Abstract
Background/Aim
Use of zebrafish models may decrease the cost of screening new irradiation protectors and mitigators.
Materials and Methods
Zebrafish (Danio rerio) models were tested for screening water-soluble radiation protectors and mitigators. Irradiation of embryos and monitoring survival, and measuring fibrosis of the caudal musculature of adults allowed for testing of acute and late effects, respectively.
Results
Incubation of zebrafish embryos either before or after irradiation in ethyl pyruvate (1 mM) increased survival. Irradiation of adults to 15 to 75 Gy, delivered in single-fraction at 13 Gy/min, showed dose-dependent fibrosis at 30 days, quantitated as physiological decrease in swimming tail movement, and histopathological detection of collagen deposition in the dorsal musculature. Continuous administration of small-molecule radioprotector drugs in the water after irradiation reduced both acute and chronic injuries.
Conclusion
The zebrafish is cost-effective for screening new radiation countermeasures.
Keywords: Radiation fibrosis, antioxidant therapy, zebrafish
A major complication of ionizing irradiation exposure is fibrosis (1, 19, 58). Patients completing a course of fractionated radiotherapy for treatment of head and neck, thoracic, and abdominal malignancies demonstrate dose-dependent appearance of late effects within 6-24 months after irradiation (59-60). While acute effects of radiation are well-documented for both total body and organ-specific exposure, a latent period following resolution of the acute effects has been difficult to relate to the onset of late effects (3, 8, 10, 19). In the C57BL/6J mouse model of radiation pulmonary fibrosis, elevation of cytokines associated with the acute effects (TGFβ, IL-1, TNFα) has been shown to resolve with resolution of acute effects at around two weeks after irradiation (3). A several-month latent period is associated with no detectable histopathological changes in the lungs, but is followed by onset of organizing alveolitis/fibrosis at around 100 to 120 days after irradiation, with concomitant elevation of TGFβ (3, 8). The molecular and cellular mechanisms associated with radiation pulmonary fibrosis, as well as fibrosis in other organs and organ systems have been difficult to elucidate (58-60).
The search for pharmacological agents to prevent or reduce radiation fibrosis has been the focus of an intense investigation. Small-molecule antioxidant inhibitors of cytokine elevation have been shown to reduce the severity of irradiation acute effects in both animal models and clinical trials (39-46). The application of pharmacological agents at the time of protection of the late effect, fibrosis, has met with incomplete success and there is need for continuous administration of anti-fibrotic agents (46, 51). The difficulty of measuring both uptake and efficacy of many test agents further complicates the evaluation of new drugs (57).
In the modern era of automated drug discovery, computational chemistry, and computer modeling of pharmacologically active small molecules, there is a need to rapidly assay potentially valuable target compounds, and then determine the structure and design of lead compounds (38, 42, 44). Lead compounds tested successfully in vitro, then require animal testing (38, 42, 44). While rodent models for acute irradiation effects have been the mainstay of clinical translational research in radiobiology, the costs of animals, animal care, and veterinary supervision services have challenged the apparent widespread use of mammalian/rodent models for clinical testing of lead compounds in many applications, including for the therapy of irradiation-induced side-effects (27).
The Zebrafish have been shown to be a valuable model for testing the effect of irradiation on teratogenetic effects (59), and potential effectiveness of radiation counter measures (59). The use of Zebrafish as a model for late irradiation effects has not been described.
In this current article, we present evidence for the efficacy of the zebrafish model in measuring radiation-induced long term effects and for screening small-molecule irradiation mitigators of late tissue fibrosis.
Materials and Methods
Zebrafish and aquatic maintenance facilities
The fish embryos and adults were maintained in conditions in accordance with the Institutional Animal Care and Use Committee of the University of Pittsburgh as previously described (59).
Irradiation procedure
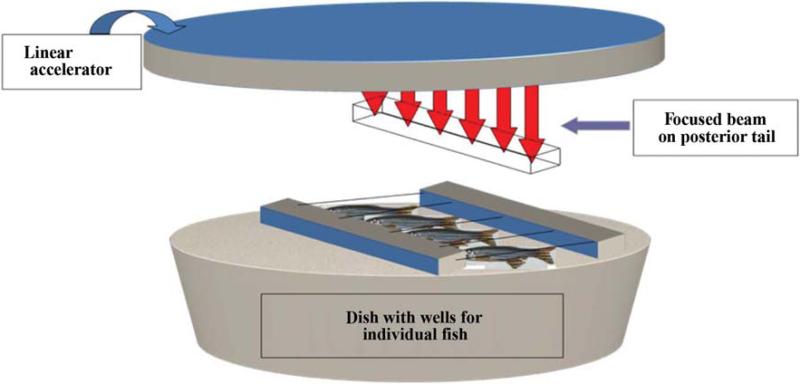
Adult zebrafish were anesthetized by placing the fish in 0.015% tricaine in water. Once the fish were sedated, they were placed in individual wells in agarose, covered with water containing 0.015% tricaine and irradiated to the caudal portion of the fish to doses up to 75 Gy (Figure 1). The maintenance of anesthetic in the water allowed movement of the gill “operculum” to facilitate oxygenation of the fish while they were irradiated.
Figure 1.
Schematic of irradiation of zebrafish caudate. Six-month old zebrafish were anesthesized in 0.015% tricaine and placed in individual wells immersed in anesthetic. The fish were irradiated at 13.6 Gy per min. The fish were shielded to protect the internal organs, but not the tail.
The holder device was placed on a series of Lucite blocks on the treatment table (patient assembly) of a Varian Linac 6 MV Varian CLINAC 2100C linear accelerator (Varian Medical Systems, Palo Alto, CA, USA). Dosimetry was carried out to deliver a 13 Gy/min dose rate of 6 MeV photons uniformly to the caudal region of the fish. Isodose curves for the radiation device and beam flatness were calculated to produce an error of <1% across the irradiated field.
The sedated fish were placed on a shallow petri dish containing agarose which had 10 slots to allow for irradiation of 10 fish at a time. The dish, filled with water containing 0.015% tricaine, was placed on a 15” stack of polystyrene on a raised LINAC table to bring the fish close to the radiation source. Source to prescription point, which was at the mid line of the fish, was kept at 55.7 cm to attain a dose rate of 13 Gy/Minute. Radiation field size of 40 cm × 7.5 cm was used to cover the entire petri dish. A 1.0-cm thick Superflab bolus material (Radiation Product Design, inc, USA) was placed above the entire irradiation area, so that the mid-line of the fish was at a depth of Dmax (1.5 cm for 6 MV beam). The area around the petri dish was filled with bolus material to maximize scatter condition at the prescription point.
A dose-response curve was created by irradiating fish in groups of 10 to doses of 15, 30, 45, 64, and 75 Gy at a dose rate of 1300 cGy per min. Fish were then allowed to recover and returned to aquarium tanks for observation. The maximum time from removal of fish to return to aquarium was approximately eight minutes. This interval has been shown to be consistent with other publications documenting the safety of Marcaine anesthesia for this interval. The fish were followed by development of fibrosis at which time they were sacrificed, fixed, sectioned, and stained with hematoxylin and Eosin (H&E) or Mallory's Trichrome Stain. All fish care protocols were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Fish care and veterinary care was provided by the Division of Laboratory Animal Research (DLAR) of the University of Pittsburgh.
Before irradiation, the anterior (cephalad) one half of the zebrafish was shielded by a 10½ value layer of lead block, so that the dose under the block was less than 10% of that in the irradiated posterior (caudal) section. This facilitated high dose irradiation of tail musculature and all associated structures.
To demonstrate that small molecules mitigate irradiation-induced fibrosis, zebrafish were irradiated to 32.5 Gy, as described above. The fish were then placed in water, containing 20 μM amifostine, 1 mM Tempol (Sigma Chemical Company, St. Louis, MO) or 20 μM EUK134 (Proteome Systems, Woburn, MA). The fish were followed for the development of fibrosis at which time they were sacrificed. In a separate experiment fish were irradiated to 30 Gy and then placed in water containing amifostine (Medimmune Prarma B.V., Nijmegen, Netherlands), tempol (Sigma/Aldrich Chemical Company, St. Louis, MO), or EUK134 (generously provided by Dr. Susan Doctrow, Proteome System, Inc., 6 Gill Street, Suite H, Woburn, MA 01801) as described above. The fish were sacrificed at day 60, fixed in paraformaldehyde, sectioned, and stained with either H&E or Mallory's Trichrome stain. The sections were examined microscopically and the percent of the caudate expressing fibrosis was determined.
Observation and detection of survival and fibrosis
Embryo survival was calculated as published (59). Adult fish were returned to aquarium maintenance and observed daily for signs of radiation morbidity. Moribund or dying fish were removed from the aquarium. Fish surviving an additional ten days were observed for signs of fibrosis detected as a decrease in tail oscillation at swimming and alteration in the structure, shape, and function of tail, dorsal and caudal fins.
Histopathology
Sagittal sections of entire zebrafish were prepared for histopathological analysis, according to previously published methods.
Staining for fibrosis using the Mallory-Trichrome stain was carried out according to previous publications. A percent of cell musculature replaced by fibrosis was calculated for each individual.
Results
Radiation fibrosis dose response curve of adult Zebrafish
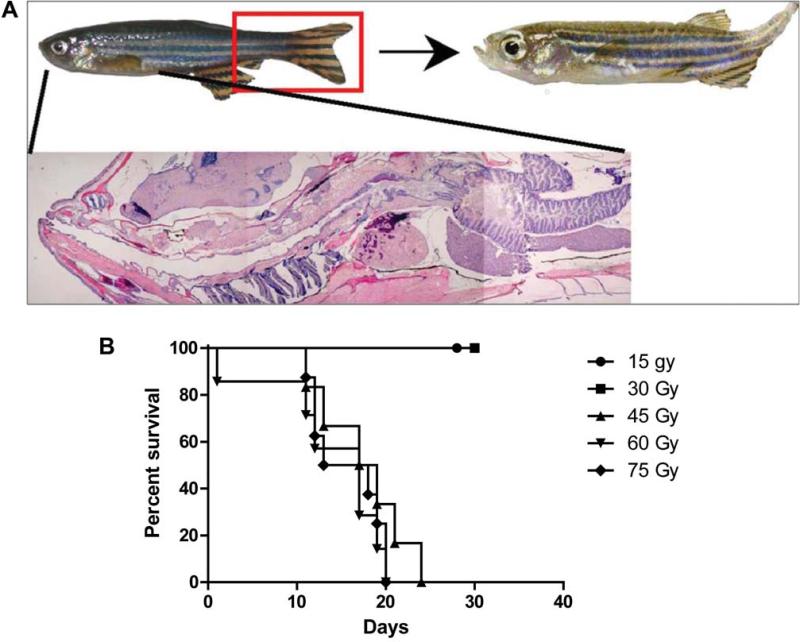
Fish receiving posterior/caudal half irradiation for doses between 15 and 75 Gy (Figure 1) were watched for development of fibrosis (Figure 2A). All fish irradiated at 45 Gy or higher developed fibrosis and were sacrificed by day 24. At 30 Gy, 25% of the fish did develop fibrosis as detected by abnormalities in swimming, stiff tail movement, and for changes in the shape and structure of tail and fin and survival (Figure 2B). Review of these results lead to the dose of 30 Gy being chosen for long-term observation as the maximum acutely tolerated dose, likely to result in irradiation late effect.
Figure 2.
Development of fibrosis in irradiated zebrafish. Zebrafish (6 months of age) were irradiated to doses ranging from 15 Gy (1500 cGy) to 75 Gy (7500 cGy) and followed for the development of fibrosis, observed as a curled stiff tail during swimming movements. This was observed at 2 months after irradiation (Figure 2A). An irradiation dose-response curve is shown in Figure 2B.
Pathological evidence of radiation-induced fibrosis
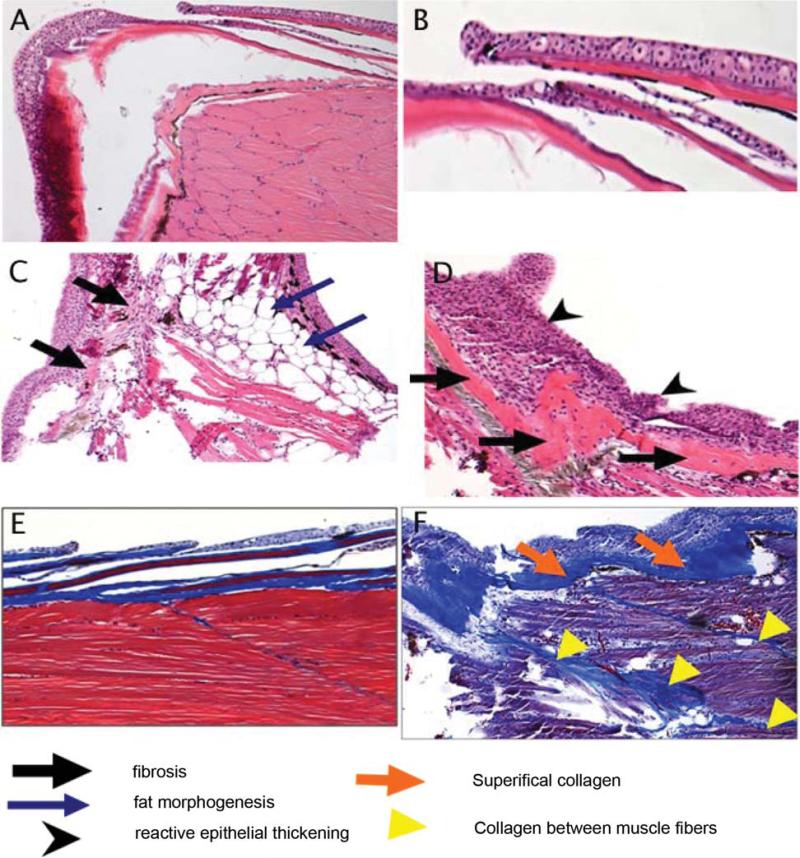
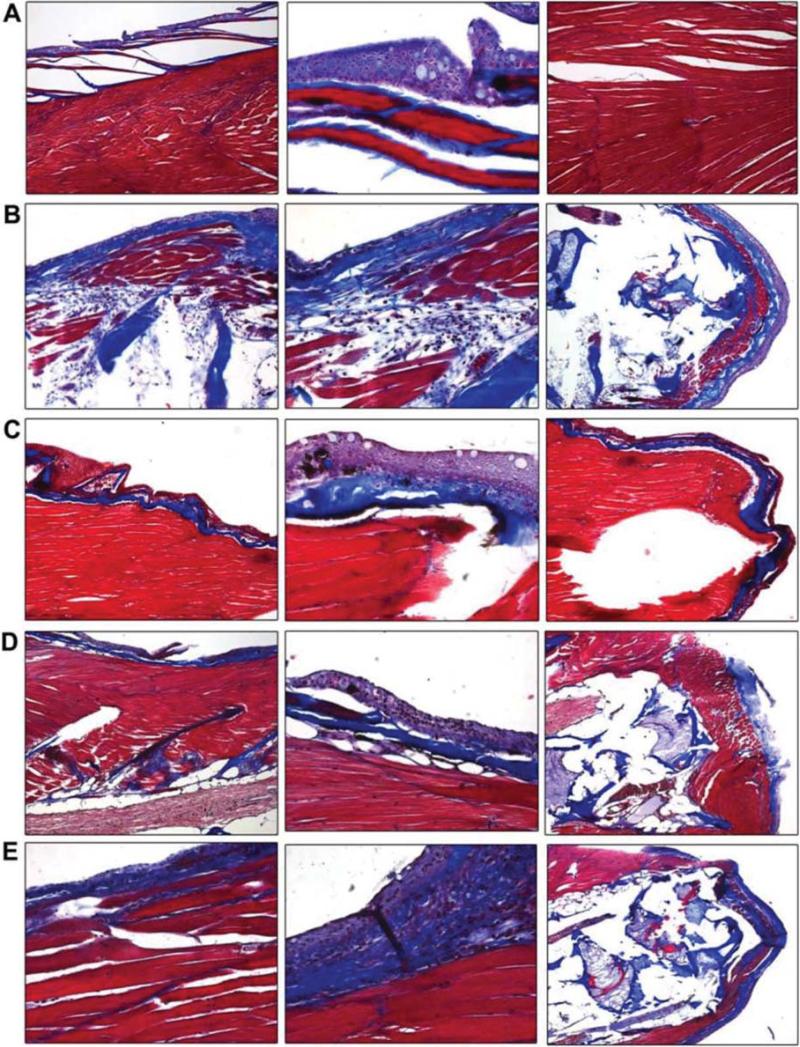
Sagittal sections of zebrafish were prepared for histopathological evaluation and Mallory trichrome staining, for evidence of fibrosis (Figure 3). Control non-irradiated muscle and skin is shown in Figure 3A and 3B, respectively. Irradiation of the caudal portion of the fish resulted in increased fibrosis as revealed by muscle atrophy, replacement of the musculature with both fatty/adipose cells, reactive skin with ongoing apoptotic cells, and abnormal layering and thickening of the skin (Figure 3C and 3D). Mallory-Trichrome staining also showed thickened areas of collagen, superficially (Figure 3E), as well as between muscle bundles (Figure 3F). These findings were similar to those seen in clinical radiation fibrosis (60-61).
Figure 3.
Histopathologic appearance of fibrotic changes in tails of irradiated zebrafish. Zebrafish were irradiated to 30 Gy and sacrificed 60 days later. The tails were removed from irradiated as well as nonirradiated control fish, fixed, sectioned and stained with H&E or Mallory's trichrome stain. Nonirradiated fish muscle and skin are shown in Figure 3A and 3B, respectively. Irradiated muscle and skin are shown in Figure 3C and 3D, respectively. Mallory's trichrome staining of collagen in nonirradiated and irradiated fish are shown in Figure 3E and 3F, respectively.
Effect of water-soluble small-molecule antioxidants on ameliorating irradiation fibrosis
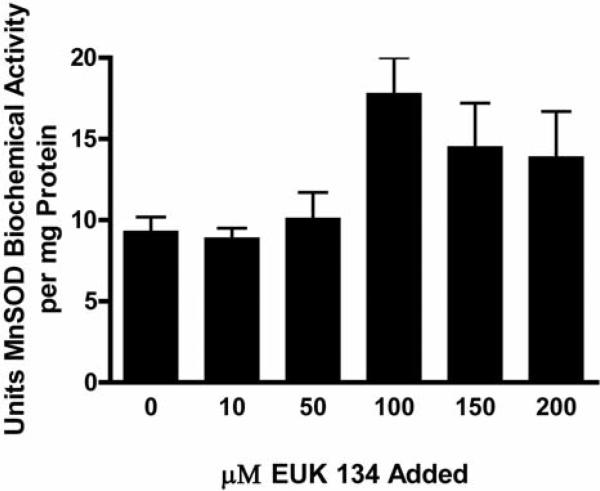
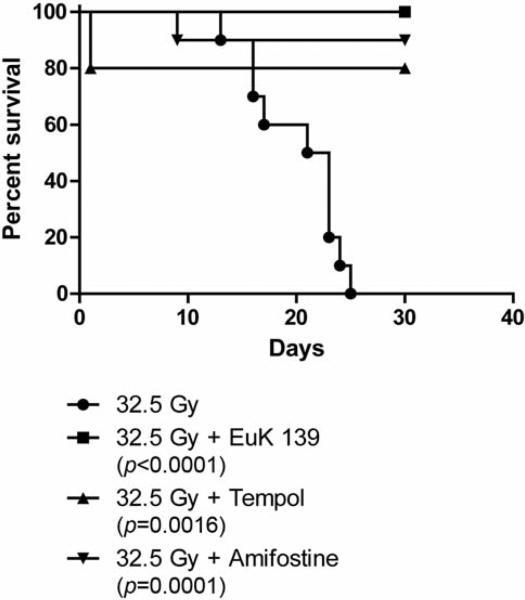
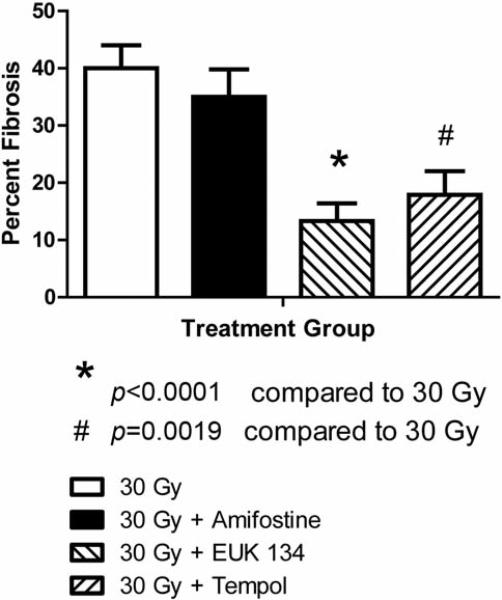
We tested the effect of Amifostine, tempol and the SOD mimetic EUK 134 in preventing radiation fibrosis by determining if this radiation toxicity ameliorating drug altered on the magnitude of radiation fibrosis in fish receiving 32.5 Gy to the posterior body half. To determine if the drugs could be concentrated in the zebrafish tissue, zebrafish were incubated in the water containing EUK 134 at concentrations ranging from 0 to 200 μM for one hour at which time the SOD activity in the muscles of the tail was determined (Figure 4). Increased SOD activity was found in fish containing 100 μM EUK (p=0.0178). To demonstrate that the small molecule antioxidants could mitigate irradiation damage, zebrafish were irradiated to 32.5 and placed in water containing amifostine, tempol, or EUK134. The fish were followed for the development of fibrosis at which time they were sacrificed. All control fish irradiated at 35 Gy were dead by 25 days (Figure 5). Fish incubated in amifostine, tempol, or EUK134 had increased survival compared to control-irradiated fish (p<0.0001, =0.0016 or =0.0001, respectively, Figure 5). Not only did EUK134 and tempol increase survival, fish irradiated to 30 Gy and incubated in tempol or EUK134 also demonstrated less fibrosis compared to control-irradiated fish at 60 days after irradiation (p<0.0001 or =0.0019, respectively, Figs. 6 and 7). Although amifostine increased survival after 32.5 Gy, it did not decrease fibrosis at 60 days after 30 Gy (Figures 6 and 7).
Figure 4.
Increased SOD activity in zebrafish maintained in EUK139. Zebrafish were maintained free swimming in concentrations of EUK139 ranging from 0 to 200 μM for 1 hr, at which time the fish were sacrificed and then frozen in liquid nitrogen. The fish were thawed and the tail was removed, homogenized and the MnSOD biochemical activity determined. Fish maintained in 100 μM drug had a significantly increased MnSOD biochemical activity compared to control untreated fish (p=0.0178).
Figure 5.
EUK139 or tempol added after irradiation improves survival of irradiated zebrafish. Fish were irradiated to 30 Gy, placed in water containing amifostine (20 μM), tempol (1 mM), EUK139 (50 μM) or vehicle control and followed for survival. Zebrafish treated with EUK139, Amifostine or Tempol after irradiation showed an increased survival compared to control fish. Drugs added pre-irradiation were toxic.
Figure 6.
EUK139 mitigates irradiation induced fibrosis. Zebrafish were irradiated to 30 Gy to the tails as described in Methods, and then placed in water containing amifostine (20 μM), tempol (1 mM), or EUK139 (50 μM). Two months later surviving fish were sacrificed, fixed, and tails stained with Mallory's trichrome stain and examined microscopically for fibrosis. Panel 6A shows the control fish, and Panel 6B is 60 days after 30 Gy. Fish placed in water containing 50 μM EUK139, after 30 Gy, are shown in Panel 6C. Panels 6D and 6E show fish maintained in tempol or amifostine, respectively, after 30 Gy. The percent of tissue showing fibrosis was determined in fish maintained in EUK139 or tempol compared to control-irradiated fish and showed significantly decreased fibrosis compared to 30-Gy, control-irradiated fish (p<0.0001 or p=0.0019, respectively, Figure 7).
Figure 7.
EUK134 or tempol prevents fibrosis in groups of 20 Zebrafish irradiated to 30 Gy to the caudal body measured at 60 days. Results are presented as the percent fibrosis±SEM from each group of fish described in the legend to Figure 6.
Discussion
One potential advantage of the zebrafish model for testing new small-molecule agents as potential clinical translational use in the prevention of radiation fibrosis is the clear economic advantage. The zebrafish model is cos-effective relative to rodent models of radiation fibrosis. The standard model for radiation pulmonary fibrosis is the C57BL/6J mouse (1, 3, 19). Since the onset of fibrosis is 100 to 120 days after irradiation, maintenance of significantly large numbers of animals (particularly 12 per group) for 120 days to 200 days, 5 to 9 thousand dollars. In contrast, the maintenance in significant numbers of zebrafish in multiple doses of a drug would be significantly less expensive.
Water-soluble agents can be monitored in a water quite effectively and this is as an advantage over taking blood or plasma samples from mice. The ability to sacrifice small numbers of fish at serial time points after administration of a drug and quantitate drug deposition in tissue relative to concentration of the water also greatly facilitates cost-effective analyses of drug uptake relative to effect. A potential disadvantage of the zebrafish system is the requirement for water solubility of small molecule agents or in the case of drug screening small molecules for potential clinical use (33, 38, 42, 44). The zebrafish model facilitates rapid translation to the clinic of agents likely to be delivered by oral and topical application which means rapid delivery to the circulation would be desirable for systemic administration. In contrast, for those agents used for organ specific radioprotection, such as Manganese Superoxide Dismutase-Plasmid Liposome protection of the lung (1), esophagus (2, 8), or oral cavity/oropharynx (16), bladder (12), a lipid-soluble and organ-specific application might be desirable (57). However, even in these situations, organ-specific radioprotection systemic tests of agents in animal models are desirable to rule-out potential systemic toxicities of a locally delivered agent. The present studies provide support for the use of the zebrafish model as a test agent for new radiation counter measures against both acute and late effects of irradiation including the prevention of late fibrosis.
Acknowledgements
Supported by NIH Grant of the NIAID U19 A168021.
References
- 1.Epperly MW, Bray JA, Kraeger S, Zwacka R, Engelhardt J, Travis E, Greenberger JS. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Therapy. 1998;5:196–208. doi: 10.1038/sj.gt.3300580. [DOI] [PubMed] [Google Scholar]
- 2.Epperly MW, Bray JA, Krager S, Berry LA, Gooding W, Engelhardt JF, Zwacka R, Travis EL, Greenberger JS. Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int J Radiat Oncol Phys. 1999;43(1):169–181. doi: 10.1016/s0360-3016(98)00355-1. [DOI] [PubMed] [Google Scholar]
- 3.Epperly MW, Travis EL, Sikora C, Greenberger JS. Magnesium superoxide dismutase (MnSOD) plasmid/liposome pulmonary radioprotective gene therapy: Modulation of irradiation-induced mRNA for IL-1, TNF-α, and TGF-ß correlates with delay of organizing alveolitis/fibrosis. Biology of Blood and Marrow Transplantation. 1999;5:204–214. doi: 10.1053/bbmt.1999.v5.pm10465100. [DOI] [PubMed] [Google Scholar]
- 4.Stickle RL, Epperly MW, Klein E, Bray JA, Greenberger JS. Prevention of irradiation-induced esophagitis by plasmid/ liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Radiat Oncol Invest Clinical & Basic Res. 1999;7(6):204–217. doi: 10.1002/(SICI)1520-6823(1999)7:4<204::AID-ROI2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Epperly MW, Sikora C, Defilippi S, Bray J, Koe G, Liggitt D, Luketich JD, Greenberger JS. Plasmid/liposome transfer of the human manganese superoxide dismutase (MnSOD) transgene prevents ionizing irradiation-induced apoptosis in human esophagus organ explant culture. Int J Cancer (Radiat Oncol Invest.) 2000;90(3):128–137. doi: 10.1002/1097-0215(20000620)90:3<128::aid-ijc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Epperly MW, Defilippi S, Sikora C, Gretton J, Kalend K, Greenberger JS. Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthotopic tumors from irradiation. Gene Ther. 2000;7(12):1011–1018. doi: 10.1038/sj.gt.3301207. [DOI] [PubMed] [Google Scholar]
- 7.Epperly MW, Epstein CJ, Travis EL, Greenberger JS. Decreased pulmonary radiation resistance of manganese superoxide dismutase (MnSOD)-deficient mice is corrected by human manganese Superoxide dismutase-plasmid/liposome (SOD2-PL) intratracheal gene therapy. Radiation Res. 2000;154(4):365–374. doi: 10.1667/0033-7587(2000)154[0365:dprrom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Epperly MW, Gretton JA, DeFilippi SJ, Sikora CA, Liggitt D, Koe G, Greenberger JS. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Epperly MW, Kagan VE, Sikora CA, Gretton JE, Defilippi SJ, Bar-Sagi D, Greenberger JS. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) administration protects mice from esophagitis associated with fractionated irradiation. Int J Cancer (Radiat Oncol Invest) 2001;96(4):221–233. doi: 10.1002/ijc.1023. [DOI] [PubMed] [Google Scholar]
- 10.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JE, Bar-Sagi D, Carlos T, Guo HL, Greenberger JS. Pulmonary irradiation-induced expression of VCAM-1 and ICAM-1 is decreased by MnSOD-PL gene therapy. Biol Blood Bone Marrow Transplant. 2002;8:175–187. doi: 10.1053/bbmt.2002.v8.pm12014807. [DOI] [PubMed] [Google Scholar]
- 11.Epperly MW, Sikora C, Defilippi S, Gretton J, Zhan Q, Kufe DW, Greenberger JS. MnSOD inhibits irradiation-induced apoptosis by stabilization of the mitochondrial membrane against the effects of SAP kinases p38 and Jnk1 translocation. Radiation Res. 2002;157:568–577. doi: 10.1667/0033-7587(2002)157[0568:msdsir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Kanai AJ, Zeidel ML, Lavelle JP, Greenberger JS, Birder LA, de Groat WC, Apodaca GL, Meyers SA, Ramage R, VanBibber MM, Epperly MW. Manganese superoxide dismutase gene therapy protects against irradiation-induced cystitis. Am J of Physiology (Renal Physiology) 2002;44:1152–1160. doi: 10.1152/ajprenal.00228.2002. [DOI] [PubMed] [Google Scholar]
- 13.Epperly MW, Defilippi S, Sikora C, Gretton J, Greenberger JS. Radioprotection of lung and esophagus by overexpression of the human manganese superoxide dismutase transgene. Military Medicine. 2002;167(1):071. [PubMed] [Google Scholar]
- 14.Epperly MW, Guo HL, Jefferson M, Wong S, Gretton J, Bernarding M, Bar-Sagi D, Greenberger JS. Cell phenotype specific duration of expression of epitope-tagged HA-MnSOD in cells of the murine lung following intratracheal plasmid liposome gene therapy. Gene Therapy. 2003;10:163–171. doi: 10.1038/sj.gt.3301852. [DOI] [PubMed] [Google Scholar]
- 15.Guo HL, Seixas-Silva JA, Epperly MW, Gretton JE, Shin DM, Greenberger JS. Prevention of irradiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (MnSOD) transgene. Radiation Research. 2003;159:361–370. doi: 10.1667/0033-7587(2003)159[0361:porioc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Guo HL, Epperly MW, Bernarding M, Nie S, Gretton J, Jefferson M, Greenberger JS. Manganese superoxide dismutaseplasmid/liposome (MnSOD-PL) intratracheal gene therapy reduction of irradiation-induced inflammatory cytokines does not protect orthotopic lewis lung carcinomas. In Vivo. 2003;17:13–22. [PubMed] [Google Scholar]
- 17.Greenberger JS, Epperly MW, Gretton J, Jefferson M, Nie S, Bernarding M, Kagan V, Guo HL. Radioprotective gene therapy. Current Gene Therapy. 2003;3:183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 18.Epperly MW, Guo HL, Bernarding M, Gretton J, Jefferson M, Greenberger JS. Delayed intratracheal injection of manganese superoxide dismutase (MnSOD)-plasmid/liposomes provides suboptimal protection against irradiation-induced pulmonary injury compared to treatment before irradiation. Gene Ther Mol Biol. 2003;7:61–68. [Google Scholar]
- 19.Epperly MW, Sikora CA, Defilippi S, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Resp Molecular Cell Biology. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 20.Epperly MW, Gretton JE, Bernarding M, Nie S, Rasul B, Greenberger JS. Mitochondrial localization of copper/zinc superoxide dismutase (Cu/ZnSOD) confers radioprotective functions in vitro and in vivo. Radiation Research. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 21.Epperly MW, Hongliang G, Shields D, Zhang X, Flanders K, Lambert P, Greenberger JS. Correlation of ionizing irradiation-induced late pulmonary fibrosis with long-term bone marrow culture fibroblast progenitor cell biology in mice homozygous deletion recombinant negative for endothelial cell adhesion molecules. In Vivo. 2004;18:1–14. [PubMed] [Google Scholar]
- 22.Greenberger JS, Epperly MW. Radioprotective antioxidant gene therapy: potential mechanisms of action. Gene Therapy and Molecular Biology (GTMB) 2004;8:31–44. [Google Scholar]
- 23.Greenberger JS, Epperly MW. Pleiotrophic stem cell and tissue effects of ionizing irradiation protection by MnSOD-plasmid liposome gene therapy. In: Columbus Frank., editor. Progress in Gene Therapy. Nova Science Publications; pp. 110–118, 2005. [Google Scholar]
- 24.Epperly MW, Goff JP, Sikora CA, Shields DS, Greenberger JS. Bone marrow origin of cells with capacity for homing and differentiation to esophageal squamous epithelium. Radiat Res. 2004;162:233–240. doi: 10.1667/rr3224. [DOI] [PubMed] [Google Scholar]
- 25.Epperly MW, Carpenter M, Agarwal A, Mitra P, Nie S, Greenberger JS. Intra-oral manganese superoxide dismutase plasmid liposome radioprotective gene therapy decreases ionizing irradiation-induced murine mucosal cell cycling and apoptosis. In Vivo. 2004;18:401–410. [PubMed] [Google Scholar]
- 26.Epperly MW, Shen H, Jefferson M, Greenberger JS. In vitro differentiation capacity of esophageal progenitor cells with capacity for homing and repopulation of the ionizing irradition damaged esophagus. In Vivo. 2004;18:675–685. [PubMed] [Google Scholar]
- 27.Stone HB, Coleman CN, Moulder JE, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS, Hauer-Jensen M, Hill RP, Kolesnick RN, MacVittie TJ, Marks C, McBride WH, Metting N, Pellmar T, Purucker M, Robbins MEC, Schiestl RH, Seed TM, Tomaszewski J, Travis EL, Wallner PE, Wolpert M, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation, and treatment of radiation injuries. Report of an NCI Workshop, December 3-4, 2003. Rad Research. 2004;162:711–718. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter M, Epperly MW, Agarwal A, Nie S, Hricisak L, Niu Y, Greenberger JS. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes (MnSOD-PL) protects the murine lung from irradiation damage. Gene Therapy. 2005;12:685–690. doi: 10.1038/sj.gt.3302468. [DOI] [PubMed] [Google Scholar]
- 29.Yaron P, Epperly MW, Fernando HC, Klein E, Finkelstein S, Greenberger JS, Luketich JD. Photodynamic therapy induced esophageal-stricture-An animal model: from mouse to pig. J Surgical Research. 2005;123:67–74. doi: 10.1016/j.jss.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Epperly MW, Zhang X, Nie S, Cao S, Kagan V, Tyurin V, Greenberger JS. MnSOD-Plasmid Liposome gene therapy effects on ionizing irradiation induced lipid peroxidation of the Esophagus. In Vivo. 2005;19:997–1004. [PubMed] [Google Scholar]
- 31.Epperly MW, Shen H, Zhang X, Nie S, Cao S, Greenberger JS. Protection of esophageal stem cells from ionizing irradiation by MnSOD-Plasmid Liposome gene therapy. In Vivo. 2005;19:965–974. [PubMed] [Google Scholar]
- 32.Epperly MW, Franicola D, Zhang X, Nie S, Wang H, Bahnson A, Shields D, Goff J, Greenberger JS. Decreased irradiation pulmonary fibrosis in Smad3−/− marrow chimeric mice correlates to reduced bone marrow stromal cell migration in vitro. In Vivo. 2006;20:573–582. [PubMed] [Google Scholar]
- 33.Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, Vlasova IL, Amoscato AA, Braslau R, Studer A, Fink MP, Greenberger JS, Wipf P, Kagan VE. Structural requirements for optimized delivery, inhibition of oxidative stress and anti-apoptotic activity of targeted nitroxides. J Pharmacology, Experimental Therapeutics. 2007;320(5):1050–1060. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- 34.Greenberger JS, Epperly MW. Antioxidant therapeutic approaches toward amelioration of the pulmonary pathophysiological damaging effects of ionizing irradiation. Current Respiratory Medicine Reviews. 2007;3:29–37. [Google Scholar]
- 35.Belikova NA, Jiang J, Tyurina YY, Zhao Q, Epperly MW, Greenberger J, Kagan VE. Cardiolipin specific peroxidase reactions of cytochrome c in mitochondria during irradiation induced apoptosis. Int J Radiat Oncol Biol Phys. 2007;69(1):176–185. doi: 10.1016/j.ijrobp.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 36.Greenberger JS. Gene therapy approaches for stem cell protection. Gene Therapy. 2008;15:100–108. doi: 10.1038/sj.gt.3303004. [DOI] [PubMed] [Google Scholar]
- 37.Fink MP, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, Kagan VE, Wipf Peter. Hemigramicidin-TEMPO Conjugates: Novel mitochondria-targeted anti-oxidants. Biochemical Pharmacology. 2007;74:801–809. doi: 10.1016/j.bcp.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Jiang J, McDonald P, Dixon TM, Franicola D, Zhang X, Nie S, Epperly LD, Kagan VE, Lazo JS, Epperly MW, Greenberger JS. Druggable genome siRNA library screening identifies Glybencamide as a novel radioprotector. Rad Res. 2009;172:414–422. doi: 10.1667/RR1674.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang J, Stoyanovsky D, Belikova NA, Tyurina YY, Zhao Q, Tungekar MA, Kapralova V, Huang Z, Mintz A, Greenberger JS, Kagan VE. A mitochondria-targeted triphenylphosphoniumconjugated nitroxide functions as a radioprotector/mitigator. Rad Res. 2009;172:706–714. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajagopalan MS, Gupta K, Epperly MW, Franicola D, Zhang X, Wang H, Zhao H, Tyurin VA, Kagan VE, Wipf P, Kanai A, Greenberger JS. The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair. In Vivo. 2009;23:717–726. [PMC free article] [PubMed] [Google Scholar]
- 41.Kagan VE, Wipf P, Stoyanovsky D, Greenberger JS, Borisenko G, Belikova NA, Yanamala N, Samhan AAK, Tungekar MA, Jiang J, Tyurina YY, Ji J, Klein-Seetharaman J, Pitt BR, Shvedovah AA, Bayir H. Mitochondrial targeting of electron scavenging antioxidants: Regulation of selective oxidation vs. random chain reactions. Advanced Drug Delivery Reviews. 2009;61(14):1375–85. doi: 10.1016/j.addr.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epperly MW, Franicola D, Shields D, Rwigema JC, Stone B, Zhang X, McBride W, Georges G, Wipf P, Greenberger JS. Screening for in vitro radiation protection and mitigation capacity of antimicrobial agents including those used in supportive care regimens for bone marrow transplant recipients. In Vivo. 2010;24(1):9–20. [PMC free article] [PubMed] [Google Scholar]
- 43.Gokhale AS, Epperly M, Glowacki J, Wang H, Wipf P, Pierce JG, Dixon T, Patrene K, Greenberger JS. Small molecule GS-nitroxide and MnSOD gene therapy ameliorate ionizing irradiation-induced delay in bone wound healing in a novel murine model. In Vivo. 2010;24:377–386. [PMC free article] [PubMed] [Google Scholar]
- 44.Mustata G, Li M, Zevola N, Bakan A, Zhang L, Epperly M, Greenberger J S, Yu J, Bahar I: Development of small-molecule PUMA inhibitors for mitigating radiation-induced cell death. Curr Med Chem Top. doi: 10.2174/156802611794072641. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koide K, Song F, Garner AL, Greenberger JS, Epperly MW. The use of 3,5,4’-Tri-O-acetylresveratrol as a potential pro-drug for Resveratrol protects mice from γ-irradiation-induced death. Am Chem Soc. doi: 10.1021/ml100159p. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epperly MW, Wang H, Jones J, Dixon T, Montesinos C, Greenberger JS. Antioxidant-chemoprevention diet ameliorates late effects of total body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Radiation Research. doi: 10.1667/RR2398.1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epperly M, Jin SQ, Nie S, Cao S, Zhang X, Franicola D, Fink M, Greenberger JS. Ethyl pyruvate, a potentially effective total body irradiation damage mitigator. Rad Res. 2007;168:552–559. doi: 10.1667/RR1009.1. [DOI] [PubMed] [Google Scholar]
- 48.Niu Y, Epperly MW, Shen H, Smith T, Lewis D, Gollin S, Greenberger JS. Intraesophageal MnSOD-Plasmid Liposome administration enhances engraftment and self-renewal capacity of bone marrow derived progenitors of esophageal squamous epithelium. Gene Therapy. 2008;15:347–356. doi: 10.1038/sj.gt.3303089. [DOI] [PubMed] [Google Scholar]
- 49.Fink M, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, Kagan VE, Wipf P. Hemigramicidin-TEMPO conjugates: Novel mitochondria-targeted antioxidants. Crit Care Med. 2007;35(9):5461–5470. doi: 10.1097/01.CCM.0000279192.96303.E7. [DOI] [PubMed] [Google Scholar]
- 50.Jiang J, Belikova NA, Xiao J, Zhao Q, Greenberger JS, Wipf P, Kagan VE. A mitochondria-targeted nitroxide/hemigramicidin S conjugate protects mouse embryonic cells against γ–irradiation. Int J Radiat Oncol Biol Phys. 2008;70(3):816–825. doi: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Epperly MW, Smith T, Wang H, Schlesselman J, Franicola D, Greenberger JS. Modulation of total body irradiation induced life shortening by systemic intravenous MnSOD-plasmid liposome gene therapy. Rad Res. 2008;170(4):437–444. doi: 10.1667/rr1286.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kagan VE, Bayir A, Bayir H, Stoyanovsky D, Borisenko GG, Tyurina YY, Wipf P, Atkinson J, Greenberger JS, Chapkin RS, Belikova NA. Mitochondria-targeted disruptors and inhibitors of cytochrome c/cardiolipin peroxidase complexes: A new strategy in anti-apoptotic drug discovery. Mol Nutr Food Res. 2009;53:104–114. doi: 10.1002/mnfr.200700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Epperly MW, Melendez A, Zhang X, Franicola D, Smith T, Greenberger BA, Komanduri P, Greenberger JS. Mitochondrial targeting of a catalase transgene product by plasmid liposomes increases radioresistance in vitro and in vivo. Radiation Research. 2009;171:588–595. doi: 10.1667/RR1424.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek P, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenberger JS. “Radioprotection”. In Vivo. 2009;23:323–336. [PMC free article] [PubMed] [Google Scholar]
- 56.Greenberger JS, Epperly M. Bone marrow-derived stem cells and radiation response. Semin Radiat Oncol. 2009;19:133–139. doi: 10.1016/j.semradonc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Epperly MW, Rwigema JCM, Li S, Gao X, Wipf P, Goff J, Wang H, Franicola D, Shen H, Kagan V, Bernard M, Greenberger JS. Intraesophageal administration of GS-nitroxide (JP4-039) protects against ionizing irradiation-induced esophagitis. In Vivo. 2010;24(6):811–821. [PMC free article] [PubMed] [Google Scholar]
- 58.Rwigema JCM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, Franicola D, Dixon T, Frantz MC, Wipf P, Tyurina Y, Kagan VE, Wang H, Greenberger JS. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80(3):860–868. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McAleer MF, Farber A, Rodeck U, Dicker AP. Differential sensitivity of zebrafish embryos to distinct classes of DNA-damaging agent associated with differential expression of DNA repair enzymes. Int J Radiat Oncol Biol Phys. 2004;60((1)Supp):S349. [Google Scholar]
- 60.Martin M, Lefaix J-L, Delanian S. TGFβ1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 61.Hall E. Radiation Biology for Radiobiologist. 4th ed. J.B. Lippincott, Inc.; Philadelphia, PA: 1999. [Google Scholar]