Abstract
Socioeconomic status is an important predictor of cognitive development and academic achievement. Late adolescence provides a unique opportunity to study how the attainment of socioeconomic status (in the form of years of education) relates to cognitive and neural development, during a time when age-related cognitive and neural development is ongoing. During late adolescence it is possible to disambiguate age- and education-related effects on the development of these processes. Here we assessed the degree to which higher educational attainment was related to performance on a cognitive control task, controlling for age. We then used diffusion tensor imaging (DTI) to assess the degree to which white matter microstructure might mediate this relationship. When covarying age, significant associations were found between educational attainment and fractional anisotropy (FA) in the superior longitudinal fasciculus (SLF) and cingulum bundle (CB). Further, when covarying age, FA in these regions was associated with cognitive control. Finally, mediation analyses revealed that the age-independent association between educational attainment and cognitive control was completely accounted for by FA in these regions. The uncinate fasciculus, a late-myelinated control region not implicated in cognitive control, did not mediate this effect.
Introduction
Socioeconomic disparities are important predictors of cognitive development and academic achievement (McLoyd, 1998). Methodologically, it is difficult to examine how socioeconomic status (SES) dynamically influences cognitive skill across the lifespan, from childhood through adulthood. Typical studies of SES in childhood measure the effects of parental factors, such as maternal education or family income, on child cognitive development (Bradley, Corwyn, Burchinal, McAdoo, & Garcia Coll, 2001). Parental SES is an important indicator of family conditions, but is inherently a “distal” factor used to account for children's experiences. In contrast, studies in adults commonly focus on the association between an individual's own educational or income attainment and cognitive performance (Gianaros et al., 2007; Scarmeas, Albert, Manly, & Stern, 2006; Stern, 2002). While this approach more proximally reflects the individual's experience, such studies commonly measure concurrent cognitive skill in adulthood, limiting conclusions about how SES influences cognitive and neural development. Although some studies of adults do include a measure of both childhood and adult SES (Gianaros et al., 2010; Singh-Manoux, Richards, & Marmot, 2005; Turrell et al., 2002), this type of investigation typically relies on the adult participant's memory of childhood SES, which may be subject to recall bias.
Late adolescence provides a unique opportunity to study the effects of educational attainment within the individual, during a time when age-related cognitive and neural development is ongoing. During late adolescence it is possible to tease apart age- and education-related effects on the development of these processes, in a way that is difficult in childhood (when age and educational attainment are highly correlated) or adulthood (when cognitive performance tends to be stable or decline). This period thus provides the ideal window for studying how the attainment of one's own SES relates to cognitive and neural development.
Executive functioning (EF) may be influenced by higher educational attainment (Van der Elst, Van Boxtel, Van Breukelen, & Jolles, 2006; Zafiri & Kosmidis, 2008). EF abilities continue to develop throughout childhood and adolescence (Casey et al., 1997; Diamond, 2002; Hughes, 2002; Liston, Watts, et al., 2006). Childhood EFs have been related to parental SES (Dilworth-Bart, Khurshid, & Vandell, 2007; Mezzacappa, 2004; Noble, McCandliss, & Farah, 2007; Noble, Norman, & Farah, 2005), while in adults, EF has been linked both to one's own educational attainment (Ardila, Ostrosky-Solis, Rosselli, & Gómez, 2000; Avila et al., 2009; Van der Elst, et al., 2006), and to parental SES (Evans & Schamberg, 2009).
The university experience – both within the classroom and without – provides students with opportunities to develop “cognitive control,” or the skills necessary for inhibiting inappropriate thoughts and actions in favor of those more appropriate to the task at hand (Liston, Cohen, Teslovich, Levenson, & Casey, 2011). Training with EF tasks has led to improvement in such abilities (Diamond, Barnett, Thomas, & Munro, 2007; Klingberg et al., 2005; Rueda, Rothbart, McCandliss, Saccomanno, & Posner, 2005).
The development of cognitive control is reflected in the protracted maturation of prefrontal and striatal circuits (Giedd, 2004; Gogtay et al., 2004; Huttenlocher, 1979; Klingberg, Vaidya, Gabrieli, Moseley, & Hedehus, 1999; Liston, Watts, et al., 2006; Sowell et al., 2003; Sowell et al., 2004; Sowell, Thompson, Tessner, & Toga, 2001). Diffusion tensor imaging (DTI) studies suggest that the development of white matter tracts in these regions are associated with various EFs during adolescence (Liston, Miller, et al., 2006; Nagy, Westerberg, & Klingberg, 2004), even when controlling for age (Liston, Miller, et al., 2006). Education may therefore lead to plasticity in prefrontal regions supporting these skills (Klingberg, et al., 2005; Rueda, et al., 2005; Springer, McIntosh, Winocur, & Grady, 2005).
Structural characteristics of several white matter tracts have been linked with cognitive control. These include the superior longitudinal fasiculus (SLF) (Ashtari et al., 2007; Burzynska et al., 2011; Charlton, Barrick, Lawes, Markus, & Morris, 2010; Karlsgodt et al., 2008; Kennedy & Raz, 2009; Konrad et al., 2010; Liston, et al., 2011; Makris et al., 2008; Olesen, Nagy, Westerberg, & Klingberg, 2003; Pavuluri et al., 2009; Vestergaard et al., 2010); cingulum bundle (Kantarci et al., 2011; Konrad, et al., 2010; Liston, Watts, et al., 2006; Makris, et al., 2008; Murphy et al., 2007; Pavuluri, et al., 2009; Schermuly et al., 2010; Skranes et al., 2009); and anterior corona radiata (ACR) (Liston, et al., 2011; Niogi et al., 2008; Pavuluri, et al., 2009). These fiber tracts have connections with the anterior cingulate gyrus (Makris, et al., 2008; Niogi, et al., 2008; Schermuly, et al., 2010), a prefrontal region implicated in cognitive control (Adleman et al., 2002; Botvinick, Braver, Barch, Carter, & Cohen, 2001; Bush et al., 1998; Casey et al., 2000).
These white matter tracts continue to develop through the teens and twenties, at which time briefly plateau and begin degenerating soon thereafter (Brickman et al., in press). Thus late adolescence – defined here as the late teens to early twenties – represents a period of ongoing cognitive and neural development in the context of wide variation in educational attainment. We hypothesized that higher educational attainment in this age group would be associated with better cognitive control, and that this would be mediated by structural differences in white matter tracts that support this skill.
Methods
Subjects
Subjects were compiled from the Brain Resource International Database, accessed via the independent BRAINnet Foundation (www.BRAINnet.net). This standardized database comprises demographic, psychometric, physiologic, and anatomic data collected on participants from 6 primary sites throughout the world (Grieve, Clark, Williams, Peduto, & Gordon, 2005). For the current study, only participants from one site in Australia (Flinders University) were included, as this was the only site to collect DTI. The full DTI dataset from this site includes 282 healthy individuals, ranging in age from 7 to 87. Participants completed WebQ, a standardized computer-based battery of questionnaires that assess medical history, demographics, and psychological function, including current or lifetime diagnosis of neurological and psychiatric conditions (Williams et al., 2009). All participants were excluded from further participation if they had history of brain injury, significant medical, neurological or psychiatric conditions, and/or drug or alcohol addiction. Individuals with first-degree family members with attention deficit hyperactivity disorder, schizophrenia, bipolar disorder, or genetic disorders were also excluded.
The present investigation included a sub-sample of 47 late adolescents (twenty-six female). This sub-sample represents all individuals in the database ranging in age from 17-23 years old, and for whom education and DTI were available. Education ranged from 11-18 years (See Table 1).
Table 1.
Demographics of Sample
| Demographic | Mean (s.d.) | Range |
|---|---|---|
| Age | 20.1 (2.2) | 17-23 |
| Years of Education | 14.1 (1.8) | 11-18 |
Of note, in Australia, a range of educational attainment is fairly common in late adolescence. School is compulsory until age 16 (corresponding to approximately 10th grade in the US), at which time the decision is made whether to complete the “senior years” (grades 11 or 12) or to seek employment (Pink & Australian Bureau of Statistics, 2010). Approximately 75% of Australians complete high school, and approximately 30% complete some type of post-secondary education (Pink & Australian Bureau of Statistics, 2010). Additionally, most universities have mechanisms for accepting talented youngsters to University early, around age 16-17, before the completion of Grade 11-12 (Victorian Government Department of Education and Early Childhood Development, 2010). Thus, among 17-23-year-olds, some variation in educational attainment would be expected at each age (see Results).
MRI scan acquisition
Magnetic resonance imaging was conducted on a 1.5 T Siemens Sonata system. The MRI protocol included a 3-D T1-weighted image (TR=9.7 ms; TE=4 ms; echo train: 7; flip angle=12°; TI=200 ms; NEX=1) and a proton-density/T2-weighted dual echo sequence (TR: 7530 ms; TE: 15/105 ms; eEcho train: 7; flip angle: 180°; NEX: 1). DTI was acquired with an echo planar imaging sequence (TR: 160 ms; TE: 88 ms; fat saturation; NEX: 4; field of view: 22 cm × 22 cm), including a baseline image (b=0) and 12 diffusion orientations with b-values of 1250. For DTI, thirty-two 6.5-mm contiguous slices were obtained with an in-plane matrix of 128x128 and resolution of 1.72 mm2.
DTI analysis
DTI analysis methodology in this cohort has been described in detail elsewhere (Grieve, Korgaonkar, Clark, & Williams, 2011). Briefly, data were preprocessed and analyzed with the fMRIB Diffusion Toolbox and tract-based spatial statistics of the fMRI Software Library (FSL 4.1.3; www.fmrib.ox.ac.uk/fsl) (Smith et al., 2006). Fractional anisotropy (FA) images generated for each participant were transformed into MNI152 1-mm3 standard space using the nonlinear registration tool FNIRT (Andersson, Jenkinson, & Smith, 2007). An average FA image was then generated and thinned to create a white matter skeleton representing the centers of all white matter tracts common to all subjects. A threshold of FA ≥ 0.2 was applied to include the major white matter pathways while avoiding peripheral tracts that are more vulnerable to intersubject variability and/or partial volume effects with gray matter. Each subject's aligned FA image was then projected onto the mean FA skeleton by assigning each skeleton voxel by the maximum FA value found in a direction perpendicular to the tract. This approach results in a standard space FA skeleton of the major white matter tracts for each subject accounting for any residual registration misalignments and variability in exact tract location between subjects (Smith, et al., 2006). We used the JHU ICBM-DTI-81 white matter labels and tractography atlases (Hua et al., 2008; Mori et al., 2008) to label sections of the skeleton corresponding to the major tracts in both hemispheres.
Selection of Regions of Interest
The superior longitudinal fasciculus (SLF), cingulum bundle (CB), and anterior coronal radiata (ACR) were chosen as regions of interest (ROIs), based on their involvement in cognitive control broadly (Burzynska, et al., 2011; Charlton, et al., 2010; Kantarci, et al., 2011; Karlsgodt, et al., 2008; Konrad, et al., 2010; Schermuly, et al., 2010; Skranes, et al., 2009; Vestergaard, et al., 2010), and the Stroop task specifically (Kennedy & Raz, 2009; Murphy, et al., 2007). Anomalies in these fiber tracts have been linked to attention-deficit-hyperactivity-disorder (ADHD), a disorder whose symptoms are closely related to deficits in cognitive control (Liston, et al., 2011).
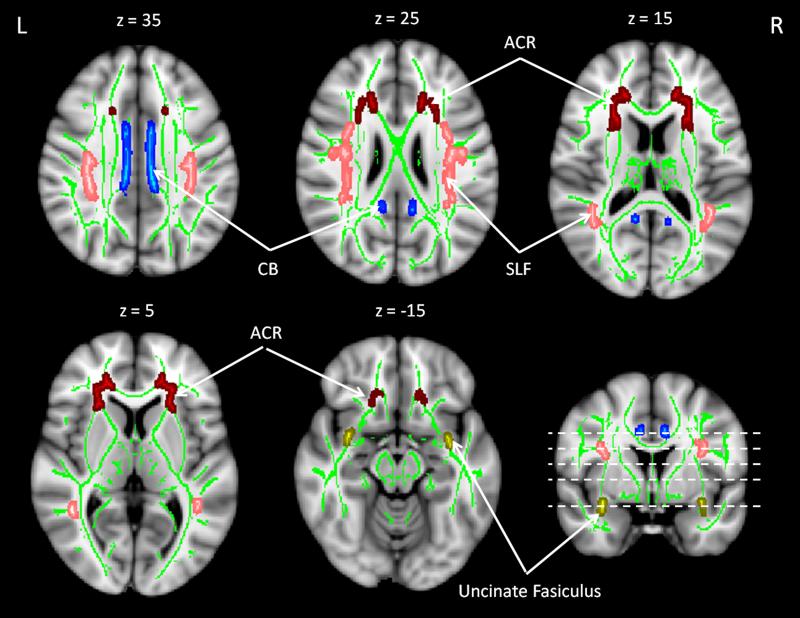
The uncinate fasciculus was selected as a control region with a similarly late-myelinating developmental trajectory (Brody, Kinney, Kloman, & Gilles, 1987; Kinney, Brody, Kloman, & Gilles, 1988; Yakovlev & Lecours, 1967), but which supports cognitive skills outside of EF (Niogi, et al., 2008). Figure 1 displays the anatomical distribution of these ROIs. As we had no predictions concerning laterality, mean FA from each hemisphere was averaged to generate a mean value for each ROI.
Figure 1.
Three regions of interest (ROIs). The superior longitudinal fasciculus (SLF) is represented in pink; the cingulum bundle (CB) is in blue; and the anterior corona radiata (ACR) is in red. The uncinate fasciculus, a late-myelinating control region, is depicted in yellow.
Cognitive Control
Participants were evaluated with IntegNeuro™, a standardized computerized neuropsychological battery that is valid and reliable (Brain Resource, 2010). A verbal interference task similar to the Stroop task (Golden, 1978) was used as an indicator of cognitive control. Subjects were presented with colored words with incongruent color-word combinations. In the first part of the task, the subject was required to identify the name of each word as quickly as possible. In the second part of the task, the subject was required to inhibit this prepotent response by identifying the color of each word as quickly as possible. The dependent variable of interest was the number of stimuli for which the font-color of the color-word was correctly selected (ignoring the name of the word) within 30 seconds (Williams et al., 2005).
This research was approved by local ethics committees and informed consent was obtained on all participants over age 18, or by parents or guardians after assent was established for individuals under age 18.
Statistical Analyses
To assess whether higher educational attainment influences cognitive control performance, and the degree to which this relationship is mediated by white matter tract integrity, we conducted a mediation analysis, defined according to Baron and Kenny's (1986) criteria (though see Salthouse (2011) for a discussion of limitations of this technique). These criteria require four separate regression analyses, which assess: (1) the degree to which educational attainment accounts for variation in cognitive control; (2) the degree to which educational attainment accounts for variation in FA; (3) the degree to which FA accounts for variation in cognitive control; and (4) the degree to which, when adjusting for FA, the association between educational attainment and cognitive control is reduced. All analyses included age as a covariate. We expected to find that FA in white matter tracts of interest would account for the effects of educational attainment on cognitive control.
Results
Educational attainment in this sample of late adolescents represented a span of secondary and higher education (see Table 1). Unsurprisingly, educational attainment was correlated with age (R=0.79; p<.000), as older individuals have had more years to obtain education. Nonetheless, Table 2 shows that this sample also exhibits a range of educational attainment at each age, typical of the Australian population, as described above.
Table 2.
Education by Age
| Age | Educational Range |
|---|---|
| 17 | 11-14 |
| 18 | 12-14 |
| 19 | 13-15 |
| 20 | 12-16 |
| 21 | 14-17 |
| 22 | 13-18 |
| 23 | 14-16 |
The first analysis investigated the degree to which educational attainment correlated with performance on the interference task, adjusting for age. Table 3 reveals that education, and not age, predicts task performance among this sample of late adolescents (R2 change=0.093, p<0.039). Despite the high correlation between age and education, these two independent variables do not represent undue multicollinearity. A problem with multicollinearity exists if tolerance, or the proportion of variance in a predictor variable that is independent of any other predictor values, is low, defined conservatively as less than 0.1-0.2 (Pedhazur, 1997). As Table 3 shows, tolerance values for these variables are higher than this, lending confidence that age and education are not redundant predictors in this sample. There were no sex differences in educational attainment (t (45) = 0.60; p=0.55), age (t (45) = -0.41; p=0.69), or performance on the interference task (t (45) = 0.04; p=0.97), and therefore analyses were not adjusted for sex.
Table 3.
Hierarchical Regression of Age and Education Predicting Cognitive Control Performance
| Step | R2 change | Sig F change | Beta | p | Tolerance | |
|---|---|---|---|---|---|---|
| 1 | Age | 0.000 | 0.948 | -0.010 | 0.948 | 1 |
| 2 | Age | -0.412 | 0.090 | 0.365 | ||
| Years of Education | 0.093 | 0.039 | 0.505 | 0.039 | 0.365 | |
A hierarchical regression was conducted with age and then years of education added as independent variables. Cognitive control, operationalized by performance on a Stroop-like task, was the dependent variable. Age did not account for variance in this cognitive control measure in this sample. When adjusting for age, years of education accounted for unique variance in this measure. Age and education did not exhibit undue multicollinearity, as evidenced by the tolerance value, suggesting that over one-third of the variance in each predictor variable is independent of the other predictor.
Next, analyses investigated the degree to which educational attainment was correlated with FA in the hypothesized tracts of interest, namely the SLF, CB, and ACR. We also investigated the degree to which educational attainment was correlated with FA in the uncinate fasciculus, a control tract not typically associated with EF. To account for multiple comparisons of 4 tracts, alpha was set at 0.0125 (Bonferroni correction of 0.05/4). All analyses adjusted for age. There were no sex differences in FA in these regions (all p's > 0.5), and therefore analyses were not adjusted for sex. Table 4 reveals that, when controlling for age, education was significantly associated with FA in the SLF (R2 change=0.189, p< 0.002), CB (R2 change=0.191, p< 0.002), and ACR (R2 change=0.182, p<0.003), but not the uncinate (R2 change=0.016, p=0.178).
Table 4.
Hierarchical Regressions of Age and Education Predicting Fractional Anisotropy
| Dependent Variable: Fractional Anistropy | Step | Model | R2 change | Sig F change | Beta | P |
|---|---|---|---|---|---|---|
| Superior Longitudinal Fasciculus | 1 | Age | 0.027 | 0.271 | 0.164 | 0.271 |
| 2 | Age | 0.738 | 0.002 | |||
| Years of Education | 0.189 | 0.002 | -0.720 | 0.002 | ||
| Cingulum Bundle | 1 | Age | 0.029 | 0.252 | 0.171 | 0.252 |
| 2 | Age | 0.747 | 0.002 | |||
| Years of Education | 0.191 | 0.002 | -0.723 | 0.002 | ||
| Anterior Corona Radiata | 1 | Age | 0.019 | 0.357 | 0.137 | 0.357 |
| 2 | Age | 0.700 | 0.002 | |||
| Years of Education | 0.182 | 0.003 | -0.706 | 0.003 | ||
| Uncinate Fasiculus | 1 | Age | 0.016 | 0.398 | 0.398 | 0.398 |
| 2 | Age | 0.572 | 0.572 | |||
| Years of Education | 0.040 | 0.178 | 0.178 | 0.178 | ||
In each region of interest (ROI), hierarchical regressions were conducted with age and then years of education as independent variables. Fractional anisotropy (FA) values in each respective ROI were the dependent variables. When adjusting for age, educational attainment significantly predicted FA in the superior longitudinal fasiculus, the cingulum bundle, and the anterior corona radiata, three ROIs that have previously been linked with cognitive control. In contrast, years of education did not account for variance in FA in the uncinate fasiculus, a control region which has not been linked to cognitive control.
The next question concerned the degree to which FA in our ROIs accounted for variation in cognitive control. Again, alpha was set at 0.0125 to account for multiple comparisons. Table 5 shows that, when adjusting for age, FA in the SLF significantly predicted interference task performance (R2 change=0.136, p<0.012). The CB showed a borderline effect (R2 change=0.107, p<0.026). Neither the ACR (R2 change=0.054, p<0.12) nor the uncinate (R2 change=0.028, p=0.264) were significant predictors of interference task performance.
Table 5.
Hierarchical Regressions of Age and Fractional Anisotropy Predicting Cognitive Control
| Dependent Variable: | Step | Model | R2 change | Sig F change | Beta | p | Tolerance |
|---|---|---|---|---|---|---|---|
| Cognitive control | 1 | Age | 0.000 | 0.948 | -.010 | 0.948 | 1 |
| 2 | Age | .052 | 0.718 | 0.973 | |||
| FA - SLF | 0.136 | 0.012 | -.374 | 0.012 | 0.973 | ||
| 1 | Age | 0.000 | 0.948 | -.010 | 0.948 | 1 | |
| 2 | Age | .047 | 0.746 | 0.971 | |||
| FA – CB | 0.107 | 0.026 | -.333 | 0.026 | 0.971 | ||
| 1 | Age | 0.000 | 0.948 | -.010 | 0.948 | 1 | |
| 2 | Age | .023 | 0.879 | 0.981 | |||
| FA – ACR | 0.054 | 0.120 | -.235 | 0.12 | 0.981 | ||
| 1 | Age | 0.000 | 0.948 | -.010 | 0.948 | 1 | |
| 2 | Age | .031 | 0.836 | 0.984 | |||
| FA - uncinate | 0.028 | 0.264 | -.170 | 0.264 | 0.984 | ||
Three hierarchical regressions were conducted, with age and then fractional anisotropy added as the independent variables. In each case, the dependent variable was performance on a Stroop-like task. When adjusting for age, fractional anisotropy in the superior longitudinal fasiculus significantly predicted cognitive control, and fractional anisotropy in the cingulum bundle showed a borderline effect when considering multiple comparisons (alpha set at 0.0125). In contrast, FA in the anterior corona radiata and uncinate fasiculus were not related to cognitive control. Age and FA do not exhibit multicollinearity. FA=fractional anisotropy. SLF=superior longitudinal fasciculus. CB= cingulum bundle. ACR=anterior corona radiata.
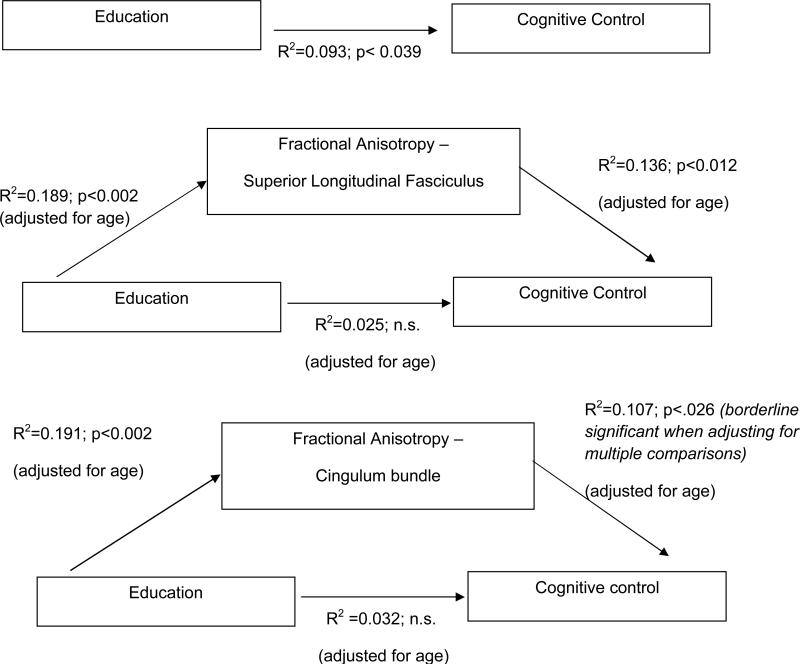
To this point, the SLF meets preliminary criteria for mediation, and the CB nearly does so. The final criterion for mediation is that FA in these ROIs accounts for the link between educational attainment and cognitive control performance. Table 6 and Figure 2 reveal that, indeed, when FA in the SLF or CB is controlled, the association between education and performance on the interference task is no longer significant. The addition of years of education to these models did not account for unique variance, once age and FA are accounted for (R2 change SLF=0.025, p=0.264; R2 change CB=0.032, p=0.215).
Table 6.
Fractional Anisotropy in the Superior Longitudinal Fasciculus and the Cingulum Bundle Mediates the Association between Educational Attainment and Cognitive Control
| Dependent Variable: | Model | R2 change | Sig F change | Beta | p | Tolerance |
|---|---|---|---|---|---|---|
| Cognitive Control | Age | -.194 | .458 | .291 | ||
| FA - SLF | -.295 | .068 | .784 | |||
| Years of Education | 0.025 | 0.264 | .292 | .264 | .294 | |
| Age | -.230 | .387 | .289 | |||
| FA – CB | -.243 | .136 | .780 | |||
| Years of Education | 0.032 | 0.215 | .329 | .215 | .293 | |
Mediation analysis assessed the degree to which the significant effect of FA in the SLF and the cingulum accounted for the relation between educational attainment and cognitive control. Two hierarchical regression analyses were conducted, with age, regional FA, and years of education added as independent variables in a step-wise fashion. The dependent variable in each case was performance on a Stroop-like task. Steps 1 and 2 are shown in Table 5; Step 3 is shown above. When adjusting for age and fractional anisotropy in the superior longitudinal fasiculus and the cingulum bundle, years of education no longer significantly predicted cognitive control task performance. Thus, FA in these regions mediates the relation between educational attainment and cognitive control, as measured by this task. Age, FA and years of education do not exhibit multicollinearity in either model. FA=fractional anisotropy. SLF=superior longitudinal fasciculus. CB= cingulum bundle
Figure 2.
The association between educational attainment and cognitive control is mediated by fractional anisotropy in the superior longitudinal fasciculus and the cingulum bundle. All analyses are adjusted for age.
Discussion
Late adolescence is a time of transition from parental SES to the attainment of one's own socioeconomic position. Here we have shown that higher educational attainment in late adolescence is associated with cognitive control, independent of age. Further, this relation is statistically mediated by white matter microstructure in several regions that support cognitive control processes. One interpretation of these results is thus that higher educational attainment may lead to age-independent changes in white matter development, which in turn supports cognitive control.
Although cognitive control continues to develop through childhood and adolescence (Diamond, 2002; Hughes, 2002; Liston, Watts, et al., 2006), it is difficult to disambiguate the effects of age and education, as these two factors tend to be extremely highly correlated in childhood (Reitan & Wolfson, 1995). Using a “natural experiment” of school cut-off age, Morrison and colleagues have shown effects of early schooling on certain cognitive skills in early childhood, controlling for age (Morrison, Smith, & Dow-Ehrensberger, 1995), though results have been mixed concerning the effect of schooling on EF (Burrage et al., 2008; Skibbe, Connor, Morrison, & Jewkes, 2011). Although such studies support the role of education in cognitive development, there is little variation in whether children ultimately complete the early school years. In late adolescence, however, there is wide variation in educational attainment across similarly aged individuals, ranging from not completing high school to obtaining an advanced degree. Thus, by studying the effects of higher educational attainment in this age group, we are in effect studying SES-in-the-making. In the present sample of late adolescents, higher education was associated with improved cognitive control, when controlling for differences in age.
The prefrontal and striatal circuits that underlie cognitive control exhibit protracted maturation (Giedd, 2004; Gogtay, et al., 2004; Huttenlocher, 1979; Klingberg, et al., 1999; Liston, Watts, et al., 2006; Sowell, et al., 2003; Sowell, et al., 2004; Sowell, et al., 2001), in part accounted for by cognitive skill (Liston, Watts, et al., 2006). However, it is difficult to disambiguate the effects of age versus education. One study showed regionally specific changes in several fronto-striatal brain structures during the first year of college (Bennett & Baird, 2006). However, whether such changes occurred as a function of age or education was unclear. The present data suggest that, independent of age, higher education is associated with differences in white matter microstructure in several regions previously associated with cognitive control, including the SLF, CB, and ACR. Moreover, these differences in the SLF and, to a lesser extent, the CB, statistically mediate the relationship between education and cognitive control.
There are many pathways by which socioeconomic disparities might lead to differences in brain development (Noble, Houston, Kan, & Sowell, 2012). For example, research suggests large differences in exposure to stress across SES (Evans, 2004). The experience of stress has important negative effects on prefrontal cortical structures, including the ACC (Liston, McEwen, & Casey, 2009; McEwen & Gianaros, 2010) . Future research is necessary to elucidate whether the white matter findings reported here result from differences in earlier childhood experiences which influence the likelihood of obtaining higher education, or conversely, whether findings are due to direct effects of higher education on brain development.
Of note, one previous report using a large sample of twins found that SES was not significantly associated with FA in white matter (though the authors did find that SES modifies the heritability of FA) (Chiang et al., 2010). Interestingly, the study sample in that paper was also drawn from Australia. Possible causes for the disparate findings across the two studies may include differences in the age of participants (late adolescents versus adults) and/or differences in characterization of SES (educational attainment versus occupational status). Certainly, more research is needed to determine whether the findings presented here may be replicated in other samples.
This study suffers from several weaknesses. Notably, we did not have a measure of parental SES in this dataset. The present data are therefore unable to disambiguate whether an individual's own educational attainment directly improves cognitive control, or whether going to college is simply more likely among adolescents from higher socioeconomic backgrounds, and that it is childhood SES conditions which have a direct effect on cognition. Of note, some work suggests that adult SES may influence cognition across the lifecourse independently of childhood SES (Turrell, et al., 2002), and that the effects of childhood SES may operate on adult cognitive achievement indirectly through adult socioeconomic position (Singh-Manoux, et al., 2005).
Secondly, this study used a cross-sectional design, which limits our ability to draw strong conclusions regarding development. Additionally, all participants in this study were from Australia, potentially limiting generalizability to other populations.
We also note the unusual pattern that in all cases, FA was negatively associated with both education and cognitive control (though not with age). Although this is not the typical pattern that would be expected, other studies have reported inverse correlations between FA and aspects of cognition, including processing speed (Tuch et al., 2005), creativity (Jung, Grazioplene, Caprihan, Chavez, & Haier, 2010), and visuospatial skills (Hoeft et al., 2007). Inverse correlations between FA and mental health have also been reported (Abe et al., 2006; Han et al., 2008; Yoo et al., 2007). Thus the picture of “optimal” white matter microstructure may not always be straightforward. Certainly this aspect of the data bears further investigation in future replications.
Finally, the small sample size in the present paper was powered to observe large effect sizes in mediation paths (Fritz & MacKinnon, 2007). It is possible that, in a larger sample, it would be possible to detect smaller (or even directionally opposite) effects in other white matter tracts.
Conclusions
In late adolescence, educational attainment is associated with cognitive control, independent of age. This association was statistically mediated by differences in white matter microstructure in several regions previously shown to support cognitive control. This has implications for how SES may influence cognition, at a time of transition from parental socioeconomic background to the attainment of one's own socioeconomic position. Future studies would benefit from longitudinal measures of neural and cognitive development in this age range, and from the simultaneous measurement of parental socioeconomic factors, to disentangle the effects of family SES and ongoing individual socioeconomic attainment as predictors of cognitive and neural development.
Acknowledgements
We acknowledge the data and support provided by BRAINnet; www.BRAINnet.net, under the governance of the BRAINnet Foundation. BRAINnet is the scientific network that coordinates access to the Brain Resource International Database for independent scientific purposes. We gratefully acknowledge Laurel Bunse and Laura Engelhardt for their helpful reviews of the literature during the development of this manuscript. We also thank the individuals who gave their time to participate in the database. Funding for this work was supported by the John M. Driscoll, MD Scholars Program awarded to KGN, and by NIH grants AG029949 and AG034189, and a grant from the Alzheimer's Association, awarded to AMB.
References
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Iwanami A, et al. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Research: Neuroimaging. 2006;146(3):231–242. doi: 10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration aka spatial normalisation. FMRIB Technical Report TR07JA2. 2007 [Google Scholar]
- Ardila A, Ostrosky-Solis F, Rosselli M, Gómez C. Age-related cognitive decline during normal aging: The complex effect of education. Archives of Clinical Neuropsychology. 2000;15(6):495–513. [PubMed] [Google Scholar]
- Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, et al. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Archives of General Psychiatry. 2007;64(11):1270–1280. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- Avila R, Moscoso MAA, Ribeiz S, Arrais J, Jaluul O, Bottino CMC. Influence of education and depressive symptoms on cognitive function in the elderly. International Psychogeriatrics. 2009;21(03):560–567. doi: 10.1017/S1041610209008928. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality & Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Baird AA. Anatomical changes in the emerging adult brain: A voxel-based morphometry study. Human Brain Mapping. 2006;27(9):766–777. doi: 10.1002/hbm.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, Burchinal M, McAdoo HP, Garcia Coll C. The home environments of children in the United States part II: Relations with behavioral development through age thirteen. Child Development. 2001;72(6):1868. doi: 10.1111/1467-8624.t01-1-00383. Article. [DOI] [PubMed] [Google Scholar]
- Brain Resource . IntegNeuro (TM) Assessment Manual (Version 1.3) Brain Resource; 2010. [Google Scholar]
- Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, et al. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2011.06.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. Journal of Neuropathology & Experimental Neurology. 1987;46(3):283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Burrage MS, Ponitz CC, McCready EA, Shah P, Sims BC, Jewkes AM, et al. Age- and schooling-related effects on executive functions in young children: A natural experiment. Child Neuropsychology. 2008;14(6):510–524. doi: 10.1080/09297040701756917. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Nagel IE, Preuschhof C, Li S-C, Lindenberger U, Bäckman L, et al. Microstructure of frontoparietal connections predicts cortical responsivity and working memory performance. Cerebral Cortex. 2011;21(10):2261–2271. doi: 10.1093/cercor/bhq293. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: An interference task specialized for functional neuroimaging -- validation study with functional MRI. Human Brain Mapping. 1998;6(4):270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, et al. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proceedings of the National Academy of Science, USA. 2000;97(18):8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance on a go-no-go task. Journal of Cognitive Neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, Lawes INC, Markus HS, Morris RG. White matter pathways associated with working memory in normal aging. Cortex. 2010;46(4):474–489. doi: 10.1016/j.cortex.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Chiang M-C, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, Toga AW, et al. Genetics of white matter development: A dti study of 705 twins and their siblings aged 12 to 29. NeuroImage Oct(Pagination) 2010 doi: 10.1016/j.neuroimage.2010.10.015. No Pagination Specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; Oxford: 2002. pp. 466–504. [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318(5855):1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth-Bart JE, Khurshid A, Vandell DL. Do maternal stress and home environment mediate the relation between early income-to-need and 54-months attentional abilities? Infant and Child Development. 2007;16(5):525–552. [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychological Science. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Social Cognitive & Affective Neuroscience. 2007;2(3):161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck SB, Sheu LK, Kuan DCH, Votruba-Drzal E, Craig AE, et al. Parental education predicts corticostriatal functionality in adulthood. Cerebral Cortex. 2010;21(4):896–910. doi: 10.1093/cercor/bhq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021(1):77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test. Stoelting Company; Wood Dale, IL: 1978. [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Human Brain Mapping. 2005;25(4):391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Clark CR, Williams LM. Regional heterogeneity in limbic maturational changes: Evidence from integrating cortical thickness, volumetric and diffusion tensor imaging measures. Neuroimage. 2011;55(3):868–879. doi: 10.1016/j.neuroimage.2010.12.087. [DOI] [PubMed] [Google Scholar]
- Han DH, Renshaw PF, Dager SR, Chung A, Hwang J, Daniels MA, et al. Altered cingulate white matter connectivity in panic disorder patients. Journal of Psychiatric Research. 2008;42(5):399–407. doi: 10.1016/j.jpsychires.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D, et al. More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams Syndrome. Journal of Neuroscience. 2007;27(44):11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. Executive functions and development: Emerging themes. Infant and Child Development. 2002;11(2):201–209. [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex — Developmental changes and effects of aging. Brain Research. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Jung RE, Grazioplene R, Caprihan A, Chavez RS, Haier RJ. White matter integrity, creativity, and psychopathology: Disentangling constructs with diffusion tensor imaging. PLoS ONE. 2010;5(3):e9818. doi: 10.1371/journal.pone.0009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77(1):26–34. doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, van Erp TGM, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biological Psychiatry. 2008;63(5):512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47(3):916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. Journal of Neuropathology & Experimental Neurology. 1988;47:217. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, et al. Computerized training of working memory in children with ADHD--a randomized, controlled trial. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JDE, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, et al. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. European Journal of Neuroscience. 2010;31(5):912–919. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- Liston C, Cohen MM, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: Pathway to disease or pathological end point? Biological Psychiatry. 2011;69(12):1168–1177. doi: 10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. PNAS. 2009;106(3) doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-Induced Alterations in Prefrontal Cortical Dendritic Morphology Predict Selective Impairments in Perceptual Attentional Set-Shifting. J. Neurosci. 2006;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, et al. Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cerebral Cortex. 2008;18(5):1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186(1):190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E. Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Development. 2004;75(5):1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison FJ, Smith L, Dow-Ehrensberger M. Education and cognitive development: A natural experiment. Developmental Psychology. 1995;31(5):789–799. [Google Scholar]
- Murphy CF, Gunning-Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, et al. White-matter integrity predicts Stroop performance in patients with geriatric depression. Biological Psychiatry. 2007;61(8):1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, et al. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131(12):3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Developmental Science. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Cognitive Brain Research. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Yang S, Kamineni K, Passarotti AM, Srinivasan G, Harral EM, et al. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;65(7):586–593. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedhazur EJ. Multiple Regression in Behavioral Research: Explanation and Prediction. 3rd ed. Harcourt Brace College Publishers; Fort Worth, TX: 1997. Chapter 10: Analysis of Effects. pp. 283–339. [Google Scholar]
- Pink B, Australian Bureau of Statistics . 2009-10 Yearbook Australia (no. 91) Australian Bureau of Statistics; Canberra: 2010. [Google Scholar]
- Reitan RM, Wolfson D. Influence of age and education on the neuropsychological test performances of older children. Child Neuropsychology. 1995;1(3):165–169. Article. [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(41):14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011;137(5):753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2006;77(3):308–316. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly I, Fellgiebel A, Wagner S, Yakushev I, Stoeter P, Schmitt R, et al. Association between cingulum bundle structure and cognitive performance: An observational study in major depression. European Psychiatry. 2010;25(6):355–360. doi: 10.1016/j.eurpsy.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Richards M, Marmot M. Socioeconomic position across the lifecourse: how does it relate to cognitive function in mid-life? Annals of Epidemiology. 2005;15(8):572–578. doi: 10.1016/j.annepidem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Skibbe LE, Connor CM, Morrison FJ, Jewkes AM. Schooling effects on preschoolers’ self-regulation, early literacy, and language growth. Early Childhood Research Quarterly. 2011;26(1):42–49. doi: 10.1016/j.ecresq.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skranes J, Lohaugen GC, Martinussen M, Indredavik MS, Dale AM, Haraldseth O, et al. White matter abnormalities and executive function in children with very low birth weight. NeuroReport. 2009;20(3):263–266. doi: 10.1097/wnr.0b013e32832027fe. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer MV, McIntosh AR, Winocur G, Grady CL. The relation between brain activity during memory tasks and years of education in young and older adults. Neuropsychology. 2005;19(2):181–192. doi: 10.1037/0894-4105.19.2.181. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(34):12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrell G, Lynch JW, Kaplan GA, Everson SA, Helkala EL, Kauhanen J, et al. Socioeconomic position across the lifecourse and cognitive function in late middle age. Journals of Gerontology Series B Psychological Sciences & Social Sciences. 2002;57(1):S43–S51. doi: 10.1093/geronb/57.1.s43. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, Van Boxtel MPJ, Van Breukelen GJP, Jolles J. The Stroop color-word test: Influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 2006;13(1):62–79. doi: 10.1177/1073191105283427. [DOI] [PubMed] [Google Scholar]
- Vestergaard M, Madsen KS, Baaré WFC, Skimminge A, Ejersbo LR, Ramsøy TZ, et al. White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. Journal of Cognitive Neuroscience. 2010;23(9):2135–2146. doi: 10.1162/jocn.2010.21592. [DOI] [PubMed] [Google Scholar]
- Victorian Government Department of Education and Early Childhood Development [4 October, 2011];Early admission into tertiary education. 2010 from http://www.education.vic.gov.au/studentlearning/programs/gifted/schooloptions/earlytertiary.htm.
- Williams LM, Gatt JM, Schofield PR, Olivieri G, Peduto A, Gordon E. ‘Negativity bias’ in risk for depression and anxiety: Brain–body fear circuitry correlates, 5-HTT-LPR and early life stress. Neuroimage. 2009;47(3):804–814. doi: 10.1016/j.neuroimage.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Williams LM, Simms E, Clark CR, Paul RH, Rowe D, Gordon E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromarker”. International Journal of Neuroscience. 2005;115(12):1605–1630. doi: 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. Regional development of the brain in early life. In: Minkowski A, editor. The Myelogenetic Cycles of Regional Maturation of the Brain. Blackwell Scientific Publications; Boston: 1967. pp. 3–65. [Google Scholar]
- Yoo SY, Jang JH, Shin YW, Kim DJ, Park HJ, Moon WJ, et al. White matter abnormalities in drug-naïve patients with obsessive-compulsive disorder: A diffusion tensor study before and after citalopram treatment. Acta Psychiatrica Scandinavica. 2007;116(3):211–219. doi: 10.1111/j.1600-0447.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Zafiri M, Kosmidis M. Effects of demographic characteristics on the “Stroop conflict”. Psychology: The Journal of the Hellenic Psychological Society. 2008;15(4):319–341. [Google Scholar]