0.0125 | (0.0094, 0.0156) | <0.0005 | |
| Age | −0.0031 | (−0.0147, 0.0086) | 0.605 | |
| Gender | 0.7005 | (0.4281, 0.9729) | <0.0005 | |
| % body fat | −0.0182 | (−0.0302, −0.0062) | 0.003 | |
Generalized Estimating Equation (GEE) analysis using Stata/SE 12.1
After controlling for age, gender, and percent body fat, the coefficient for fructose intake increased to 0.0125 [95% CI: (0.0094, 0.0156); table 4]. Gender (p < 0.0005) and total percent body fat measured by DEXA (p = 0.003) were also significant predictors of fructose excretion.
The ranges of fructose intake were distinct and narrow for each diet (Figure 2 A). We performed subgroup analyses (high and low GL diets) unadjusted and adjusted for age, gender, and percent body fat. With low GL diet, the natural log transformed 24-h urinary fructose was significantly associated with last day total fructose intake in the unadjusted model [β = −0.0058, 95% CI: (−0.01166, −0.00002), p = 0.049]. All other analyses did not show statistically significant associations of urinary fructose with fructose intake (data not shown).
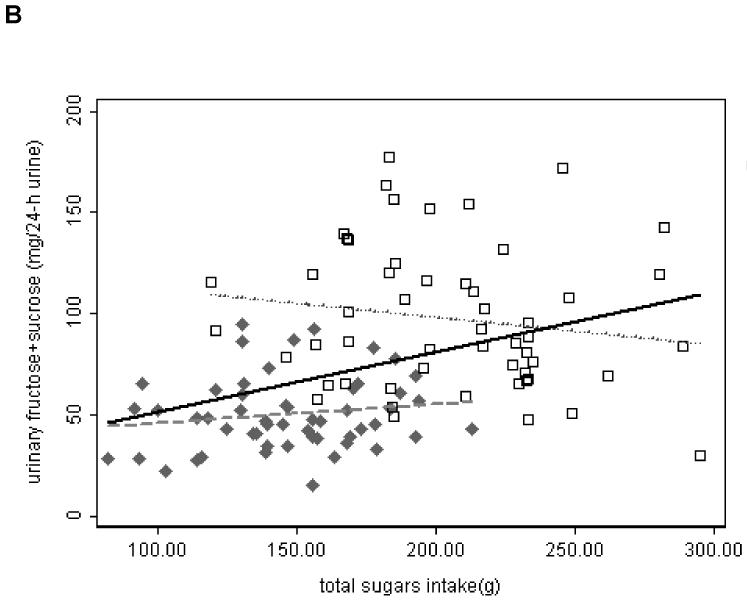
Fig. 2.
Scatter plot of 24-h urinary sugars measured by GC method in 106 samples from 53 participants who had complete urine collection versus intake of sugars. Solid Diamond – samples from high GL diet; Open Square – samples from low GL diet. Linear regression lines are shown. Solid line – overall; dash line – high GL; dot line – low GL.
A. urinary fructose vs. intake of fructose. B. sum of urinary fructose and sucrose vs. intake of total sugars.
We also performed subgroup analyses by BMI (normal weight and overweight/obese). The natural log transformed 24-h urinary fructose was significantly associated with last day total fructose intake in both unadjusted and adjusted models in each BMI group (data not shown).
3.3. The relationship between the sum of urinary fructose & sucrose excretion and intake of total sugars
Last day intake of total sugars ranged from 82 g to 295 g with a mean of 176 g (Table 3). The natural log transformed sum of 24-h sucrose and fructose excretion was significantly associated with last day intake of total sugars (table 5). After adjusting for age, gender, and percent body fat, the β coefficient changed from 0.0054 in the unadjusted model to 0.0077. Therefore, if we compared two groups of people with a 10 g difference of total sugars intake, we would expect median 24-h urinary fructose plus sucrose of the higher intake group to be 5.57% and 8.05% higher than that of the lower intake group in the unadjusted and adjusted models respectively. Gender and total percent body fat measured by DEXA were also significant predictors of the sum of urinary fructose & sucrose (p < 0.0005 for both).
Table 5.
Association between 24-h urinary fructose plus sucrose excretion (natural log transformed) and intake of dietary total sugars in 53 participants after 2 different diets
| GC method |
||||
|---|---|---|---|---|
| Parameter | β | 95% CI | p | |
| Unadjusted model |
||||
| Intake of total sugars |
0.0054 | (0.0034, 0.0074) | <0.0005 | |
| Adjusted model |
||||
| Intake of total sugars |
0.0077 | (0.0057, 0.0098) | <0.0005 | |
| Age | −0.0040 | (−0.0135, 0.0055) | 0.411 | |
| Gender | 0.7201 | (0.4840, 0.9562) | <0.0005 | |
| % body fat | −0.0169 | (−0.0262, −0.0076) | <0.0005 | |
Generalized Estimating Equation (GEE) analysis using Stata/SE 12.1
The ranges of total sugars intake were distinct for each diet (Figure 2 B). We performed subgroup analysis (high and low GL diets) unadjusted and adjusted for age, gender, and percent body fat. With high GL diet, the natural log transformed sum of 24-h urinary fructose and sucrose was significantly associated with last day total sugars intake in the adjusted model [β = 0.0055, 95% CI: (0.0015, 0.0095), p = 0.008]. All other analyses did not show statistically significant association of sum of urinary fructose and sucrose with total sugars intake (data not shown).
We also performed subgroup analyses by BMI (normal weight and overweight/obese). The natural log transformed sum of 24-h urinary fructose and sucrose was significantly associated with last day total sugars intake in both unadjusted and adjusted models in each BMI group (data not shown).
4. Discussion
In this study we compared GC and enzymatic methods to measure urinary fructose and sucrose. There was a statistical significant difference between the means of urinary sugars measured by the two methods. However, it is not uncommon for the absolute quantity of an analyte to differ when different analytical methods are used. Further, the reagent kit for the enzymatic method was designed for food analysis, not for biological samples. Inhibitor or other materials present in urine may interfere with the assay, and non-specificity of the enzymes could have given artificially higher values. For example β-fructosidase hydrolyzes the β-fructosidic bond in not only sucrose but also other glycosides. Nonetheless, the correlation between the two methods was good for fructose, but weak for sucrose. LOQ of the enzymatic method was 0.005 mg/ml and GC method 0.001 mg/ml. The enzymatic method failed to measure sucrose concentration in 33 of the 157 (21%) urine samples. Results of the GC method indicated that 17 of the 33 samples should have been above the LOQ of the enzymatic method. The enzymatic method relies on three different enzyme reactions, which can introduce variation at several steps in the assay. In contrast, the GC method is relatively simple, measures multiple sugars in the same run, and reads the analytes’ concentration directly off the standard curve. In addition, the GC method has better sensitivity and the starting urine volume can be potentially increased to accommodate samples with low concentrations of sugars. Thus, our hypothesis of GC as a better method is supported by the data presented in this study.
We showed the utility of this GC method of measuring urinary fructose and sucrose in a controlled feeding study, the CARB study. Means ± SD urinary recovery (as percentage of intake) was 0.024 ± 0.013% (range 0.001 - 0.094%) and 0.075 ± 0.039% (range 0.013% - 0.211%) for sucrose and fructose respectively of the 106 samples from the 53 participants who had complete urine collection. The sum of urinary sucrose and fructose as percentage of total sugars intake ranged from 0.010% to 0.097% with a mean of 0.022% (SD 0.043%). Further analysis of the 106 samples indicated that 24-hour urinary fructose excretion was statistically significantly associated with fructose intake. The sum of 24-hour urinary fructose and sucrose was statistically significantly associated with total sugars consumption. The association was attenuated in subgroup analyses stratified by high/low GL diets presumably due to narrow ranges of sugars intake in each diet. The results from the overall analyses of the 106 samples and from subgroup analyses stratified by BMI confirmed our hypothesis that urinary sugars excretion is significantly associated with dietary sugars intake and were consistent with those previously reported by Tasevska et al [19].
Despite the significant positive association between urinary sugars excretion and intake of dietary sugars, fructose intake alone explained only 24.3% of urinary fructose excretion, and intake of total sugars explained only 16.3% of the sum of urinary fructose and sucrose in our CARB study samples. These are comparable to other dietary biomarkers such as serum folic acid and carotenoids [31, 32], but substantially lower than those reported by Tasevska et al [19, 22]. In their “dose-response study”, intake of total sugars explained 74% of the variability in urinary sugars (sum of sucrose and fructose) and in their “habitual varying diet study”, 72% [19]. In another study, the authors reported that extrinsic sugars explained 64% of the variability in urinary sugars in their “habitual varying diet study” subjects [22]. There are several important differences between our study and theirs. Our study participants were free-living, reporting to the FHCRC Prevention Center daily, whereas the participants in the studies of Tasevska et al. lived in the volunteer suite of the Medical Research Council Dunn Human Nutrition Unit — a more controlled environment. Our statistical models included data from a very heterogeneous group of 53 people. Their studies had a smaller sample size with 12 male participants in the dose-response study and 13 (7 males and 6 females) in the habitual varying diet study. It is possible their participants were more homogeneous in terms of currently unknown factors that determine urinary sugars excretion. In addition, for regression analyses they used the mean values of multiple day dietary intake and urinary excretion while we used single individual values (last day of each of the 28-d study diet periods).
We used CV of intra-individual creatinine excretion ≤15% as a criterion for completeness of 24-h urine collection. Since creatinine was used as within person criterion, we were not concerned with endogenous factors such as age, gender and muscularity that affect creatinine elimination. Meat content is the primary dietary factor on urinary creatinine elimination. In our study, meat source and amount remained the same for each calorie level, i.e. for each participant. 13 (72%) of the 18 participants who recorded incomplete urine collection for at least one of the two time points did not pass the creatinine criterion, indicating using creatinine criterion was adequate. Additional 10 participants were eliminated by the creatinine criterion. This could have been attributed to mis-reporting by study participants or we could have excluded participants unnecessarily.
There are several strengths of our study. Participants consumed a variety of food in a controlled, experimental setting. The isocaloric study diets provided a good range of intakes of sugars. Participants represented a diverse population with a range of adiposity. We used two analytical methods to measure urinary sugars contents and chose the results from the preferred method, GC, for regression analysis. The limitation of our study is that we had a single 24-h urine collection on the last day of each of the 28-d study diets. Therefore we were not able to evaluate the intra-person variation of urinary sugars excretion while consuming the same amount of sugars. However this reflects the reality we often face using biomarkers in observational studies in which we may have only single day or spot urine collection. Another limitation is that urine specimens were not kept cold while participants transported them from their homes to FHCRC clinic. Due to lack of preservative, there is a chance of sugars degradation during transportation. However, since all participants lived nearby, the duration samples were not in cold storage was limited.
In conclusion, we used a GC method to further evaluate the utility of urinary sucrose and fructose as biomarkers of sugars intake. Compared to the enzymatic method used previously, the GC method was more sensitive and less cumbersome, lending itself well to measuring large numbers of samples from observational studies. However, very small amounts of fructose and sucrose (0.075% and 0.024% of intake respectively) were excreted in urine and intake of sugars only explained a modest amount of urinary fructose and sucrose excretion. Even in our adjusted models, which controlled for age, gender, and percent body fat, only 44.3% of the variation in urinary fructose and 41.7% of the variation in the sum of urinary fructose and sucrose were explained. This suggests that other factors are contributing to the variation in urinary sugars excretion. More studies are needed to understand what other factors determine the excretion of sugars and to establish a better prediction model that includes these other determinants.
Acknowledgment
This work was supported by grants U54 CA116847 and P30 CA015704 from the NIH National Cancer Institute and by Fred Hutchinson Cancer Research Center.
Abbreviations
- GC
gas chromatography
- HDR
- hour dietary recalls
- FFQ
food frequency questionnaires
- OPEN
Observing Protein and Energy Nutrition
- ME
measurement error
- GL
glycemic load
- CARB
Carbohydrates and Related Biomarkers
- BMI
body mass index
- FHCRC
Fred Hutchinson Cancer Research Center
- CV
coefficient of variation
- LOQ
limit of quantitation
- GEE
Generalized Estimating Equation
- DEXA
dual-energy X-ray absorptiometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Krebs-Smith SM. Choose beverages and foods to moderate your intake of sugars: measurement requires quantification. J Nutr. 2001;131:527S–35S. doi: 10.1093/jn/131.2.527S. [DOI] [PubMed] [Google Scholar]
- [2].Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape journal of medicine. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- [3].Agriculture USDo, Communications Oo Agriculture Fact Book. 2000 Internet: http://www.usda.gov/documents/factbook2000.pdf. [Google Scholar]
- [4].Wells HF, Buzby JC. Dietary Assessment of Major Trends in U.S. Food Consumption, 1970-2005. Economic Information Bulletin No (EIB-33) 2008 [Google Scholar]
- [5].Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. Journal of the American Dietetic Association. 2010;110:1477–84. doi: 10.1016/j.jada.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes care. 2010;33:2477–83. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- [9].Aune D, Chan DS, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, et al. Dietary fructose, carbohydrates, glycemic indices and pancreatic cancer risk: a systematic review and meta-analysis of cohort studies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:2536–46. doi: 10.1093/annonc/mds076. [DOI] [PubMed] [Google Scholar]
- [10].Bao Y, Stolzenberg-Solomon R, Jiao L, Silverman DT, Subar AF, Park Y, et al. Added sugar and sugar-sweetened foods and beverages and the risk of pancreatic cancer in the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr. 2008;88:431–40. doi: 10.1093/ajcn/88.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cust AE, Slimani N, Kaaks R, van Bakel M, Biessy C, Ferrari P, et al. Dietary carbohydrates, glycemic index, glycemic load, and endometrial cancer risk within the European Prospective Investigation into Cancer and Nutrition cohort. Am J Epidemiol. 2007;166:912–23. doi: 10.1093/aje/kwm161. [DOI] [PubMed] [Google Scholar]
- [12].Parnell W, Wilson N, Alexander D, Wohlers M, Williden M, Mann J, et al. Exploring the relationship between sugars and obesity. Public health nutrition. 2008;11:860–6. doi: 10.1017/S1368980007000948. [DOI] [PubMed] [Google Scholar]
- [13].Sigman-Grant M, Morita J. Defining and interpreting intakes of sugars. Am J Clin Nutr. 2003;78:815S–26S. doi: 10.1093/ajcn/78.4.815S. [DOI] [PubMed] [Google Scholar]
- [14].Yeung EH, Saudek CD, Jahren AH, Kao WH, Islas M, Kraft R, et al. Evaluation of a novel isotope biomarker for dietary consumption of sweets. Am J Epidemiol. 2010;172:1045–52. doi: 10.1093/aje/kwq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. International journal of epidemiology. 1989;18:858–67. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- [16].Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158:1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
- [17].Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Horn LV, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. Am J Epidemiol. 2008;167:1247–59. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- [18].Luceri C, Caderni G, Lodovici M, Spagnesi MT, Monserrat C, Lancioni L, et al. Urinary excretion of sucrose and fructose as a predictor of sucrose intake in dietary intervention studies. Cancer Epidemiol Biomarkers Prev. 1996;5:167–71. [PubMed] [Google Scholar]
- [19].Tasevska N, Runswick SA, McTaggart A, Bingham SA. Urinary sucrose and fructose as biomarkers for sugar consumption. Cancer Epidemiol Biomarkers Prev. 2005;14:1287–94. doi: 10.1158/1055-9965.EPI-04-0827. [DOI] [PubMed] [Google Scholar]
- [20].Bingham S, Luben R, Welch A, Tasevska N, Wareham N, Khaw KT. Epidemiologic assessment of sugars consumption using biomarkers: comparisons of obese and nonobese individuals in the European prospective investigation of cancer Norfolk. Cancer Epidemiol Biomarkers Prev. 2007;16:1651–4. doi: 10.1158/1055-9965.EPI-06-1050. [DOI] [PubMed] [Google Scholar]
- [21].Joosen AM, Kuhnle GG, Runswick SA, Bingham SA. Urinary sucrose and fructose as biomarkers of sugar consumption: comparison of normal weight and obese volunteers. International journal of obesity. 2008;32:1736–40. doi: 10.1038/ijo.2008.145. [DOI] [PubMed] [Google Scholar]
- [22].Tasevska N, Runswick SA, Welch AA, McTaggart A, Bingham SA. Urinary sugars biomarker relates better to extrinsic than to intrinsic sugars intake in a metabolic study with volunteers consuming their normal diet. Eur J Clin Nutr. 2009;63:653–9. doi: 10.1038/ejcn.2008.21. [DOI] [PubMed] [Google Scholar]
- [23].Johner SA, Libuda L, Shi L, Retzlaff A, Joslowski G, Remer T. Urinary fructose: a potential biomarker for dietary fructose intake in children. Eur J Clin Nutr. 2010;64:1365–70. doi: 10.1038/ejcn.2010.160. [DOI] [PubMed] [Google Scholar]
- [24].Tasevska N, Midthune D, Potischman N, Subar AF, Cross AJ, Bingham SA, et al. Use of the predictive sugars biomarker to evaluate self-reported total sugars intake in the Observing Protein and Energy Nutrition (OPEN) study. Cancer Epidemiol Biomarkers Prev. 2011;20:490–500. doi: 10.1158/1055-9965.EPI-10-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nakamura H, Tamura Z. Gas chromatographic analysis of mono- and disaccharides in human blood and urine after oral administration of disaccharides. Clinica chimica acta; international journal of clinical chemistry. 1972;39:367–81. doi: 10.1016/0009-8981(72)90055-1. [DOI] [PubMed] [Google Scholar]
- [26].Celli M, D’Eufemia P, Dommarco R, Finocchiaro R, Aprigliano D, Martino F, et al. Rapid gas-chromatographic assay of lactulose and mannitol for estimating intestinal permeability. Clin Chem. 1995;41:752–6. [PubMed] [Google Scholar]
- [27].Farhadi A, Keshavarzian A, Fields JZ, Sheikh M, Banan A. Resolution of common dietary sugars from probe sugars for test of intestinal permeability using capillary column gas chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;836:63–8. doi: 10.1016/j.jchromb.2006.03.046. [DOI] [PubMed] [Google Scholar]
- [28].Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang CY, et al. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142:369–74. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mifflin MD, Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–7. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- [30].Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. American Industrial Hygiene Association journal. 1993;54:615–27. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- [31].Rock CL, Thornquist MD, Kristal AR, Patterson RE, Cooper DA, Neuhouser ML, et al. Demographic, dietary and lifestyle factors differentially explain variability in serum carotenoids and fat-soluble vitamins: baseline results from the sentinel site of the Olestra Post-Marketing Surveillance Study. J Nutr. 1999;129:855–64. doi: 10.1093/jn/129.4.855. [DOI] [PubMed] [Google Scholar]
- [32].Bailey RL, Mills JL, Yetley EA, Gahche JJ, Pfeiffer CM, Dwyer JT, et al. Unmetabolized serum folic acid and its relation to folic acid intake from diet and supplements in a nationally representative sample of adults aged > or =60 y in the United States. Am J Clin Nutr. 2010;92:383–9. doi: 10.3945/ajcn.2010.29499. [DOI] [PMC free article] [PubMed] [Google Scholar]