Abstract
Before the discovery of corneal cross-linking (CXL), patients with keratoconus would have had to undergo corneal transplantation, or wear rigid gas permeable lenses (RGPs) that would temporarily flatten the cone, thereby improving the vision. The RGP contact lens (CL) would not however alter the corneal stability and if the keratoconus was progressive, the continued steepening of the cone would occur under the RGP CL. To date, the Siena Eye has been the largest study to investigate long term effects of standard CXL. Three hundred and sixty-three eyes were treated and monitored over 4 years, producing reliable long-term results proving long-term stability of the cornea by halting the progression of keratoconus, and proving the safety of the procedure. Traditionally, CXL requires epithelial removal prior to corneal soakage of a dextran-based 0.1% riboflavin solution, followed by exposure of ultraviolet-A (UV-A) light for 30 min with an intensity of 3 mW/cm2. A series of in vitro investigations on human and porcine corneas examined the best treatment parameters for standard CXL, such as riboflavin concentration, intensity, wavelength of UV-A light, and duration of treatment. Photochemically, CXL is achieved by the generation of chemical bonds within the corneal stroma through localized photopolymerization, strengthening the cornea whilst minimizing exposure to the surrounding structures of the eye. In vitro studies have shown that CXL has an effect on the biomechanical properties of the cornea, with an increased corneal rigidity of approximately 70%. This is a result of the creation of new chemical bonds within the stroma.
Keywords: Corneal cross-linking techniques, new technologies, riboflavin
Corneal cross-linking (CXL) is an established method for the treatment of keratoconus and corneal ectasia. CXL has been proven to strengthen the corneal structure, inhibiting the progression of keratoconus[1] [Fig. 1].
Figure 1.
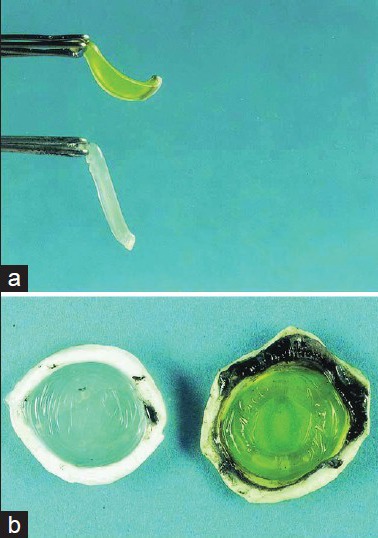
Porcine cornea (a) showing the stiffening effect after cross-linking (CXL), compared to an untreated cornea (b) Source: Wollensak et al .[6]
Before the discovery of CXL, patients with keratoconus would have had to undergo corneal transplantation, or wear rigid gas permeable lenses (RGPs) that would temporarily flatten the cone, thereby improving the vision. The RGP contact lens (CL) would not however alter the corneal stability and if the keratoconus was progressive, the continued steepening of the cone would occur under the RGP CL. To date, the Siena Eye Study[2] has been the largest study to investigate long-term effects of standard CXL. Three hundred and sixty-three eyes were treated and monitored over 4 years, producing reliable long-term results proving the efficacy of the procedure in terms of long-term stability of the cornea by halting the progression of keratoconus, and proving the safety of the procedure.
Traditionally, CXL requires epithelial removal prior to corneal soakage of a dextran-based 0.1% riboflavin solution, followed by exposure of ultraviolet-A (UV-A) light for 30 min with an intensity of 3 mW/cm2. A series of in vitro investigations on human and porcine corneas examined the best treatment parameters for standard CXL, such as riboflavin concentration, intensity, wavelength of UV-A light, and duration of treatment.[3] Photochemically, CXL is achieved by the generation of chemical bonds within the corneal stroma through localized photopolymerization, strengthening the cornea whilst minimizing exposure to the surrounding structures of the eye.[4] In vitro studies have shown that CXL has an effect on the biomechanical properties of the cornea, with an increased corneal rigidity of approximately 70%.[5] This is a result of the creation of new chemical bonds within the stroma.
The Role of Riboflavin
Before UV light illumination, the stroma needs to be soaked with a photosensitizer—riboflavin. A limiting factor is the epithelium with a thickness of approximately 50 μm that forms a barrier to both riboflavin and UV-A penetration. Removal of the corneal epithelium enhances penetration and allows proper absorption of riboflavin into the cornea and anterior chamber in order for the UV-A light to efficiently illuminate the cornea and excite the riboflavin. Treatments performed with epithelium removal have been named “epi-OFF” treatments. New formulations aim to improve the diffusion of riboflavin molecules through epithelium into the stroma and such approaches have been named as “epi-ON” treatments.
During the UV light illumination, riboflavin acts further as a shield during irradiation to the cornea, protecting deeper ocular structures such as the endothelium, lens, and retina from UV-A irradiances that are too high.[3] The combination of riboflavin and UV-A light creates 80–95% absorption into the cornea during cross-linking[5] depending on the concentration and the corneal thickness. Throughout the CXL procedure, the constant irradiation dose used is 5.4 J/cm2. Photochemical processes that occur in the corneal stroma are dependent on the radiant exposure of UV light. To avoid damage to the endothelium caused by UV-A light, effective CXL should only occur in the first 200–250 μm of the corneal stroma.[3] Using a wavelength of 360–370 nm, UV intensity of 3 mW/cm2 and 5.4 J/cm2 of energy ensures exposure of UV light on the cornea is below harmful levels. Wollensak et al.,[6] determined the damage threshold at the corneal endothelium to be 0.36 mW/cm2 (0.65 J/cm2); however this intensity may cause damage if corneal thickness is below 400 μm.
Another important role of riboflavin is to prevent corneal dehydration during exposure.[7] If corneas are particularly thin before CXL, hypoosmolar riboflavin 0.1% artificially swells the cornea to at least 400 μm to reduce the cytotoxic risk of UV-A to the endothelium.[8] Isoosmolar riboflavin 0.1% solution containing 20% dextran has a temporary dehydrating effect resulting in thinning of the cornea, and this is the solution that is typically used with standard CXL.
Epi-ON or transepithelial CXL has been reported to be less painful for the patient and to reduce the risk of infection postoperatively by keeping the epithelium intact. Although the short-term effects of epi-ON seem positive, this does not give us significant evidence to suggest long-term success in halting the progression of keratoconus. A study with a follow-up time of 3 years found a reduction in the steepest keratometry to be more prominent in corneas after epi-OFF CXL compared to transepithelial CXL.[9] Hafezi[10] found the concentration of riboflavin in the stroma to be 40 times less during transepithelial CXL, reducing the long term cross-linking effect on the corneal shape. This corresponds with the findings of Wollensak et al.,[11] demonstrating a reduction in biomechanical changes by one-fifth using transepithelial CXL compared to standard CXL studies as reported here.
Higher Intensities for Short Treatment Times
A shorter treatment time for CXL would be beneficial for both patient and surgeon. The Bunsen-Roscoe (BL) law of reciprocity states a certain biological effect is directly proportional to the total energy dose, irrespective of time. Ex vivo experiments have shown biomechanical stiffening of corneal tissue after exposure to 10 mW/cm2, correlating with the outcomes seen after treatment with standard CXL.[12] Recent ex vivo measurements found a steady increase in stiffness after exposure to illumination intensities of 40–45 mW/cm2. Interestingly, this study found no statistical significant stiffness increase in intensities ranging from 50 mW/cm2 up to 90 mW/cm2, proving a higher intensity cross-linking may not be as effective if illumination duration is less than 7 min. Further laboratory work done showed that 3 and 10 mW/cm2 CXL treatments had similar effects on porcine corneas that had been treated with CXL and then imaged with second harmonic (SH) imaging. Also, 30 and 100 mW/cm2 treatments had less of an effect on the porcine corneas and the effect was more superficial.[13] In the living model, this would suggest that the treatment effect would be more superficial with the higher fluency treatments.
Clinical Experience
The Wellington Eye Clinic has been performing CXL for keratoconus and post-LASIK ectasia since January of 2007. Overall, CXL has been a positive experience for both patient and physician. The initial indications were two-fold:
CXL for any patient demonstrating signs of progression of the keratoconus (topographic evidence of the average K-value and/or K-Max becoming steeper over time). This group was typically younger, from the late teens to late 20s.
CXL for any patient that was wearing RGP CL's, but had now found that they were no longer possible to wear due to comfort issues or fitting issues. Traditionally, this patient would now be facing the prospect of keratoplasty. The typical age in this group would be late 30s to early 50s.
In the above two scenarios, CXL has proven to be very effective with 95% of patients having the keratoconus progression arrested (or reversed to some extent) and the great majority of patients in group 2 being able to return to RGP CL wear without the need for keratoplasty.
Following the 7th International CXL Meeting (www.cxl-congress.com) held in Milan in January of 2011, the guidelines adopted at that meeting are currently being followed: CXL is indicated for any patient younger than 27 years with ectatic disease, while patients older than 27 years can be monitored for signs of progression.
CXL stabilizes the corneal shape while not impacting vision much in our experience. For someone who has a distorted corneal shape to start with, CXL will in all likelihood prevent the corneal shape from deteriorating, but will not improve it much.[14] This is the very reason that combination procedures have been developed in conjunction with CXL. We combine CXL with topography-guided photorefractive keratectomy (PRK) (SimLC), with thermal keratoplasty procedures such as Keraflex (Avedro, Inc.), conductive keratoplasty (Refractec Inc), and intracorneal rings (e.g., Intacs®).
The indications for a combined procedure have mostly to do with visual performance. If the uncorrected visual acuity (UCVA) and/or best corrected visual acuity (BCVA) are reasonably good (6/10 or better), then CXL as a standalone procedure is advised most often. If the BCVA is less than 6/12, combination procedures are then considered.
SimLC (Simultaneous topography-guided PRK and CXL). Our 6 year data shows that this is an excellent procedure in terms of improving the corneal shape and maintaining the improvement. The average amount of corneal flattening is 5.9 D with SimLC, while for those that do flatten with CXL alone (70% of eyes in our experience) flatten by 2.1 D. The stability that is provided by CXL following laser ablative procedures (phototherapeutic keratectomy) seems to be permanent. The Wellington Eye Clinic has collected 6 years of data on SimLC cases, and it appears that the result achieved at the 1 year interval is maintained through longer follow-up including 3, 4, and 5 years. It is well-known that PRK reduces the corneal strength by approximately 5–10%, while the CXL strengthens the cornea by around 70%. It is obvious from this that the final corneal shape is also a stronger cornea promising more stability.
-
Thermal keratoplasty procedures:
- Keraflex: This is a microwave-based procedure that heats the cornea at 150 μm below the surface to around 65°C for less than a few milliseconds to induce a flattening over this area. The application is always central irrespective of where the cone is located as the annular scar created by the treatment needs to be placed directly in front of the pupil so that light rays can pass through the annular ring and pupil. The flattening effect can be titrated according to the refraction that is required to be treated. CXL is then done afterwards in an attempt to stabilize the flattening effect.
- Conductive keratoplasty (CK): A study at the Wellington Eye Clinic is currently evaluating the effect of CXL to stabilize CK treatments for keratoconus. Spots can be applied over the apex of the cone in order to flatten it or superiorly in a more peripheral location in order to steepen the superior cornea. The advantages of CK include being able to place the spots exactly where they are required unless the steepest point is central and within the pupillary area. CXL is also performed either the next day or a few days later in order to stabilize the flattening effect.
-
Intrastromal rings: This modality is used least in the Wellington Eye Clinic as it is the most expensive of the treatment options on offer and somewhat unpredictable. Many patients are not candidates because their corneas are either too thin, too steep, or both.
CXL is used in conjunction with the above-mentioned procedures in order to stabilize their effects or as a standalone procedure. Most of the published literature is with the Dresden protocol as described in the introduction and this requires the epithelium to be removed. The two biggest changes in the way that CXL is carried out nowadays relates to the fluence (with higher energies and shorter treatment times) and to the preservation of the corneal epithelium during the CXL procedure.
A number of clinical trials are currently underway at the Wellington Eye Clinic designed to answer both these questions:
- Are the higher fluency treatments as safe and effective as the 3 mW × 30 min Dresden protocol procedure?
-
Can we achieve corneal shape stability using epi-ON procedures?Enrolment was recently completed for the accelerated CXL study aimed at reducing treatment time to 10 min with an intensity of 9 mW/cm2 using the IROC UV-X 2000 device (IROC Innocross AG, z). The beam profile is optimized with 9 mW/cm2 centrally and 12 mW/cm2 in the periphery. The initial analysis suggests that the 10 min procedure is as safe as the conventional 30 min 3 mW/cm2 Dresden protocol procedure. A first interim analysis has provided an encouraging result demonstrating a stronger flattening effect with the optimized beam profile compared to the standard Dresden protocol.
A study comparing epi-OFF CXL to epi-ON CXL is also currently under investigation. It is prospective and in all cases the IROC UV-X 1000, IROC Innocross AG, Zurich, Switerland device is being used delivering 3 mW/cm2 for 30 min. The epithelium is prepared by wiping it down with a Weck cell sponge to remove the phospholipid layer from the surface. The riboflavin soak is with hypoosmolar riboflavin 0.5% for around 12–15 min. As per the routine, the cornea and anterior chamber are examined at the slit-lamp to ensure that the riboflavin has indeed soaked into the cornea. If it has, the illumination is commenced for 30 min. If it has not soaked well enough, then additional soak time is added until the cornea contains enough riboflavin. Results here are very interesting with no surprise in terms of increased patient comfort, much quicker return to preoperative levels of vision, much less time off work/school and increased safety. What has been most surprising however is the flattening of the cornea seen at 1 month with further flattening at 3 months postop. Patients that have reached the 6 month interval are demonstrating stability of the flattening effect achieved by the 3 month visit. With epi-OFF Dresden protocol CXL we expect to see the corneal metrics deteriorate at the 1 month interval, but then improve quite slowly so that the 3 month postop levels look quite similar to the preoperative measurements. The 6 month visit normally shows flattening when compared to the preoperative and 3 month postoperative levels.
Discussion
CXL has been widely used to treat keratoconus and ectasia following LASIK with much success that is widely published in the peer-reviewed literature. Even though it may look a lot more sophisticated in years to come, the current procedure has contributed greatly to the management of keratoconus today. The incidence of penetrating keratoplasty and lamellar keratoplasty for keratoconus has greatly diminished since the advent of CXL.[15]
The thermal procedures are a different matter altogether and we are still learning about the ideal time to apply the CXL in order to achieve maximal stability. Data published at the American Academy of Ophthalmology (AAO) of 2012[16] showed that the timing of CXL in relation to the thermal application was of vital importance. It appears that directly after the initial flattening effect brought about by the thermal shrinkage of collagen fibers in the annulus, the cornea starts rebounding again. This rebound effect is greater at the start and then slows down after a couple of hours. Serial hourly Pentacam (Allegro Oculyzer) examinations after Keraflex was performed and flattened the cornea by anything from 15–30 diopters, the rebound of corneal steepness would occur mostly in the first hour at around 3–5 diopters and then in hour 2 and 3, it slowed down further to 1–2 diopters per hour. After 3 h the rebound effect was very minimal and after 5–6 h in many cases it was becoming slightly flatter again. Our 1 year data has shown that cases where CXL was done 5 h post-Keraflex tended to be most stable at the 1 year interval. It is our belief that if the CXL is applied during the active rebound phase (first 5 h), the newly formed cross-links are simply overcome by the stronger forces of the rebounding fibres. On the other hand, if the CXL is applied 6 h or more after the Keraflex, then the odds are that the effect will be more stable as more cross links will survive the rebound effect. Two other parameters played a role in our study: The longer the corneal riboflavin soak was, the more likely the effect would last. Nowadays, we simply soak for as long as it takes to ensure that there is riboflavin in the cornea before commencing illumination. Some of the Keraflex treatments were followed by accelerated CXL using the Avedro system and 30 mW was used for 3 min.
Conclusion
During the past few years, surgeons have gained substantial clinical experience of corneal cross-linking. New treatment strategies have been reported mainly focusing on shorter treatment times, better efficacy and vision rehabilitation. New light profiles have been shown to provide an improved efficacy; however, the underlying physical characteristics of the CXL process seem to limit the reduction of the treatment time. Combining CXL with refractive procedures is required to provide patients with visual rehabilitation, however, the current strategies are manifold and their application is very dependent on the patient's clinical situation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Ex Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 2.Caporossi A, Mazzotta C, Baiocchi S, Caparossi T. Long-term results of riboflavin a corneal cross-linking for keratoconus in Italy: The Siena eye cross study. Am J Ophthalmol. 2010;149:585–93. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal cross-linking. J Cataract Refract Surg. 2009;35:1358–62. doi: 10.1016/j.jcrs.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Hafezi F, Randleman JB. Published by SLACK Incorporated; 2013. Corneal Collagen Cross-Linking. 55-60. [Google Scholar]
- 5.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–83. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 6.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 7.Aurich H, Wirbelauer C, Jaroszewski J, Hartmann C, Pham DT. Continuous measurement of corneal dehydration with online optical coherence pachymetry. Cornea. 2006;25:182–4. doi: 10.1097/01.ico.0000176610.50461.77. [DOI] [PubMed] [Google Scholar]
- 8.Hafezi F, Mrochen M, Iseli HP, Seiler T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg. 2009;35:621–4. doi: 10.1016/j.jcrs.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 9.Gualdi L. Paper. CXL Congress; 2012. EPI-ON cross-linking: 3 years results and suggestions for the selection of the patient. [Google Scholar]
- 10.Hafezi F. Paper. CXL Congress; 2012. CXL: Epithelium On or Off? [Google Scholar]
- 11.Wollensak G, Iomdina E. Biomechanical and histological changes after corneal cross-linking with and without epithelial debridement. J Cataract Refract Surg. 2009;35:540–6. doi: 10.1016/j.jcrs.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety Of UVA riboflavin cross-linking of the cornea. Cornea. 2007;26:385–9. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci. 2011;52:9048–52. doi: 10.1167/iovs.11-7818. [DOI] [PubMed] [Google Scholar]
- 14.Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:149–60.15. doi: 10.1016/j.jcrs.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Kanellopoulos AJ, Binder PS. Collagen cross-linking (CCL) with sequential topography-guided PRK: A temporizing alternative for keratoconus to penetrating keratoplasty. Cornea. 2007;26:891–5. doi: 10.1097/ICO.0b013e318074e424. [DOI] [PubMed] [Google Scholar]
- 16.Cummings AB. Factors affecting the stability of Keraflex treatments for keratoconus Presented as a poster at the American Academy of Ophthalmology, Chicago. 2012 [Google Scholar]


