Abstract
Aim
We determined whether absence of caspase-1 altered the stress response of hematopoietic and bone marrow stromal cells in vitro.
Materials and Methods
Long-term bone marrow cultures from caspase-1 –/– and control caspase-1 +/+ mice were established and the derived bone marrow stromal and interleukin-3 (Il-3)-dependent hematopoietic progenitor cell lines were evaluated for radiosensitivity.
Results
Long-term bone marrow cultures from caspase-1 –/– mice generated hematopoietic cells for over 30 weeks in vitro, significantly longer than controls did (p=0.0018). Bone marrow stromal (mesenchymal stem cell) and Il-3-dependent hematopoietic progenitor cell lines from caspase-1–/– marrow cultures compared to caspase-1 +/+ were radioresistant (p=0.0486 and p=0.0235 respectively). Total-body irradiated caspase-1 –/– mice were not significantly radioresistant compared to controls (p=0.6542).
Conclusion
Caspase-1 deletion increases hematopoiesis and radioresistance of bone marrow cells in vitro.
Keywords: Caspase-1, radiosensitivity, radioresistance, long-term bone marrow cultures
Multiple molecular biological pathways have been demonstrated to be involved in cell killing by ionizing irradiation, including intramitotic death, autophagy, necroptosis, and apoptosis (1-3). The process of apoptosis is initiated by both external and internal cellular processes. For example, extracellular binding of tumor necrosis factor-α (TNF-α) to its receptor on the cell membrane initiates activation of the apoptotic pathway through caspase-1 (1). One form of intracellular activation of apoptosis involves ionizing irradiation-induced DNA strand breaks, which while repaired in minutes, initiate a process of translocation of nuclear proteins to the mitochondria including stress activated protein kinases, which lead to mitochondrial membrane depolarization, separation of cytochrome c from cardiolipin at the inner mitochondrial membrane, and leakage of cytochrome c into the cytoplasm, where activation of the caspase pathway follows (13-15). Caspase pathway activation leads to poly-ADP-ribosyl-polymerase activation, DNA fragmentation, and apoptosis (13).
Agents which activate apoptosis include those involved in the oxidative stress response of cells, tissues, and organs (16). Ionizing irradiation is prominent in the induction of radical oxygen species (ROS), including superoxide, nitric oxide, and peroxynitrite (15), which consume cellular antioxidant stores and accelerate mitochondrial damage, leading to cytochrome c leakage and activation of the caspase pathway (13).
Caspase-1 deficiency has been shown to lead to resistance to other forms of oxidative stress, including that associated with neurodegeneration (2). To determine whether caspase-1 is involved in the oxidative stress response of bone marrow of caspase-1 –/– mice, we evaluated oxidative stress induced by hematopoiesis in continuous bone marrow cultures, radiosensitivity of bone marrow stromal and interleukin (Il-3)-dependent hematopoietic cell lines in vitro, and the response to total-body irradiation.
Materials and Methods
Mice
Homologous recombinant-negative caspase-1 –/– mice on a C57BL/6 background were developed by BASF Research (Charlotte, NC, USA) and obtained from Dr. F. Wong (17). The caspase-1 +/+ mouse strain used in these experiments was C57BL/6NTac (Taconic Farms, Hudson, NY, USA).
Mice were housed five per cage and fed standard Purina Laboratory Chow according to IACUC Institutional regulations (IACUC protocol 1201406). All experimental protocols were approved by the University of Pittsburgh IACUC (Assurance Number A3187-01). Veterinary care was provided by the Division of Laboratory Animal Research of the University of Pittsburgh.
Mouse strain genotyping
To confirm that the mice and cell lines were caspase-1 –/– or caspase-1 +/+, DNA was isolated from the Caspase-1–/– mice, C57BL/6NTac, stromal cell lines, and Il-3 dependent cell lines. Polymerase Chain Reaction (PCR) was used to genotype the mice or cell lines using primers specific for caspase-1 –/– gene or Caspase-1 +/+ gene. The caspase-1 –/– primers were ICER7; 5′- CCT GGT GTT GAA GAG CAG AA -3′ and NEUF: 5′- TGC TCC TGC CGA GAA AGT AT -3′ with a PCR product of 1400 base pairs. The caspase-1 +/+ primers were ICEF6: 5′- ATC CAG GAG GGA ATA TGT GG -3′ and ICER7: 5′- CCT GGT GTT GAA GAG CAG AA -3′ with a PCR product of 700 base pairs. The PCR reaction mix contained a final concentration of 2.5 mM MgCl2 for the caspase-1 –/– reaction. The PCR reactions was as follows: 95°C for 5 min, 94°C for 45 s, 58°C for 1 min, 72°C for 2 min, back to step 2 for 35 cycles, 72°C for 10 min, and 4°C for soaking.
Long-term bone marrow cultures (LTBMCs)
The methods for establishment of LTMCs have been published previously (4, 5). Briefly, the contents of adult mouse femurs and tibias were flushed through a 17-gauge needle into 25 cm2 plastic flasks (Corning Incorporated, Corning, NY, USA) and maintained in a high humidity incubator with 7% CO2, in Fisher's medium supplemented with 15% heat-inactivated fetal calf serum with penicillin and streptomycin. Cultures were supplemented with 10–5 M hydrocortisone sodium hemisuccinate (4). Fresh corticosteroid was added weekly according to these published methods (4, 5).
Non-adherent cells were removed weekly and total cells counted, and then plated in 0.8% methylcellulose-containing medium supplemented with growth factors for hematopoiesis, according to published methods (6-10). Day 7 and day 14 colony forming units-granulocyte-macrophage (CFU-GM) colonies were counted using an inverted microscope for each time point (4, 5). For the experiments described, eight cultures were established from four mice (one tibia and one femur per flask) for each of the genotypes studied. Cultures were scored weekly for the percentage of surface area covered with adherent cells, number of cobblestone islands (representative of adherent hematopoietic stem cell containing cell populations), as well as for the non-adherent cells produced per flask, and number of non-adherent cells producing day 7 and day 14 CFU-GM (11, 12).
Data for weekly cobblestone island numbers, non-adherent cell numbers, percentage of confluence of adherent cells, day 7 colony counts and day 14 colony counts were collected at week 1 through week 51. At each week, the cobblestone numbers for each mouse (collected from two flasks) were averaged. Three mice were compared in each group (caspase-1 –/– or control caspase-1 +/+) (total six data points). The two groups were compared each week with the two-sided two-sample t-test, using the averaged numbers. Similar calculations were performed for non-adherent cell numbers and for the percentage of confluence of adherent cells. For day 7 and day 14 colony-forming progenitor cell counts, observations were compared between the two groups each week with the two-sided two-sample t-test.
Derivation of bone marrow stromal cell lines
Adherent cells from LTBMCs at 20 weeks were transferred by trypsinization to plastic petri dishes and grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, with penicillin and streptomycin (11, 12). Cell lines were expanded conservatively and then cloned by Poissen distribution methods according to published procedures (12). Clonal lines of caspase-1 –/– and caspase-1 +/+ bone marrow stromal cell cultures were expanded according to published methods (12).
Derivation of Il-3 dependent hematopoietic progenitor cell lines
Non-adherent cells were harvested from caspase-1 –/– and caspase-1 +/+ mouse LTBMCs at week 4 and cultured in six-well tissue culture plate with 4.0 ml of Iscove's modified Eagle's medium supplemented with 20% fetal calf serum and 1.0 ng/ml of recombinant Il-3 (Peprotech, Rocky Hill, NJ, USA). The cells were passaged weekly by cytocentrifugation at 300 ×g and replenished with 4.0 ml fresh medium. Cells were kept at high density and passaged weekly by this method for 10 weeks, at which time the mixture was split into two. From the passage at week 10, the cells were frozen at –80°C for one week, and thawed for culture in the same medium as above. The re-cultured cells were termed as primary Il-3-dependent cell lines and split for colony assay and subcloning (6, 7).
Clonogenic radiation survival curves
The methods for radiation survival curves for adherent cell lines (12) and non-adherent hematopoietic progenitor cell lines (13, 14) have been published previously. Briefly, cells were irradiated to doses between 0 and 800 cGy, using a JL Shepherd Mark I Model 68 cesium irradiator (JL Shepherd and Associates, San Fernando, CA, USA) at 70 cGy per minute. Adherent cells were plated in quadruplicate and colonies of 50 cells or more scored at seven days. Non-adherent Il-3-dependent cell lines were plated in triplicate in methylcellulose containing recombinant mouse stem cell factor (SCF), Il-3, Il-6, and erythropoietin (epo) (Stem Cell Technologies, Vancouver, BC, Canada) and CFU-GM colonies were scored on day 7. Survival curves were analyzed by linear regression and single-hit multi-target analysis according to published methods (19).
DNA repair measurements by the Comet assay
Measurement of DNA strand breaks after irradiation was performed as described previously (19). Cells of the caspase-1 –/– and caspase-1 +/+ cell lines were irradiated and incubated at 37°C for 0, 10 min, 1 h, 6 h, or 24 h at which time the cells were rapidly chilled to 4°C to stop DNA repair. The cells were mixed in low-melt agarose, and 500 cells were placed on the sample area of a CometSlide (Comet Assay 4250-050-K; Travigen, Inc., Gaithersburg, MD, USA). The slides were rapidly chilled to 4°C and kept in the dark to prevent DNA repair. The slides were then placed in pre-chilled lysis solution and kept at 4°C for 60 min, followed by washing in neutral electrophoresis buffer for 30 min, at 4°C, and electrophoresis at 21 V for 1 h at 4°C, then immersed in DNA precipitation solution for 30 min at room temperature, immersed in 70% ethanol for 30 min, and dried at 45°C for 15 min. The cells were then stained with SYBR Green 1 (Travigen, Inc., Gaithersburg, MD, USA) and examined under a fluorescence microscope. The comet tails for each of at least 150 cells were quantified using the Comet Assay IV software (19).
Measurements of antioxidant stores by Trolox assay
Total antioxidant status of the adherent caspase-1 –/– and caspase-1 +/+, and the non-adherent Il-3-dependent cell lines was determined using an antioxidant reductive capacity assay (Northwest Life Science Specialties, Vancouver, WA) as previously published (19). Cells were assayed 24 h after irradiation to 0, 5, or 10 Gy. The net absorbance values were compared to a standard curve and results reported as millimolar Trolox equivalents (19).
Total-body irradiation of mice
Total-body irradiation was carried out according to published methods (9, 10). Caspase-1 –/– and Caspase-1 +/+ (C57BL/6NTac) mice were irradiated to 9.25 Gy according to published methods using a JL Shepherd Mark I, model 68 cesium irradiator (JL Shepherd and Associates, San Fernando, CA, USA) at an irradiation dose rate of 70 cGy/min (9). Statistical analysis was performed using a log-rank test.
Statistical analysis of long term bone marrow cultures and radiation survival curves
For bone marrow culture data at each week, the cobblestone numbers for each mouse culture were collected from two flasks, and calculated by averaging the two numbers. The two groups were compared each week with the two-sided two-sample t-test, using the averaged numbers. As this was an exploratory study, p-values were not adjusted for multiple comparisons. Similar calculations and tests were performed for the weekly and cumulative non-adherent cell numbers and percentage confluence of the adherent cell layer. For the weekly and cumulative day 7 colony-forming cell progenitor production, the observations were compared between the two groups each week with the two-sided two-sample t-test. For the day 14 colony-forming progenitor cell production, the observations were compared between the two groups each week with the two-sided two-sample t-test. In all these tests, a p-value of less than 0.05 was regarded as significant. We did not adjust p-values for multiple comparisons. These analyses were carried out with SAS software (SAS Institute, Inc., Cary, NC, USA). Radiation survival curves were analyzed using log-rank test as published elsewhere (10).
Statistical analysis of cell line antioxidant levels and comet assays
The comet tail intensity was summarized by median and inter-quartile range (IQR) for each stromal cell or Il-3-dependent cell line and radiation dose (0 or 5 Gy) at different times (10 min, 20 min, or 30 min) after irradiation (19). The data distribution was skewed to the right. The two-sided Wilcoxon rank sum test was used to compare between the cell lines at different radiation doses and different times after irradiation. The data were then log-transformed so that their distribution was close to normal and a linear mixed model was built on the log-transformed data of each irradiated group, using cell line and time of measurement, and their interaction term as fixed explanatory variables. Each experiment was considered as a random effect, therefore, a total of six experiments were carried out. The F-test in this model was used to compare slopes between the cell lines and a significant p-value indicates a significant difference in the change of tail intensity with time after radiation.
Student's t-test was used to analyze statistical differences in the comet assay and antioxidant levels. In all these tests, a p-value of less than 0.05 was regarded as significant. We did not adjust p-values for multiple comparisons. These analyses were carried out with SAS software (SAS Institute, Inc., Cary, NC, USA).
Results
Genotyping of mice
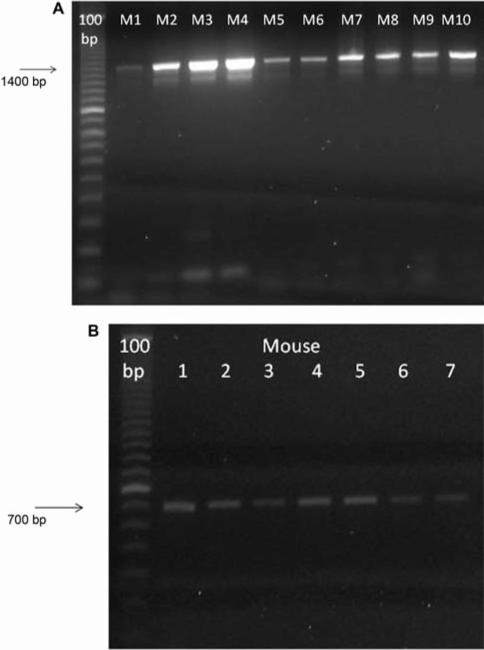
To ensure that the mice were caspase-1 –/–, PCR was used to genotype individual mice using primers specific for the caspase-1 gene (Figure 1).
Figure 1.
Genotype confirmation of caspase-1 –/– and caspase-1 +/+ mice. Polymerase Chain Reaction (PCR) on DNA isolated from caspase-1 –/– or C57BL/6NTac mice using primers specific for caspase-1–/– mice (1400 bp band) (A) and individual wild-type caspase-1 +/+ (700 bp band) (B). Numbers represent individual mice.
Continuous caspase-1 –/– bone marrow cultures demonstrate increased longevity
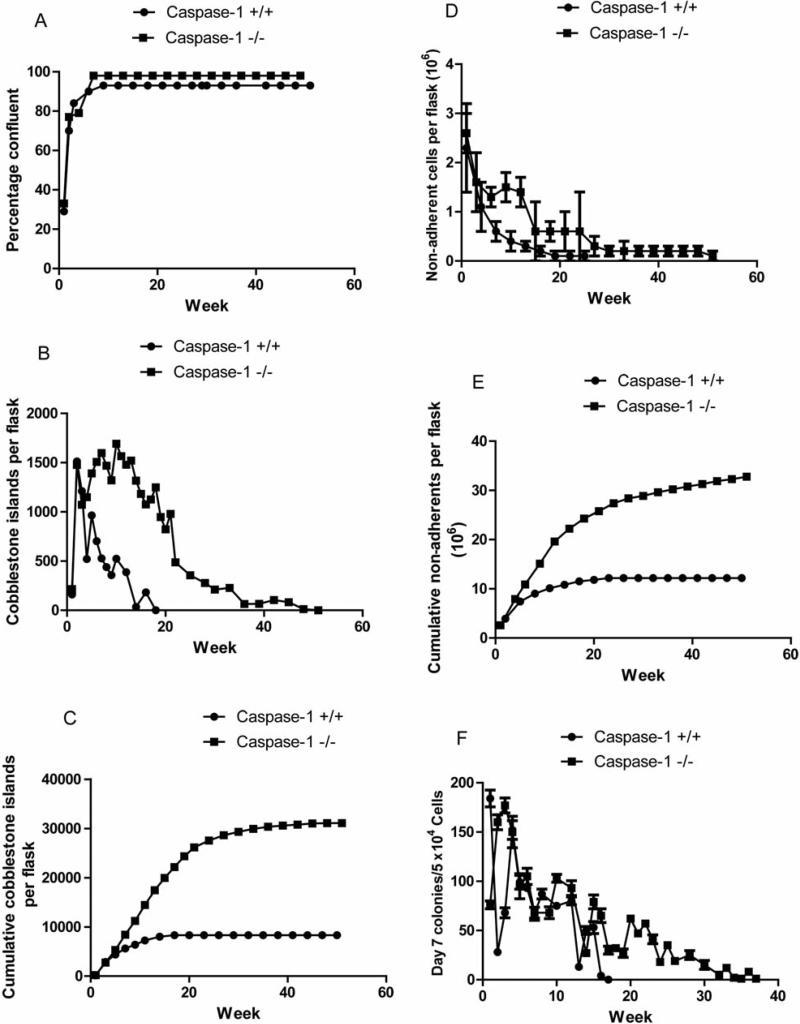
As shown in Figure 2A and Table I, LTBMCs for both C57BL/6NTac control caspase-1 +/+ and caspase-1 –/– mice reached a plateau phase of the adherent layer, with 100% confluence by week 5. These results are similar to those with LTBMCs from most mouse strains and stocks (4, 5). An assay of the adherent layer for flattened colonies of hematopoietic cells attached to the adherent stromal cell layer (cobblestone islands) was next evaluated according to published methods (12).
Figure 2.
Increased longevity of hematopoiesis in long-term bone marrow cultures from caspase-1 –/– compared to caspase-1 +/+ C57BL/6NTac mice. Eight cultures per group were tested weekly for percentage confluence of adherent layer (A); cobblestone islands scored weekly (B); cumulative cobblestone islands (C); nonadherent cells/flask (D); cumulative nonadherent colony-forming cells (E); weekly nonadherent day 7 colony-forming cells (F); cumulative day 7 colony-forming cells (G); weekly nonadherent day 14 colony-forming cells (H); cumulative day 14 colony-forming cells (I). (Statistical analysis is shown in Tables I-V).
Table I.
Analysis of hematopoiesis in long term culture of caspase-1 –/– mouse bone marrow cells: weekly confluence of adherent cell layer (%).
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
|---|---|---|---|---|---|
| Caspase 1 –/v | 32.5±6.1 (n=4) | 76.9±6.9 (n=4) | 78.8±3.2 (n=4) | 81.3±1.4 (n=4) | 80.6±1.2 (n=4) |
| Caspase 1 +/+ | 29.4±3.1 (n=4) | 75.0±4.1 (n=4) | 81.3±1.4 (n=4) | 80.6±1.3 (n=4) | 80.0±0.0 (n=4) |
| p-value | 0.3990 | 0.6559 | 0.2070 | 0.5370 | 0.3910 |
| Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | |
| Caspase 1 –/– | 90.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) |
| Caspase 1 +/+ | 90.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) |
| p-value | 1 | 1 | 1 | 1 | 1 |
| Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | |
| Caspase 1 –/– | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) |
| Caspase 1 +/+ | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) |
| p-value | 1 | 1 | 1 | 1 | 1 |
| Week 16 | Week 17 | Week 18 | Week 19 | Week 20 | |
| Caspase 1 –/– | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) |
| Caspase 1 +/+ | 95.0±0.0 (n=4) | 95.0±0.0 (n=3) | 95.0±0.0 (n=3) | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) |
| p-value | 1 | 1 | 1 | 1 | 1 |
| Week 21 | Week 22 | Week 23 | Week 24 | Week 25 | |
| Caspase 1 –/– | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) |
| Caspase 1 +/+ | 95.0±0.0 (n=1) | 95.0±0.0 (n=1) | 95.0±0.0 (n=1) | 95.0±0.0 (n=1) | 95.0±0.0 (n=1) |
| p-value | 1 | 1 | 1 | 1 | 1 |
| Week 26 | Week 27 | Week 28 | Week 29 | Week 30 | |
| Caspase 1 –/– | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) | 95.0±0.0 (n=4) |
| Caspase 1 +/+ | 95.0±0.0 (n=1) | CT | CT | CT | CT |
| p-value | 1 | ||||
| Week 31 | Week 32 | Week 33 | Week 34 | Week 35 | |
| Caspase 1 –/– | 95.0±0.0 (n=4) | 95.0±0.0 (n=3) | 95.0±0.0 (n=3) | 95.0±0.0 (n=3) | 95.0±0.0 (n=3) |
| Week 36 | Week 37 | Week 38 | Week 39 | Week 40 | |
| Caspase 1 –/– | 95.0±0.0 (n=3) | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) |
| Week 41 | Week 42 | Week 43 | Week 44 | Week 45 | |
| Caspase 1 –/– | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) |
| Week 46 | Week 47 | Week 48 | Week 49 | Week 50 | |
| Caspase 1 –/– | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) | 95.0±0.0 (n=2) |
| Week 51 | |||||
| Caspase 1 –/– | 95.0±0.0 (n=2) |
Data are summarized as mean±standard deviation, n is the number of mice used, and p-values were calculated with the two-sided two-sample t-test. Significant p-values are shown in bold. Due to a lack of hematopoietic activity beginning at week 27, the caspase-1 +/+ LTBMCs were terminated (CT).
Weekly-scored cobblestone islands from the flasks (eight cultures per group) demonstrated a clear decrease in the number of islands beginning at week 5 for caspase-1 +/+ mouse controls (Figure 2B and Table II).
Table II.
Analysis of hematopoiesis in long term cultures of caspase-1 –/– mouse bone marrow cells: weekly cobblestone numbers.
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
|---|---|---|---|---|---|
| Caspase-1 –/– | 215.8±75.2 (n=4) | 1478.0±227.3 (n=4) | 1072.5±263.2 (n=4) | 1149.9±172.6 (n=4) | 1391.9±264.5 (n=4) |
| Caspase-1 +/+ | 161.0±51.3 (n=4) | 1513.5±345.8 n=4) | 1211.0±351.9 (n=4) | 520.8±132.5 (n=4) | 964.5±422.2 (n=4) |
| p-value | 0.2743 | 0.8694 | 0.5517 | 0.0012 | 0.1371 |
| Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | |
| Caspase-1 –/– | 1507.3±197.0 (n=4) | 1597.5±156.5 (n=4) | 1469.0±167.3 (n=4) | 1320.0±36.4 (n=4) | 1693.0±321.0 (n=4) |
| Caspase-1 +/+ | 702.5±159.6 (n=4) | 527.5±151.7 (n=4) | 439.0±104.8 (n=4) | 357.5±114.9 (n=4) | 524.5±168.3 (n=4) |
| p-value | 0.0007 | <0.0001 | <0.0001 | <0.0001 | 0.0007 |
| Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | |
| Caspase-1 –/– | 1566.0±348.9 (n=4) | 1479.5±255.4 (n=4) | 1521.0±121.2 (n=4) | 1318.5±219.0 (n=4) | 1182.5±346.0 (n=4) |
| Caspase-1 +/+ | 327.5±67.4 (n=4) | 387.0±157.7 (n=4) | 307.5±250.2 (n=4) | 30.5±26.6 (n=4) | 51.8±100.2 (n=4) |
| p-value | 0.0048 | 0.0003 | 0.0001 | <0.0001 | 0.0008 |
| Week 16 | Week 17 | Week 18 | Week 19 | Week 20 | |
| Caspase-1 –/– | 1075.0±425.8(n=4) | 1125.8±374.7(n=4) | 1249.0±545.6(n=4) | 948.0±392.0 (n=4) | 822.0±429.9 (n=4) |
| Caspase-1 +/+ | 159.0±318.0 (n=4) | 55.3±95.8 (n=3) | 0.0±0.0 (n=3) | 0.0±0.0 (n=2) | 0.0±0.0 (n=2) |
| p-value | 0.0137 | 0.0052 | 0.0196 | 0.0169 | 0.0315 |
| Week 21 | Week 22 | Week 23 | Week 24 | Week 25 | |
| Caspase-1 –/– | 979.8±220.3 (n=4) | 487.0±105.0 (n=4) | 461.3±222.7 (n=4) | 434.4±405.1 (n=4) | 355.5±415.8 (n=4) |
| Caspase-1 +/+ | 0.0±0.0 (n=2) | 0.0±0.0 (n=1) | 0.0±0.0 (n=1) | 0.0±0.0 (n=1) | 0.0±0.0. (n=1) |
| p-value | 0.0030 | ||||
| Week 26 | Week 27 | Week 28 | Week 29 | Week 30 | |
| Caspase-1 –/– | 405.0±469.9 (n=4) | 308.0±346.2 (n=4) | 277.0±346.2 (n=4) | 232.5±341.9 (n=4) | 210.3±291.1 (n=4) |
| Caspase-1 +/+ | 0.0±0.0 (n=1) | CT | CT | CT | CT |
| Week 31 | Week 32 | Week 33 | Week 34 | Week 35 | |
| Caspase-1 –/– | 190.5±243.7 (n=4) | 180.5±244.1 (n=4) | 227.3±279.9 (n=3) | 210.0±195.2 (n=3) | 96.7±90.0 (n=3) |
| Week 36 | Week 37 | Week 38 | Week 39 | Week 40 | |
| Caspase-1 –/– | 53.7±46.9 (n=3) | 67.3±108.1 (n=3) | 60.0±73.5 (n=2) | 66.0±93.3 (n=2) | 57.0±80.6 (n=2) |
| Week 41 | Week 42 | Week 43 | Week 44 | Week 45 | |
| Caspase-1 –/– | 48.0±67.9 (n=2) | 78.0±110.3 (n=2) | 54.5±77.1 (n=2) | 54.0±76.4 (n=2) | 62.0±87.7 (n=2) |
| Week 46 | Week 47 | Week 48 | Week 49 | Week 50 | |
| Caspase-1 –/– | 16.5±23.3 (n=2) | 12.5±17.7 (n=2) | 9.0±12.7 (n=2) | 4.5±6.4 (n=2) | 2.5±3.5 (n=2) |
| Week 51 | |||||
| Caspase-1 –/– | 0.0±0.0 (n=2) |
Data are summarized as mean±standard deviation, n is the number of mice used, and p-values were calculated with the two-sided two-sample t-test. Significant p-values are shown in bold. Due to a lack of hematopoiesis by the caspase-1 +/+ LTBMCs beginning at week 27, the cultures were terminated (CT).
The disappearance of cobblestone islands by week 19 is consistent with previous publications on the longevity of hematopoiesis in C57BL/6J mouse marrow cultures (5). In striking contrast, caspase-1 –/– LTBMCs demonstrated continuous presence of cobblestone islands through week 28 and small numbers were still detected at week 42 (Figure 2B). These results demonstrate a striking increase in the longevity of hematopoiesis in the caspase-1 –/– LTBMCs and predicted increased continuous production of non-adherent cells in hematopoietic progenitor cell lines by these cultures compared to those from the caspase-1 +/+ mice.
As shown in Figure 2C, cumulative detection of cobblestone islands demonstrated a significant increase in persistence in caspase-1 –/– LTBMCs compared to controls. Production of increased numbers of non-adherent cells on a weekly basis (Figure 2D) and cumulative basis (Figure 2E) in caspase-1 –/– LTBMCs was detected and was consistent with the increased longevity of hematopoiesis reflected in the maintenance of cobblestone islands in the adherent layer. These differences in increased caspase-1 –/– culture cell production number were also highly significant (Table III).
Table III.
Analysis of hematopoiesis in long term cultures of caspase-1 –/– mouse bone marrow cells: weekly non-adherent cell numbers.
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
|---|---|---|---|---|---|
| Caspase 1 –/– | 26.12±3.25 (n=4) | 15.75±1.94 (n=4) | 16.25±6.43 (n=4) | 20.53±6.19 (n=4) | 17.45±3.85 (n=4) |
| Caspase 1 +/+ | 23.18±1.83 (n=4) | 15.35±1.59 (n=4) | 11.15±1.75 (n=4) | 11.42±5.27 (n=4) | 12.88±5.56 (n=4) |
| p-value | 0.1667 | 0.7616 | 0.1768 | 0.0663 | 0.2250 |
| Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | |
| Caspase 1 –/– | 12.86±1.58 (n=4) | 12.86±1.58 (n=4) | 13.63±2.84 (n=4) | 15.53±3.25 (n=4) | 15.79±3.44 (n=4) |
| Caspase 1 +/+ | 5.97±1.36 (n=4) | 5.97±1.36 (n=4) | 3.99±0.42 (n=4) | 3.63±0.75 (n=4) | 4.07±1.39 (n=4) |
| p-value | 0.0006 | 0.0006 | 0.0059 | 0.0040 | 0.0007 |
| Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | |
| Caspase 1 –/– | 14.67±2.17 (n=4) | 14.20±2.49 (n=4) | 11.97±3.64 (n=4) | 9.24±2.06 (n=4) | 5.79±1.13 (n=4) |
| Caspase 1 +/+ | 3.62±1.35 (n=4) | 2.55±0.83 (n=4) | 3.44±1.13 (n=4) | 1.85±0.47 (n=4) | 2.33±0.83 (n=4) |
| p-value | 0.0001 | 0.0001 | 0.0042 | 0.0044 | 0.0025 |
| Week 16 | Week 17 | Week 18 | Week 19 | Week 20 | |
| Caspase 1 –/– | 7.84±1.64 (n=4) | 6.54±1.31 (n=4) | 5.53±1.69 (n=4) | 5.34±2.06 (n=4) | 4.23±1.04 (n=4) |
| Caspase 1 +/+ | 2.18±0.93 (n=3) | 1.87±0.89 (n=3) | 1.08±0.35 (n=3) | 1.28±0.34 (n=2) | 1.14±0.64 (n=2) |
| p-value | 0.0032 | 0.0033 | 0.0071 | 0.0592 | 0.0206 |
| Week 21 | Week 22 | Week 23 | Week 24 | Week 25 | |
| Caspase 1 –/– | 6.33±2.37 (n=4) | 5.09±3.88 (n=4) | 5.22±5.46 (n=4) | 5.55±6.10 (n=4) | 4.08±3.29 (n=4) |
| Caspase 1 +/+ | 1.30±. (n=1) | 1.11±. (n=1) | 1.10±. (n=1) | 0.88±. (n=1) | 1.02±. (n=1) |
| p-value | |||||
| Week 26 | Week 27 | Week 28 | Week 29 | Week 30 | |
| Caspase 1 –/– | 3.17±2.03 (n=4) | 2.74±1.59 (n=4) | 2.13±0.85 (n=4) | 1.55±1.00 (n=4) | 1.56±0.55 (n=4) |
| Caspase 1 +/+ | 0.94±. (n=1) | 0.82±. (n=1) | CT | CT | CT |
| Week 31 | Week 32 | Week 33 | Week 34 | Week 35 | |
| Caspase 1 –/– | 1.78±1.03 (n=4) | 2.03±1.03 (n=3) | 2.21±1.04 (n=3) | 2.10±1.07 (n=3) | 2.33±0.73 (n=3) |
| Week 36 | Week 37 | Week 38 | Week 39 | Week 40 | |
| Caspase 1 –/– | 1.86±0.44 (n=3) | 2.25±0.50 (n=2) | 1.76±0.16 (n=2) | 1.72±0.24 (n=2) | 1.49±0.27 (n=2) |
| Week 41 | Week 42 | Week 43 | Week 44 | Week 45 | |
| Caspase 1 –/– | 1.15±0.37 (n=2) | 1.90±0.71 (n=2) | 1.76±0.43 (n=2) | 1.96±0.66 (n=2) | 1.96±0.44 (n=2) |
| Week 46 | Week 47 | Week 48 | Week 49 | Week 50 | |
| Caspase 1 –/– | 1.58±0.30 (n=2) | 1.51±0.12 (n=2) | 1.54±0.22 (n=2) | 1.63±0.15 (n=2) | 1.57±0.27 (n=2) |
| Week 51 | |||||
| Caspase 1 –/– | 1.38±. (n=1) |
Data are summarized as mean±standard deviation, n is the number of mice used, and p-values were calculated with the two-sided two-sample t-test. Significant p-values are shown in bold. Numbers are (×105). Due to a lack of hematopoiesis by the caspase-1 +/+ LTBMCs beginning at week 28, the cultures were terminated (CT).
The production of non-adherent cells does not always reflect production of hematopoietic progenitor cells capable of forming colonies in the secondary culture in semi-solid medium containing hematopoietic growth factors (5). Assays for hematopoietic progenitors were next carried out scoring differentiated granulocyte macrophage colonies at day 7 in the secondary culture, and the more primitive CFU-GM colonies, including erythroid-containing colonies, at day 14. As shown in Figure 2F, weekly production of day 7 CFU-GM and cumulative production of day 7 CFU-GM (Figure 2G), respectively, revealed an increased longevity of hematopoiesis in caspase-1 –/– LTBMCs compared to control caspase-1 +/+ LTBMCs (Table IV).
Table IV.
Analysis of hematopoiesis in long term cultures of caspase-1 –/– mouse bone marrow cells: weekly production of day 7 colony-forming progenitor cells.
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
|---|---|---|---|---|---|
| Caspase 1 –/– | 76.0±4.0 (n=3) | 160.3±7.6 (n=3) | 177.3±7.6 (n=3) | 149.7±16.0 (n=3) | 94.7±12.7 (n=3) |
| Caspase 1 +/+ | 184.0±8.5 (n=3) | 28.0±2.0 (n=3) | 68.3±5.1 (n=3) | 152.0±9.5 (n=3) | 98.7±7.0 (n=3) |
| p-value | <0.0001 | <0.0001 | <0.0001 | 0.8389 | 0.6573 |
| Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | |
| Caspase 1 –/– | 105.0±8.2 (n=3) | 67.7±5.5 (n=3) | 74.0±6.0 (n=3) | 68.0±6.0 (n=3) | 103.3±4.0 (n=3) |
| Caspase 1 +/+ | 93.0±3.0 (n=3) | 67.0±6.6 (n=3) | 86.7±5.0 (n=3) | 81.0±5.0 (n=3) | 74.7±3.2 (n=3) |
| p-value | 0.0756 | 0.8993 | 0.0487 | 0.0449 | 0.0007 |
| Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | |
| Caspase 1 –/– | 94.3±8.7 (n=3) | 93.0±7.5 (n=3) | 52.0±6.0 (n=3) | 27.3±3.5 (n=3) | 79.0±7.0 (n=3) |
| Caspase 1 +/+ | 80.0±6.0 (n=3) | 80.3±4.0 (n=3) | 13.3±2.5 (n=3) | 50.0±4.0 (n=3) | 53.3±6.1 (n=3) |
| p-value | 0.0792 | 0.0625 | 0.0005 | 0.0018 | 0.0087 |
| Week 16 | Week 17 | Week 18 | Week 19 | Week 20 | |
| Caspase 1 –/– | 64.7±7.0 (n=3) | 30.3±4.0 (n=3) | 32.3±2.1 (n=3) | 27.3±4.2 (n=3) | 62.3±2.5 (n=3) |
| Caspase 1 +/+ | 4.0±1.0 (n=3) | ND | ND | ND | ND |
| p-value | 0.0038 | ||||
| Week 21 | Week 22 | Week 23 | Week 24 | Week 25 | |
| Caspase 1 –/– | 47.3±2.5 (n=3) | 57.0±3.0 (n=3) | 40.7±4.5 (n=3) | 18.3±2.5 (n=3) | 35.3±2.1 (n=3) |
| Week 26 | Week 27 | Week 28 | Week 29 | Week 30 | |
| Caspase 1 –/– | 19.3±1.5 (n=3) | 14.3±1.5 (n=3) | 24.7±4.5 (n=3) | 30.7±2.5 (n=3) | 14.7±4.5 (n=3) |
| Week 31 | Week 32 | Week 33 | Week 34 | Week 35 | |
| Caspase 1 –/– | 11.7±2.5 (n=3) | 5.0±2.0 (n=3) | 12.0±2.6 (n=3) | 2.0±1.0 (n=3) | 1.0±1.0 (n=3) |
| Week 36 | Week 37 | Week 38 | Week 39 | Week 40 | |
| Caspase 1 –/– | 7.7±3.5 (n=3) | 1.3±2.3 (n=3) |
Data are summarized as mean±standard deviation, n is the number of observations, and p-values were calculated with the two-sided two-sample t-test. Significant p-values are shown in bold. (5×104 c/ml). Beginning at week 17 no day 7 colonies in the caspase-1 +/+ LTBMCs were detected (ND).
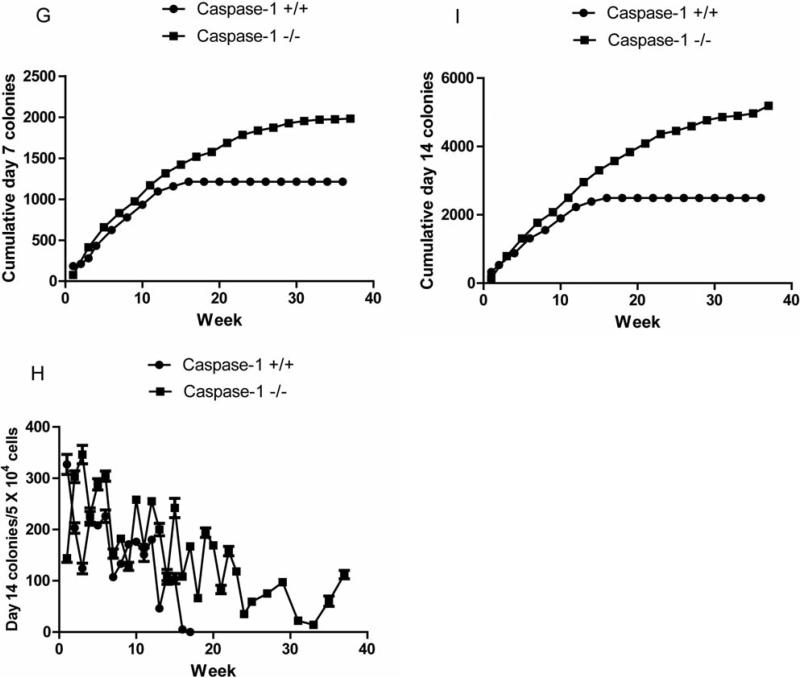
The more primitive CFU-GM assay at day 14 showed an even more striking persistence of primitive hematopoietic progenitor cells in caspase-1 –/– LTBMCs (Figure 2H) (Table V) and cumulative production was significantly higher in caspase-1 –/– LTBMCs (Figure 2I).
Table V.
Analysis of hematopoiesis in long term cultures of caspase-1 –/– mouse bone marrow cells: weekly production of day 14 colony-forming progenitor cells.
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
|---|---|---|---|---|---|
| Caspase 1 –/– | 144.3±7.8 (n=3) | 302.7±11.4 (n=3) | 346.0±18.0 (n=3) | 228.0±14.0 (n=3) | 288.3±11.0 (n=3) |
| Caspase 1 +/+ | 327.0±19.5 (n=3) | 203.3±10.1 (n=3) | 123.7±10.4 (n=3) | 222.3±13.6 (n=3) | 208.0±6.0 (n=3) |
| p-value | 0.0001 | 0.0003 | <0.0001 | 0.6412 | 0.0004 |
| Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | |
| Caspase 1 –/– | 303.7±10.0 (n=3) | 153.0±9.0 (n=3) | 181.7±7.5 (n=3) | 127.7±8.0 (n=3) | 257.7±6.5 (n=3) |
| Caspase 1 +/+ | 225.7±12.3 (n=3) | 107.3±5.0 (n=3) | 132.7±7.0 (n=3) | 171.0±7.0 (n=3) | 176.0±7.5 (n=3) |
| p-value | 0.0011 | 0.0016 | 0.0012 | 0.0021 | 0.0001 |
| Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | |
| Caspase 1 –/– | 166.3±5.0 (n=3) | 255.0±7.0 (n=3) | 200.3±12.3 (n=3) | 104.3±11.0 (n=3) | 242.0±18.7 (n=3) |
| Caspase 1 +/+ | 150.7±13.3 (n=3) | 179.7±7.1 (n=3) | 46.3±6.5 (n=3) | 111.3±11.0 (n=3) | 104.3±10.0 (n=3) |
| p-value | 0.1293 | 0.0002 | <0.0001 | 0.4790 | 0.0004 |
| Week 16 | Week 17 | Week 18 | Week 19 | Week 20 | |
| Caspase 1 –/– | 108.0±6.6 (n=3) | 167.0±4.6 (n=3) | 66.0±6.0 (n=3) | 193.7±9.3 (n=3) | 169.0±5.0 (n=3) |
| Caspase 1 +/+ | 5.0±1.0 (n=3) | ND | ND | ND | |
| p-value | <0.0001 | ||||
| Week 21 | Week 22 | Week 23 | Week 24 | Week 25 | |
| Caspase 1 –/– | 82.7±8.3 (n=3) | 157.7±9.1 (n=3) | 118.0±4.6 (n=3) | 35.3±5.5 (n=3) | 59.3±5.0 (n=3) |
| Week 26 | Week 27 | Week 28 | Week 29 | Week 30 | |
| Caspase 1 –/– | 60.7±7.5 (n=3) | 75.3±4.5 (n=3) | 77.3±5.0 (n=3) | 97.0±6.6 (n=3) | 73.0±7.5 (n=3) |
| Week 31 | Week 32 | Week 33 | Week 34 | Week 35 | |
| Caspase 1 –/– | 22.3±2.5 (n=3) | 18.7±5.0 (n=3) | 14.3±3.2 (n=3) | 8.7±2.3 (n=3) | 60.0±10.0 (n=3) |
| Week 36 | Week 37 | Week 38 | Week 39 | Week 40 | |
| Caspase 1 –/– | 114.7±7.0 (n=3) | 111.7±8.0 (n=3) |
Data are summarized as mean±standard deviation, n is the number of observations, and p-values were calculated with the two-sided two sample t-test. Significant p-values are shown in bold. Beginning at week 17 no day 7 colonies in the caspase-1 +/+ LTBMCs were detected (ND).
These results indicated a strong effect of deletion of caspase-1 on the persistence of hematopoiesis in marrow cultures and suggested that absence of this pro-apoptotic gene product conferred either resistance to the oxidative stress of long-term hematopoiesis or was associated with the absence of negative regulatory factors in LTBMCs.
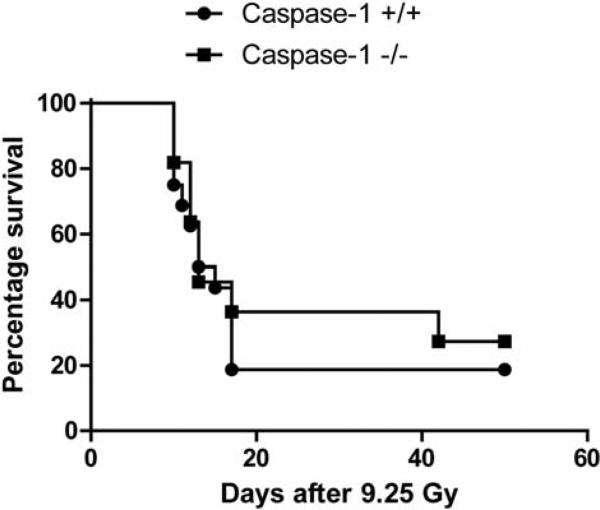
Absence of total body irradiation resistance of caspase-1 –/– mice
We next tested irradiation resistance of caspase-1 –/– mice to the lethal dose (LD) 50/30 dose of 9.25 Gy. Caspase-1 –/– mice showed no significant increase in survival after the LD 50/30 dose of total-body irradiation compared to age-matched C57BL/6NTac control caspase-1 +/+ mice (Figure 3).
Figure 3.
Survival after total-body irradiation of caspase-1 –/– and caspase-1 +/+ C57BL/6NTac mice. Mice in groups of 15 were irradiated to 9.25 Gy total-body dose, as described (9). Mice were followed-up for development of hematopoietic syndrome as identified by a loss of 10% body weight, and lethargy, at which time the mice were sacrificed according to IACUC-approved protocols. There was no significant change in development of hematopoietic syndrome in the two mouse strains. p=0.0680.
Radioresistance of caspase-1 –/– bone marrow stromal and Il-3-dependent hematopoietic progenitor cell lines
We tested the radiosensitivity in clonogenic survival curve assays of stromal and hematopoietic progenitor cell lines derived from marrow cultures. As shown in Figure 4A, caspase-1 –/– LTBMCs were radioresistant by D0 compared to control mouse stromal cell lines. There was a significant increase in the D0 of 1.85±0.06 Gy for the caspase-1 –/– cells compared to 1.57±0.10 for the caspase-1 +/+ cells (p=0.0486). Il-3-dependent hematopoietic progenitor cell lines derived from caspase-1 –/– marrow cultures were also radioresistant relative to caspase-1 +/+ cell lines by increased ñ (Figure 4B) (p=0.0235). Plating efficiencies for all experiments were not significantly different (Figure 4A, B).
Figure 4.
Irradiation survival curves of caspase-1 –/– mouse bone marrow stromal and Il-3 dependent hematopoietic progenitor cell lines. Bone marrow stromal cell (A) and Il-3-dependent cell lines (B) were derived from Long Term Bone Marrow Cultures (LTBMCs) of control C57BL/6NTac and caspase-1 –/– mice. Cell lines from stromal cells removed at week 4 from LTBMCs established from C57BL/6NTac or caspase-1 –/– mice were irradiated to doses ranging from 0 to 8 Gy, plated in 4-well tissue culture plates, incubated for seven days at 37°C, and then stained with crystal violet. Colonies of greater than 50 cells were counted and data analyzed using linear quadratic and single-hit multi-target models.
Comparable kinetics of DNA repair in caspase-1 –/– LTBMCs
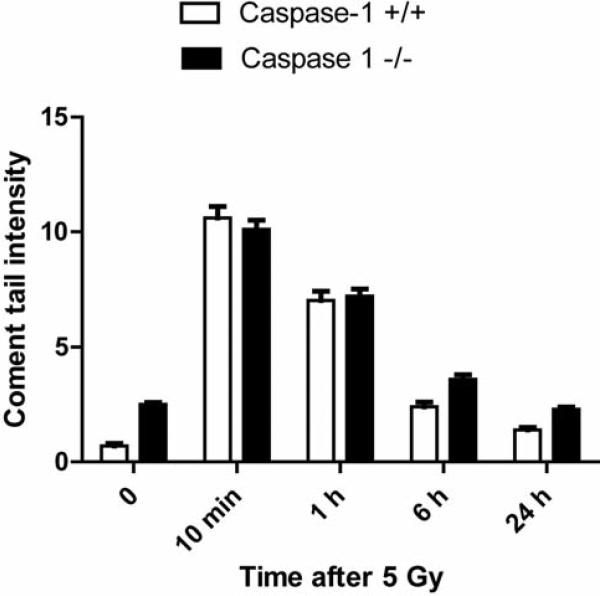
The comet assay in multiple experiments showed no significant differences in the kinetics of DNA repair between caspase-1 –/– compared to caspase-1 +/+LTBMCs. We irradiated both cell lines to a dose of 5 Gy in the logarhythmic phase (Figure 5). These data suggest that the radioresistance of caspase-1 –/– cell line in vitro may have been attributable to post-nuclear/DNA repair events, such as mitochondrial oxidative stress-related events.
Figure 5.
DNA repair measurement in irradiated bone marrow stromal cells from caspase-1 –/– or caspase-1 +/+ C57BL/6NTac mice by comet assay. Stromal cells from caspase-1 –/– and caspase-1 +/+ C57BL/6NTac mice were irradiated to 5 Gy and comet tails measured at 0, 10 min, 1 h, 6 h, and 24 h. There was no difference in comet tails between the two cell lines in two replicate experiments.
Measurement of caspase-1 –/– cell line antioxidant stores
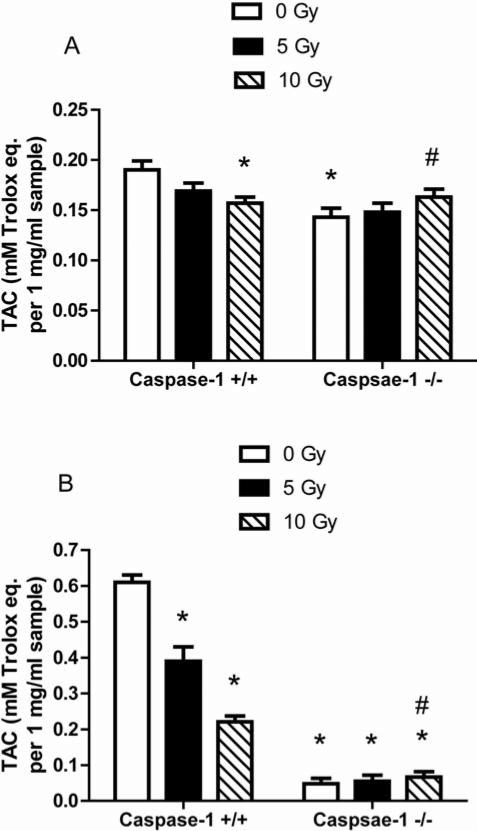
We next measured baseline and post-irradiation stress responses in the cell lines using an assay for antioxidant levels. Caspase-1 –/– stromal cells had significantly lower baseline antioxidant stores before irradiation compared to caspase-1 +/+ cells (p=0.0105) (Figure 6A). There was a significant decrease in antioxidant stores in caspase-1 +/+ stromal cells 24 h after 10 Gy irradiation (p=0.0333) (Figure 6A). However, in caspase-1 –/– cells, there was a reversed pattern showing a significant increase in antioxidant levels following 10 Gy compared to pre-irradiation levels (Figure 6A) (p=0.0441). The same pattern of reversed oxidative stress responses was observed with caspase-1 –/– Il-3-dependent hematopoietic progenitor cell lines (Figure 6B). The data were consistent with the relative radioresistance of caspase-1 –/– cells in vitro.
Figure 6.
Antioxidant stores in caspase-1 –/– cell lines as measured by Trolox assay. Bone marrow stromal cells (A) and Il3-dependent cells (B) were analyzed for total antioxidant capacity (TAC) 24 h after irradiation with 0, 5 Gy, and 10 Gy. In stromal cells, there was a significant decrease in the antioxidant levels in the caspase-1 +/+ cells following 10 Gy compared to 0 Gy expression (p=0.0333). While caspase-1 –/– stromal cells had significantly lower antioxidant stores before irradiation (p=0.0105), antioxidant level significantly increased following irradiation at 10 Gy (p=0.0441). In hematopoietic cells, the same pattern was seen. *p<0.05 compared to caspase-1 +/+ at 0 Gy. #p<0.05 compared to caspase-1 –/– at 0 Gy.
Discussion
The present results demonstrate relative resistance to oxidative stress of caspase-1 –/– mouse bone marrow cells in vitro relative to control caspase-1 +/+ mice. In both the assay for response to oxidative stress in the high oxygen environment of LTBMCs (10-12), and in the assay for clonogenic survival of irradiated bone marrow stromal and Il-3-dependent hematopoietic cells, caspase-1 –/– mouse marrow demonstrated resistance.
In LTBMC caspase-1 –/– marrow showed an increased production of hematopoietic cells and total cumulative production of colony forming hematopoietic progenitors at day 7 and day 14 colonies in secondary culture. Improved longevity of hematopoiesis in LTBMCs has been shown to be mouse strain-dependent and reflects genetic determinants of resistance to oxidative stress. In some strains, such as Akr/J and CBA mice, longevity of LTBMCs was associated with Mendelian-heritable factors borne-out in F1, F2, and backcrossed mouse strains (5). Multiple factors regulate hematopoietic longevity in LTBMCs. Mice that were genetically recombinant deletion-negative for nitric oxide synthase-1, an enzyme-responsible for generation of nitric oxide in neurons, had increased longevity of hematopoiesis in LTBMCs (9). Abrogation of the transforming growth factor-β (TGF-β) signal transduction pathway by deletion of SMAD-3 (11) also increased longevity of hematopoiesis. In contrast, marrow from senescent accelerated SAMP6 mice placed in long-term marrow cultures showed reduced longevity (12). In each example, longevity of hematopoiesis in LTBMCs was associated with relative resistance of hematopoietic stem cells and/or bone marrow stromal cells to the oxidative stress of the high oxygen incubator. That caspase-1 –/– mouse marrow demonstrated increased longevity of hematopoiesis in LTBMCs suggests that the pathway of oxidative stress response involving apoptosis is involved (2, 3, 11).
Caspase-1 –/– stromal and hematopoietic cells showed clear radioresistance in clonogenic radiation survival curve assays. The mechanism by which caspase-1 –/– marrow cultures derived bone marrow stromal and Il-3-dependent hematopoietic cell lines are radioresistant compared to their wild-type littermate cell lines is not yet known. In the present studies, cells were irradiated in culture in doses up to 8 Gy and then re-plated in colony assay in which seven cell divisions are required before a 50-cell colony is detectable, and scored as a surviving colony forming cell. This assay measures the ability of cells to survive not only the first cell doubling after ionizing irradiation, but seven cell doublings (14, 15). Thus, the clonogenic survival curve is representative of the relative capacity of cells to survive not only initial radiation killing, but proliferative stress required for seven cell doublings. The mechanism by which caspase-1 –/– stromal, and hematopoietic cell lines were radioresistant did not involve change in DNA repair by the comet assay, suggesting a post-nuclear step or mitochondrial step in the antiapoptotic pathway. Caspase-1 –/– cell lines showed no differences in DNA repair by comet assay but both stromal and hematopoietic cells showed a reverse pattern of low baseline, and post-irradiation elevation of antioxidant stores. These data are consistent with a mitochondrial mechanism of radioresistance.
In contrast to in vitro studies, total-body irradiation of caspase-1–/–mice did not reveal increased survival compared to the background mouse strain, C57BL/6NTac. The lethal irradiation dose for 50% of mice at 30 days (LD 50/30) represents that causing the hematopoietic syndrome in which cell death is reversible by marrow transplant (9). The absence of in vivo radioresistance of caspase-1 –/– mice may be attributable to a counteracting or compensating mechanism for caspase-1 deletion in other critical tissues such as intestine, lung, and liver. Further studies are required to determine whether caspase-1 may be a target for drug development of novel radiation mitigators.
Acknowledgements
Supported by Research Grant NIH/NIAID (CMCR) 1U19A168021. This project used the UPCI Hillman Cancer Center Animal Facility that is supported in part by award P30CA047904.
References
- 1.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA. Molecular cloning of the interleukin-1 β converting enzyme. Science. 1992;256(5053):97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 2.Guegan C, Vila M, Teismann P, Chen C, Onteniente B, Li M, Friedlander RM, Przedborski S. Instrumental activation of Bid by caspase-1 in a transgenic mouse model of ALS. Mol Cell Neurosci. 2002;20(4):553–562. doi: 10.1006/mcne.2002.1136. [DOI] [PubMed] [Google Scholar]
- 3.Malik AF, Hoque R, Ouyang X, Ghani A, Hong E, Khan K, Moore LB, Ng G, Munro F, Flavell RA, Shi Y, Kyriakides TR, Mehal WZ. Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response. Proc Natl Acad Sci USA. 2011;108:20095–20100. doi: 10.1073/pnas.1105152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberger JS. Sensitivity of corticosteroid-dependent, insulin-resistant lipogenesis in marrow preadipocytes of mutation-diabetic obese mice. Nature. 1978;275:752–754. doi: 10.1038/275752a0. [DOI] [PubMed] [Google Scholar]
- 5.Sakakeeny MA, Greenberger JS. Granulopoiesis longevity in continuous bone marrow cultures and factor-dependent cell line generation: Significant variation among 28 inbred mouse strains and outbred stocks. J Nat Canc Inst. 1982;68:305–317. [PubMed] [Google Scholar]
- 6.Greenberger JS, Sakakeeny MA, Humphries KC, Eaves CG, Eckner RJ. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci USA. 1983;80:2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberger JS, Sakakeeny MA, Davis LM, Moloney WC, Reid D. Biologic properties of factor independent nonadherent hematopoietic and adherent preadipocyte cell lines derived from continuous bone marrow cultures. Leukemia Res. 1984;8:363–375. doi: 10.1016/0145-2126(84)90076-6. [DOI] [PubMed] [Google Scholar]
- 8.Rwigema J-CM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, Franicola D, Dixon T, Frantz M-C, Wipf P, Tyurina Y, Kagan VE, Wang H, Greenberger JS. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80(3):860–868. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajagopalan MS, Stone B, Rwigema J-C, Salimi U, Epperly MW, Goff J, Franicola D, Dixon T, Cao S, Zhang X, Buchholz BM, Bauer AJ, Choi S, Bakkenist C, Wang H, Greenberger JS. Intraesophageal manganese superoxide dismutase-plasmid liposomes ameliorates novel total body and thoracic irradiation sensitivity of homologous deletion recombinant negative nitric oxide synthase-1 (Nos1 −/−) mice. Radiat Res. 2010;174:297–312. doi: 10.1667/RR2019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epperly MW, Cao S, Zhang X, Franicola D, Kanai AJ, Greenberger EE, Greenberger JS. Increased longevity of hematopoiesis in continuous bone marrow cultures derived from mtNos −/− homozygous recombinant negative mice correlates with increased radioresistance of hematopoietic and bone marrow stromal cells. Exp Hemat. 2007;35:137–145. doi: 10.1016/j.exphem.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Epperly MW, Cao S, Goff J, Shields D, Zhou S, Glowacki J, Greenberger JS. Increased longevity of hematopoiesis in continuous bone marrow cultures and adipocytogenesis in marrow stromal cells derived from SMAD3 −/− mice. Exp Hemat. 2005;33:353–362. doi: 10.1016/j.exphem.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 12.O'Sullivan R, Goff J, Shields D, Epperly M, Greenberger JS, Glowacki J. Cell biologic parameters of accelerated osteoporosis in SAMP6 mice are demonstrated in long-term bone marrow culture senescence and in the biology of bone marrow stromal cell lines. Exp Hemat. 2012;40:499–509. [Google Scholar]
- 13.Epperly MW, Sikora C, Defilippi S, Gretton J, Zhan Q, Kufe DW, Greenberger JS. MnSOD inhibits irradiation-induced apoptosis by stabilization of the mitochondrial membrane against the effects of SAP kinases p38 and JNK1 translocation. Radiat Res. 2002;157:568–577. doi: 10.1667/0033-7587(2002)157[0568:msdsir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Epperly MW, Gretton JE, Bernarding M, Nie S, Rasul B, Greenberger JS. Mitochondrial localization of copper/zinc superoxide dismutase (CuZnSOD) confers radioprotective functions in vitro and in vivo. Radiat Res. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 15.Epperly MW, Osipov AN, Martin I, Kawai K, Borisenko GG, Jefferson M, Bernarding M, Greenberger JS, Kagan VE. Ascorbate as a redox-sensor and protector against irradiation-induced oxidative stress in 32D cl 3 hematopoietic cells and subclones overexpressing human manganese superoxide dismutase. Int J Radiat Oncol Biol Phys. 2004;58:851–861. doi: 10.1016/j.ijrobp.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Epperly MW, Bernarding M, Gretton J, Jefferson M, Nie S, Greenberger JS. Overexpression of the transgene for manganese superoxide dismutase (MnSOD) in 32D cl 3 cells prevents apoptosis induction by TNF-α;, IL-3 withdrawal and ionizing irradiation. Exp Hemat. 2003;31:465–474. doi: 10.1016/s0301-472x(03)00041-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhang WH, Wang X, Narayanan M, Zhang Y, Huo C, Reed JC, Friedlander RM. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Natl Acad Sci USA. 2003;100(26):16012–16017. doi: 10.1073/pnas.2534856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernard ME, Kim H, Berhane H, Epperly MW, Franicola D, Zhang X, Houghton F, Shields D, Wang H, Bakkenist CJ, Frantz M-C. Wipf P and Greenberger JS: GS-nitroxide (JP4-039) mediated radioprotection of human Fanconi anemia cell lines. Radiat Res. 2011;176:603–612. doi: 10.1667/rr2624.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epperly MW, Chaillet JR, Kalash R, Shaffer B, Goff J, Shields D, Dixon T, Wang H, Berhane H, Kim J-H, Greenberger JS. Conditional radioresistance of tet-inducible manganese superoxide dismutase bone marrow stromal cells. Radiat Res. doi: 10.1667/RR3177.1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]