Abstract
An 83-year-old male presented with dyspnoea and stridor. He had undergone pneumonectomy 40 years ago. CT scan revealed gross shift of mediastinum (post-pneumonectomy syndrome) with tortuous trachea kinked at the thoracic inlet. Fibre optic bronchoscopy showed a near total expiratory closure of trachea, right main bronchus, and segmental bronchi confirming tracheobronchomalacia. He was managed with long length, low tracheostomy in view of his poor general condition of permitting more invasive procedures. He showed adequate clinical improvement and was discharged home. Tracheobronchomalacia in post-pneumonectomy syndrome requires emergent management. Its occurrence after 40 years is very rare and may be easily missed. It can be diagnosed with dynamic CT and FOB. Although invasive management with stenting or surgical methods is routinely advised, conservative care can be effective in selected cases.
Keywords: Post-pneumonectomy, Post pneumonectomy syndrome, Stridor, Tacheobronchomalacia
INTRODUCTION
Tracheobronchomalacia is defined as weakness of the cartilaginous structures of the tracheo-bronchial wall. The trachea and the main bronchi lose their usual degree of rigidity with the airway walls coming closer together. This reduces the calibre of the airway lumen, particularly during expiration, and results in dynamic airway obstruction. Symptomatic tracheobronchomalacia is a life-threatening condition, with grave anxiety to sufferer and observer. It requires individualized treatment usually with invasive procedures. Rarely, as in our patient, conservative management may result in satisfactory abatement of symptoms; however, it should be reserved only for patients with high risk for invasive procedures.
CASE REPORT
An 83-year-old male presented with barking cough with greenish expectoration, shortness of breath (grade III MMRC) and wheezing for 3 days. For last 3 years he has been under treatment for recurrent exacerbations of bronchial asthma, required multiple admissions at various hospitals and was suspected to have brittle asthma. He was a diabetic and hypertensive and a lifelong non-smoker. He had undergone left pneumonectomy 40 years ago for pulmonary tuberculosis. On general examination he was conscious, alert, afebrile, pulse was regular, 120 per minute, BP was 130/80 mm Hg. He had tachypnoea with respiratory rate of 28/min and had hypoxia with SpO2 of 89% on room air. Examination of respiratory system revealed shift of trachea to left, audible stridor which increased on lying down, absent air entry on the left side and conducted sounds on the right side. Arterial Blood Gas analysis showed; pH–7.28, pCO2–53 mm Hg, pO2–75.1 mm Hg, HCO3–22.1.
2D Echocardiography showed normal LV function, Mild RV dysfunction, dilated RA/RV (TAPSE: 1.24 cm), mild TR and plethoric IVC. CBP, Serum sodium, potassium, and magnesium, were normal. Serum calcium-6.8 mg/dl, phosphate-2.7 mg/dl, parathyroid hormone-179.4 pg/ml, Vit D-3.58, and serum albumin-2.7 mg/dl.
Patient was given intravenous hydrocortisone, calcium gluconate infusion, and NIV-BiPAP support. CT scan of Neck and Chest were done which showed left post-pneumonectomy status, thickening and calcification of left pleura, tortuous trachea with acute angulation at the thoracic inlet, gross shift of mediastinum to the left, multiple spiculated lesions with surrounding fibrosis (granulomas) in anterior and posterior segments of RUL, multiple calcified pretracheal lymph nodes and compensatory hyperinflation of right lung [Figure 1]. In view of worsening of arterial blood gas values and respiratory failure, he was intubated and mechanically ventilated. Fibreoptic bronchoscopy showed severe narrowing of trachea during inspiration and near total closure during expiration suggestive of tracheomalacia [Figure 2]. Similar dynamic airflow obstruction was visualized up to segmental bronchi, though of progressively lesser severity. Patient was considered unsuitable for invasive surgical procedures in view of poor general condition, high risk for anesthetic complications, and generalized debility. He was unsuitable for placement of stent due to long segment tracheobronchial involvement. He refused further invasive procedures. Long length tracheostomy tube (Portex 8.0) was placed with tip of tracheostomy tube 2 - 3 cm from the level of carina as checked with FOB post-placement. Patency of lower trachea was maintained and chest radiography showed well-aerated right lung. He was discharged with tracheostomy used as interface for BiPAP ventilator with no requirement of supplemental oxygen.
Figure 1.
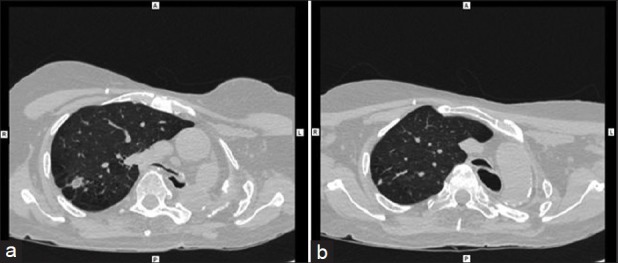
(a) CT scan showing left post pneumonectomy status, and tortuous trachea with acute angulation at thoracic inlet. (b) CT Scan
Figure 2.
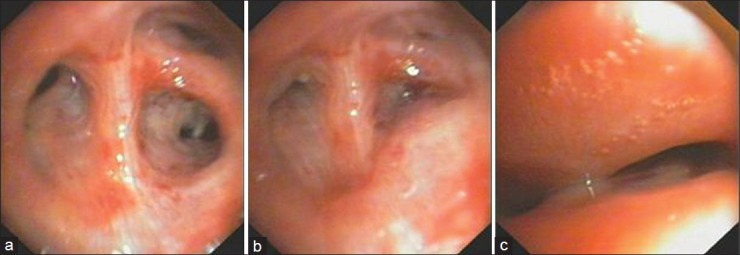
(a) Fiber optic bronchoscopy with airway open during inspiration. (b) FOB with airway closed during expiration. (c) Airway collapse during expiration
DISCUSSION
Tracheomalacia is a process characterized by flaccidity of the supporting tracheal cartilage, widening of the posterior membranous wall, and reduced anterior-posterior airway caliber. These factors cause tracheal collapse, in expiration and especially during times of increased airflow, such as coughing, crying, or feeding. If the condition extends further to the bronchi, it is termed tracheobronchomalacia. The usual symptom of tracheomalacia is inspiratory stridor or laryngeal crow.
Tracheomalacia may be congenital or acquired. The adult forms of tracheobronchomalacia are classified as either idiopathic or acquired during the course of other illnesses, and are usually disorders of middle-aged and older persons. Malacia may occur alone or may be accompanied by excessive dynamic airway collapse caused by increased invagination of the membranous posterior airway wall during expiration. Aetiologies include chronic inflammation; systemic diseases such as relapsing polychondritis; recurrent infections; sequelae from infections such as tuberculosis; pressure necrosis from overinflated tracheotomy or endotracheal tube cuffs; unrecognized tracheobronchial fracture; impaired blood supply after lung transplantation; and airway compression from substernal goitres, mediastinal tumours, or vascular anomalies. The cases of acquired tracheomalacia often are not recognized clearly.
Long-standing compression of the cartilaginous rings of the tracheobronchial tree between a great vessel (aorta or pulmonary artery) and the vertebral bodies due to postpneumonectomy syndrome may account for rare cases of secondary tracheobronchomalacia.[1,2] It is a rare delayed complication after pneumonectomy caused by marked mediastinal shift and compression of the airways by the thoracic spine, descending thoracic aorta, ligamentum arteriosum, or pulmonary artery. In these patients with difficulty in clearing secretions chronic airway infection and inflammation also contribute to the pathology. Malacia is usually noted in the main bronchus and lower trachea. Typically, the onset of symptoms related to postpneumonectomy syndrome coincides with the mediastinal shift,[3,4] which usually occurs within the first year after pneumonectomy. Our patient had noticed symptoms for more than a few years and was managed as a patient of asthma for the past 3 years (labelled variously as difficult-to-treat or brittle asthma). However, this is a known misdiagnosis in patients with tracheobronchomalacia and has been mentioned often. Our patient was very unusual as he presented so late i.e., 40 years after the pneumonectomy.
Therapeutic options for patients with tracheobronchomalacia[5,6] include disease-specific medical treatment; bronchodilators and methods of secretion clearance. NIV–PPV is used as adjunctive therapy in patients with residual or multifocal disease to provide pneumatic stenting. Open surgical techniques such as membranous wall tracheoplasty may be used in good surgical candidates in experienced centers. An incidental finding of tracheobronchomalacia does not warrant treatment whereas in symptomatic patients, therapy has to be individualized. The choice of therapy depends on the etiology, extent, type, and severity of airway abnormalities.
The treatment of tracheobronchomalacia in the setting of postpneumonectomy syndrome[4] deserves special attention because published literature is very limited. Malacia is usually an indicator of poor outcome. Surgical repositioning of the mediastinal structures may be curative if malacia is minimal or not accompanied by obstructed airways. In patients with significant malacia surgical treatment can prevent recurrent mediastinal shift but may not reverse dynamic central airway obstruction. Resections of malacic tracheobronchial segments and aortic division with bypass have been attempted. The use of airway stents appears a good option for postpneumonectomy syndrome but is sparingly reported[2,3] and has been used mainly in cases of respiratory failure or when surgery was declined.
Minimally invasive methods such as placement of stents, and surgical techniques require the patient's general condition to permit use of general anaesthesia. Our patient was first given a trial of non-invasive ventilation but poor response necessitated placement of endotracheal tube for invasive mechanical ventilation. The patient was grossly debilitated and was deemed high risk for general anaesthesia or surgery. Airway stenting was offered, but patient refused any further invasive interventions. We had no recourse but to try conservative management. Use of low tracheostomy with a long length tube, bypassing the upper airway resistance, along with specialty BiPAP ventilator to provide dynamic pneumatic stenting gave fairly satisfactory results. Patient had relief from symptomatology with resolution of acute respiratory failure. He was successfully discharged home with long length low tracheostomy used as interface for specialty BiPAP and did not require supplemental oxygen.
This is a rare case of an unusual complication viz. post-pneumonectomy tracheobronchomalacia. We wish to report it because we could satisfactorily manage him with the minimally invasive method of tracheostomy which has not been reported in recent literature. In the absence of access to highly advanced procedures like airway stenting or airway surgery and in the situations where surgery cannot be undertaken due to poor patient condition, conservative management can be offered to reduce the hospital stay with significant symptomatic relief to the patient.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology. 2006;11:388–406. doi: 10.1111/j.1440-1843.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 2.Murgu SD, Colt HG. A 68-year-old man with intractable dyspnea and wheezing 45 years after a pneumonectomy. Chest. 2006;129:1107–11. doi: 10.1378/chest.129.4.1107. [DOI] [PubMed] [Google Scholar]
- 3.Jeong SH, Cho HJ, Lee HN, Lee HS, Sheen SS, Oh YJ, et al. A case of postpneumonectomy syndrome treated with endobronchial stent. Tuberc Respir Dis. 2002;53:325–31. [Google Scholar]
- 4.Jansen JP, Brutel de la Rivière A, Alting MP, Westermann CJ, Bergstein PG, Duurkens VA. Postpneumonectomy syndrome in adulthood. Surgical correction using an expandable prosthesis. Chest. 1992;101:1167–70. doi: 10.1378/chest.101.4.1167. [DOI] [PubMed] [Google Scholar]
- 5.Valji AM, Maziak DE, Shamji FM, Matzinger FR. Postpneumonectomy syndrome: Recognition and management. Chest. 1998;114:1766–9. doi: 10.1378/chest.114.6.1766. [DOI] [PubMed] [Google Scholar]
- 6.Rakovich G, Bussieres J, Frechette E. Postpneumonectomy syndrome. MMCTS 2009. MMCTS 2008.003475. doi: 10.1510/mmcts.2008.003475. [DOI] [PubMed] [Google Scholar]


