Abstract
Pediatric keratoconus demonstrates several distinctive management issues in comparison with adult keratoconus with respect to under-diagnosis, poor compliance and modifications in treatment patterns. The major concerns comprise of the accelerated progression of the disease in the pediatric age group and management of co-morbidities such as vernal keratoconjuntivitis. Visual impairment in pediatric patients may affect social and educational development and overall negatively impact their quality of life. The treatment algorithm between adults and pediatric keratoconus has been similar; comprising mainly of visual rehabilitation with spectacles, contacts lenses (soft or rigid) and keratoplasty (lamellar or penetrating) depending on the stage of the disease. There is a paradigm shift in the management of keratoconus, a new treatment modality, corneal collagen crosslinking (CXL), has been utilized in adult keratoconic patients halting the progression of the disease. CXL has been utilized for over a 10 year period and based on the evidence of efficacy and safety in the adult population; this treatment has been recently utilized in management of pediatric keratoconus. This article will present an update about current management of pediatric keratoconus with special focus on CXL in this age group.
Keywords: Corneal collagen cross linking, corneal transplant, keratoconus, pediatric keratoconus
Keratoconus is a progressive, frequently asymmetric, non-inflammatory corneal thinning disorder characterized by changes in the structure and organization of corneal collagen.[1,2,3,4] The disease classically manifests in the 2nd decade of life when the cornea assumes an increasingly conical shape owing to its biomechanical instability. This leads to irregular astigmatism and subsequent decrease in visual acuity.[5] Management of keratoconus has mainly consisted of visual rehabilitation by means of spectacles, contact lenses and intracorneal ring segments (ICRS) implantation for early to moderate stages and lamellar or penetrating keratoplasty in advanced stages with contact lens intolerance and/or corneal scar.[5,6,7,8]
The introduction of corneal collagen cross-linking (CXL) in routine clinical practice has changed the management of keratoconus in the adult population. CXL is a technique that uses ultraviolet A (UVA) light and riboflavin (photosensitizer, Vitamin B2); the photochemical reaction between the two within the corneal stroma leads to the development of chemical bonds between collagen fibrils and thereby CXL strengthens the cornea and slows or stops the progression of keratoconus and other corneal ectasia (such as post-laser in situ keratomileusis [LASIK] ectasia and pellucid marginal degeneration [PMD]). Therefore CXL is probably the only ‘true’ treatment for corneal ectasia which directly addresses the disease pathology and potentially avoids the need for corneal transplantation.[9] Due to its success in adult keratoconus patients, very recently, CXL has been attempted to stop or slow progression of keratoconus in the pediatric age group.[10,11,12,13,14,15,16,17,18]
Validation of Efficacy and Safety in Adults
Almost all studies examining the role of CXL in management of keratoconus, have been conducted in adults. This is because it is more appropriate to test an investigational treatment in a group of patients who are old enough to understand nature of treatment, the informed consent process, and potential benefits as well as possible complications. In addition, patient cooperation during (pre and post-operative) evaluations and the procedure itself is superior in adults compared to children which is mandatory for reliable methodology to study safety and efficacy of any new treatment.
Initially, in-vitro laboratory studies were performed to evaluate the effect of riboflavin–UVA combination on the biomechanical properties of cornea. The photodynamic therapy was reported to increase the rigidity of porcine and human corneal tissue by a factor of 4.6 and 4.5 respectively.[19,20] After initial encouraging laboratory evidence, a pilot study by Wollensak et al., on humans (23 eyes of 22 keratoconic patients) started in 1998 and results demonstrating successful halting of keratoconus progression were published in 2003.[21] Since, this first report by Wollensak et al., there are numerous publications in peer-reviewed literature over last decade with a variety of methodologies (retrospective, prospective uncontrolled and randomized controlled trials), addressing safety and efficacy of CXL in treatment of keratoconus as well as other corneal ectatic conditions.[22,23,24,25,26]
These studies have provided sufficient evidence that CXL is successful in slowing or halting keratoconus progression and may even demonstrate visual, topographic and aberrometric improvement by induced corneal flattening and reduction in irregular astigmatism. Importantly, medium and long-term studies have validated an excellent safety profile for standard CXL (epithelium-off Dresden protocol), with respect to the health of corneal endothelium, intra-ocular structures and an acceptable complication rate when strict inclusion criteria (corneal thickness at least 400 um) are adhered to in the adult keratoconic population.[22,23] Due to the success of standard CXL, variations in the treatment protocol [transepithelial (TE) CXL, accelerated (ACC) CXL][27] and its application in pediatric keratoconic patients have been under evaluation.[10,11,12,13,14,15,16,17,18]
Rationale for Cxl in Pediatric Keratoconus
Although keratoconus is most frequently diagnosed after adolescence, the corneal ectasia process starts at a much younger age.[5] Pediatric keratoconus (keratoconus manifesting in childhood [less than 18 years of age] or adolescence [between 10 to 19 years of age]) exhibits several unique characteristics. Studies have shown that pediatric keratoconus demonstrates a higher rate (88% of keratoconic eyes) and speed of keratoconus progression as compared to adult keratoconus.[15,28,29,30] Léoni-Mesplié et al.,[28] conducted a retrospective study to assess the severity of keratoconus at diagnosis and its scalability over a period of 2 years in children compared to adults. Keratoconus in children was significantly more severe at diagnosis, with 27.8% being stage 4 vs 7.8% of adults and keratoconus evolved faster in children as compared with adult group. In addition, the biomechanical rigidity of the cornea is inversely related to age and children with keratoconus are frequent eye rubbers, especially the subgroup of children with coexisting vernal keratoconjunctivitis (VKC). Therefore keratoconus progression in children is aggressive and may not halt on its own.[31,32,33] This may lead to progressive visual impairment in pediatric patients and affect the social as well as educational development of the child and thus, negatively affecting their quality of life.
Treating patients with mild keratoconus at an earlier age could be of greater benefit than waiting until patients are older and have more advanced disease requiring corneal transplantation. As corneal transplantation in children carries a poorer prognosis than in adults,[34] a treatment to halt the progression and potentially avoid keratoplasty offers immense benefits in long run. CXL is effective in halting the progression of keratoconus with an excellent safety profile in adults. For this reason, CXL has been very recently utilized and evaluated it in children.
Review of Published Clinical Studies
There have been 9 published studies presenting outcomes of CXL treatments (standard epithelium off: 8 and trans-epithelial: 2, as one study comprises of comparative evaluation of epi-on and epi-off techiques) in pediatric and adolescent keratoconic patients under the age of 19 years.[10,11,12,13,14,15,16,17,18] The studies have followed a variety of methodologies (case series, retrospective non comparative, retrospective comparative and prospective studies) and demonstrated outcomes of CXL in terms of efficacy and safety over the follow up range from one year to three years after CXL. The studies are described in Table 1. Soeters et al.,[10] was the first to report outcomes of CXL in documented progressive keratoconus in a pediatric age group (10 to 16 years). In this case series comprising of five eyes of four patients, standard CXL was successful in stabilizing keratoconus and avoiding corneal transplant in four eyes while three eyes demonstrated significant visual and topographic improvement. However, one eye which underwent CXL despite superficial pre-existing haze, required keratoplasty to treat the stromal scar.
Table 1.
Chief characteristics of published studies regarding outcomes of corneal collagen cross linking in pediatric keratoconus
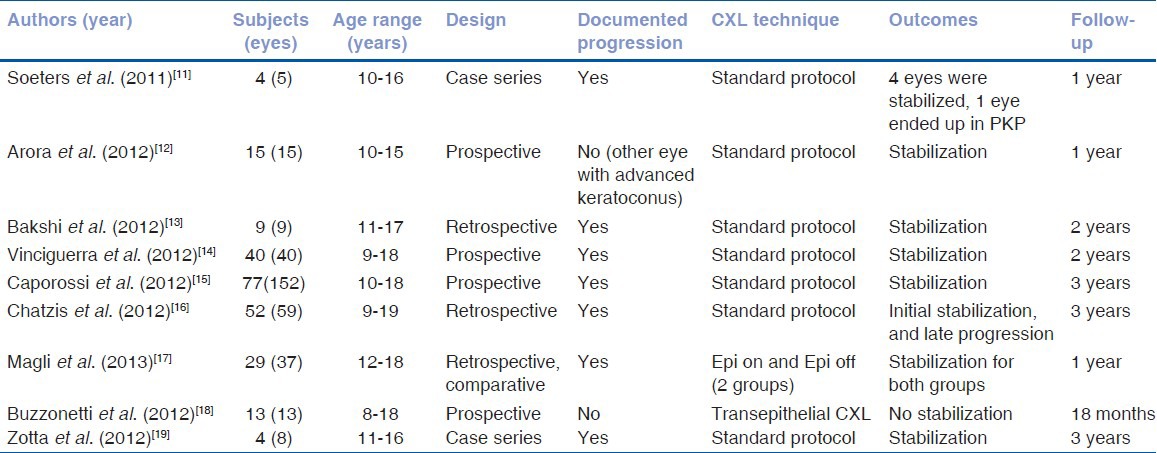
Arora et al.,[11] included 15 eyes of 15 pediatric keratoconus patients (10 to 15 years) in their prospective study and reported results with follow up of a year. The authors did not document keratoconus progression prior to CXL and they based their decision of performing CXL on status of the fellow eye (all fellow eyes had advanced keratoconus demonstrating either hydrops or required corneal transplant). At the end of a year after CXL, mean flattening of apical keratometry (K) was 1.01 ± 2.40 diopters, mean uncorrected distance visual acuity (UDVA) improved significantly from 20/200 to 20/100 (P = 0.035) and mean corrected distant visual acuity (CDVA) from 20/70 to 20/40 (P = 0.003).
Bakshi et al.,[12] in their retrospective study enrolled nine eyes of nine consecutive progressive keratoconic pediatric patients (11 to 17 years) and after CXL, patients were followed for up to two years. Most patients (7 of 9, 77.8%) showed a long-term stability. Improvement in CDVA, keratometry indices and refraction were noted, however they did not reach statistical significance, which may be due to small sample size. Only one eye demonstrated corneal haze as a post-operative complication, which resolved on topical steroid therapy.
Vinciguerra et al.,[13] demonstrated outcomes of standard CXL in 40 eyes of 40 pediatric (9 to 18 years) patients in a prospective study with up to 2 years of follow up. After CXL, all keratoconic eyes were stabilized; furthermore there was a statistically significant improvement in UDVA, CDVA, refraction and keratometric indices. Furthermore, for a 3mm pupil, there was a statistically significant improvement in both total and corneal higher-order aberrations including coma. Importantly, endothelial cell density was not altered and none of the patients experienced visually significant complications. In 62% eyes, CXL-specific striae developed and only 6.9% eyes had grade one haze, which regressed after a month of topical steroid therapy.
Prospective study report from Caporrossi et al.,[14] (Siena CXL Pediatrics trial) involving 152 eyes of 77 patients (10 to 18 years) remains the largest series with the longest follow up (3 years). The study demonstrated after CXL, keratoconus stabilized and demonstrated rapid significant visual function improvement in these pediatric patients. There was a better and faster visual recovery in eyes with less than 450 um corneal thickness as compared with thicker cornea group. No adverse events (infections or scars) were recorded in this pediatric series, however they noticed transient corneal edema (55%) and slight to moderate haze (9.8%) which resolved without significantly influencing visual outcomes.
Chatzis and Hafezi[15] conducted a retrospective analysis of 59 eyes from 42 children and adolescents (aged 9 to 19 years) with confirmed keratoconus with up to 3 years follow up. Fifty-two of the 59 eyes enrolled in this study showed progression, corresponding to a progression rate of 88%. Forty-six eyes underwent CXL. Maximal keratometry, CDVA, and K index showed significant changes over the follow up period. However, significant Kmax reduction observed up to two years after CXL lost significance at three years. They suggested performing CXL as soon as diagnosis of pediatric keratoconus is made due to the very high rate of keratoconus progression in their study. Furthermore, CXL was safe with no visually significant complications. However, they suggested the effect of arrest of disease progression might not be as long lasting as in adults. One eye in a habitual eye rubber required a re-treatment to stabilize the keratoconus.
Two studies, Magli et al.,[16] and Buzzonetti et al.,[17] looked at role of trans-epithelial CXL in pediatric keratoconus with variable outcomes. Magli et al., conducted comparative analysis of standard CXL (epi-off) and trans-epithelial CXL (TE CXL) protocols in a retrospective comparative evaluation of 37 eyes of 29 patients (12-18 years). They demonstrated stability of keratoconus in both groups, and TE CXL demonstrated fewer eyes with corneal edema and less pain as compared with standard CXL up to 1 year of follow up. In contrast to this study, Buzzonetti et al., performed a prospective analysis of TE CXL for pediatric keratoconus (8 to 18 years age) in 13 eyes of 13 patients and demonstrated that K readings and HOA aberrations significantly worsened during follow up. Confocal microscopy demonstrated demarcation line at depth of only 105um in contrast to the demarcation line typically seen at 300um in standard CXL treatment. They concluded that TE CXL appears to be safe but does not effectively halt keratoconus progression as compared with standard CXL.
These publications have demonstrated that visual, refractive and topographic stabilization and improvements after pediatric CXL are similar to that reported for adult treatment, with stability or improvement maintained for up to three years follow up when treated with the standard protocol. Chatzis and Hafezi[15] found similar outcomes over two years; however, they noted some keratometric progression at three years of follow up. It suggests that pediatric CXL may not provide long-term stability comparable to adult treatment and may require re-treatment especially in the subset of patients who persists eye rubbing.
Personal Experience
Our group has reported outcomes of pediatric CXL following standard Dresden Protocol in eight eyes of four children (11 to 16 years), who were followed for three years.[18] Stabilization of keratometric indices were demonstrated in all cases throughout follow-up (from the first post-operative interval), while visual acuity improved in six eyes and remained stable in the remaining two eyes. Manifest refraction remained stable, and corneal thickness decreased at the first post-operative month with gradual return to preoperative values during follow up. These results are similar to other pediatric CXL studies following the standard CXL protocol and demonstrate feasibility and safety of CXL in treating pediatric keratoconus. There were no complications.
Special Concerns
Timing of treatment
Chatzis and Hafezi[15] found that 88% of pediatric keratoconic patients demonstrated progression from their initial evaluation and Soeters et al.,[10] reported rapid progression of the keratoconus ranging from 2.6 D in seven weeks to 5.0 D over a year. These findings suggest that treatment at onset of diagnosis may be appropriate as opposed to waiting for signs of progression as is commonly done in adults, because keratoplasty in the younger age group may be imminent. A well-designed randomized contra-lateral eye controlled trial should be undertaken to answer this question. We suggest, it should be recommended to evaluate pediatric keratoconus patients very closely (3 months as against 6 months in adults) to identify the earliest signs of progression and offer them CXL. If longer-term follow up demonstrates continued efficacy and, more importantly, continued safety of CXL in pediatric age, taking into account very high rate and speed of progression, performing CXL without waiting for definite progression might become the standard of care.
Treatment protocol
We suggest following standard CXL (epithelium off - Dresden protocol) in pediatric keratoconus, which has been shown to be successful in stabilization in most studies. Nevertheless, Chatzis and Hafezi et al.,[15] have reported stabilization for 2 years and late regression of the ‘standard CXL’ effect at 3 years follow up, indicating possibility of re-treatment. Considering lesser biomechanical rigidity in the younger pediatric population, the use of a higher total power compared to adult CXL (5.4 J/cm 2) is currently under investigation. With the current protocol, the need for re-treatment should be discussed with parents.
Management of comorbidities
Many pediatric keratoconus patients suffer from ocular comorbidities such as surface allergy especially vernal kerato-conjunctivitis (VKC).[11] VKC compounds the problems with keratoconus as continued surface inflammation and the tendency toward eye rubbing further accelerates keratoconus progression and may lead to advanced disease in young age. Furthermore, many eyes with VKC demonstrate partial limbal cell deficiency which may result in delayed epithelial healing after standard CXL treatment.[35] Therefore, it is recommended that VKC should be controlled aggressively in these eyes prior to CXL and patients as well as parents should be counseled about avoiding eye rubbing. In addition, care should be taken to protect limbal stem cells during irradiation (eg., using a PMMA ring and maintaining centration during the procedure.)
Epithelium off or epithelium on cxl
Studies have shown that the corneal epithelium is a significant barrier for penetration of riboflavin and UVA light.[36,37] A variety of transepithelial or modified epithelial removal approaches have been attempted to improve riboflavin penetration across intact epithelium; however, to date, none have been close to reaching the efficacy of epithelium off technique.[38] TE CXL may be of limited value especially in the pediatric age group as keratoconus is more aggressive in this group of patients. Buzzonetti and Petrocelli[17] reported sub-optimal outcomes after transepithelial pediatric CXL, in which K readings and HOAs showed statistically significant worsening and a demarcation line depth of only 105 um. We recommend following standard epithelium off CXL in pediatric eyes. The method of epithelial removal may be manual, alcohol assisted or trans-epithelial PTK. In our study on adult CXL, the group with PTK demonstrated statistically significant topographic and visual outcomes as compared with manual method.[39] If the corneal thickness is less than 400 um, then TE CXL may be attempted in these eyes. Special care should be taken in pediatric patients regarding post-operative pain, possibility of microbial keratitis in epithelium off technique.
Choice of anesthesia
According to patient's age, mental state and co-operation ability, either general or topical anesthesia can be utilized. If successfully validated in treatment of adult keratoconus, rapid riboflavin delivery by iontophoresis and accelerated UVA exposure (ACC CXL) may be utilized in pediatric keratoconus in the future to reduce treatment time for CXL. It will be of immense benefit in reducing general anesthesia time and risk and will result in better cooperation when topical anesthesia is used in pediatric patients.
Management of Visual Impairment
Currently, management of keratoconus consists of a two-pronged approach, first to halt keratoconus progression with CXL and equally important, visual rehabilitation for improving functional vision. Management of visual impairment in pediatric patients does not significantly differ from adult keratoconic patients. In the initial stages, patients can be managed with spectacles and toric soft contact lenses, as the cornea still demonstrates regular astigmatism. As the disease progresses and the corneal biomechanical rigidity worsens, high irregular astigmatism that cannot be corrected with spectacles and soft lenses anymore, in such cases a good fitting rigid gas permeable contact lenses (RGPCL) should be utilized. The RGPCL regular surface replaces the irregular cornea as the anterior refractive surface improving functional vision.[5] In pediatric patients, RGPCLs lenses with high oxygen permeability should be preferred and lenses should be replaced more frequently.
Approximately 20% of keratoconus patients demonstrate contact lens intolerance, and depending on the corneal thickness and the presence of corneal scarring, the decision to utilize of intra-corneal ring segment (ICRS) implantation (requiring 400 u thickness at corneal mid-periphery with clear central cornea) or corneal transplant (very thin cornea and/or presence of stromal scar) is advocated in adult keratoconus.[6,7,8] ICRS are not preferred in the pediatric age group for variety of reasons e.g., aggressive nature of keratoconus, tendency of eye rubbing and non-compliance. Although the option of ICRS (less invasive) is not commonly utilized in pediatric eyes, in adolescent patients with end stage keratcoconus and imminent keratoplaty (more invasive), this option may be worth considering. ICRS are crescent-shaped polymethyl methacrylate implants that are inserted in intra-stromal channels (created either manually or with femtosecond lasers) at 70% depth of thinnest pachymetry underlying the segments. This results in an arc shortening effect and redistribution of corneal peripheral lamellae to produce flattening of the central cornea. This reversible procedure works by flattening and regularizing central cornea and has demonstrated improvement in UDVA and CDVA as well as improvement in RGPCL tolerance.[40] Compared to the manual technique, the femtosecond laser makes tunnel creation faster, easier and more reproducible as well as offering accurate tunnel dimensions.[41]
In a very advanced disease, corneal transplant to replace the pathological keratoconic corneal tissue with healthy donor cornea is advocated. In children, penetrating keratoplasty (PK) has been the standard of care until recently. PK performed for keratoconus seems to enjoy a very high rate of success and survival.[42,43] However, the outcomes of penetrating keratoplasty in children are poor compared with adults because of the higher incidence of endothelial graft rejection and lower rate of rejection reversal.[34] Deep anterior lamellar keratoplasty (DALK) selectively replaces pathological corneal stroma in keratoconic eyes, while preserving patients own corneal endothelium. The most important advantage of DALK over PK comprises of retention of most endothelial cells compared to the rapid endothelial cell loss in PK and elimination or reduction in graft rejection rate, thus providing better and longer graft survival rate.[44] This is of immense benefit in the pediatric age group. Additional benefits include the possibility of early steroid withdrawal (reducing steroid related morbidities such as glaucoma and cataract), early suture removal, better tectonic support and extra-ocular nature of the procedure.[44]
Conclusions
Although with limited evidence in pediatric population, CXL may be considered in the management of progressive pediatric keratoconus, especially due to the higher rate and speed of progression in this age group. The timing of CXL remains a topic of debate, however these patients should be kept under very close follow up to look for the earliest signs of progression and upon which, CXL should be promptly offered. After evaluations regarding comparison of risk of visually significant complications from CXL and the risk of visual loss from accelerated progression of keratoconus in young age, it will be clear whether it is appropriate to offer CXL without waiting for progression. Standard epithelium-off CXL protocol should be followed until evidence of equal efficacy of TE CXL and ACC CXL protocols. Parents should be informed about the off-label nature, possibility of short lasting effect and need for re-treatment especially in very aggressive forms. The utilization of more total power than current protocol is a topic of future research to potentially improve efficacy of procedure avoid possible re-treatments. The comprehensive management should include visual rehabilitation with optimum methods (spectacles, RGPCL or ICRS) according to the stage of keratoconus. In advanced keratoconus, the management still consists of lamellar or full thickness corneal transplant.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Zadnik K, Barr JT, Gordon MO, Edrington TB. Biomicroscopic signs and disease severity in keratoconus. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Cornea. 1996;15:139–46. doi: 10.1097/00003226-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Zadnik K, Steger-May K, Fink BA, Joslin CE, Nichols JJ, Rosenstiel CE, et al. CLEK Study Group. Collaborative longitudinal evaluation of keratoconus. Between-eye asymmetry in keratoconus. Cornea. 2002;21:671–9. doi: 10.1097/00003226-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Cheng EL, Maruyama I, Sundar Raj N, Sugar J, Federer RS, Yue BY. Expression of type XII collagen and hemidesmosome- associated proteins in keratoconus corneas. Curr Eye Res. 2001;22:333–40. doi: 10.1076/ceyr.22.5.333.5491. [DOI] [PubMed] [Google Scholar]
- 4.Radner W, Zehemayer M, Skorpik C, Mallinger R. Altered organization of collagen in apex of keratoconus corneas. Ophthalmic Res. 1998;30:327–32. doi: 10.1159/000055492. [DOI] [PubMed] [Google Scholar]
- 5.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 6.Zare MA, Hashemi H, Salari MR. Intracorneal ring segment implantation for the management of keratoconus: Safety and efficacy. J Cataract Refract Surg. 2007;33:1886–91. doi: 10.1016/j.jcrs.2007.06.055. [DOI] [PubMed] [Google Scholar]
- 7.Bahar I, Kaiserman I, Srinivasan S, Ya-Ping J, Slomovic AR, Rootman DS. Comparison of three different techniques of corneal transplantation for keratoconus. Am J Ophthalmol. 2008;146:905–12. doi: 10.1016/j.ajo.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Bromley JG, Randleman JB. Treatment strategies for corneal ectasia. Curr Opin Ophthalmol. 2010;21:255–8. doi: 10.1097/ICU.0b013e32833a8bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suri K, Hammersmith KM, Nagra PK. Corneal collagen cross-linking: Ectasia and beyond. Curr Opin Ophthalmol. 2012;23:280–7. doi: 10.1097/ICU.0b013e328354865e. [DOI] [PubMed] [Google Scholar]
- 10.Soeters N, Van der Lelij A, van der Valk R, Tahzib NG. Corneal crosslinking for progressive keratoconus in four children. J Pediatr Ophthalmol Strabismus. 2011;21:48. doi: 10.3928/01913913-20110614-02. [DOI] [PubMed] [Google Scholar]
- 11.Arora R, Gupta D, Goyal JL, Jain P. Results of corneal collagen cross-linking in pediatric patients. J Refract Surg. 2012;28:759–62. doi: 10.3928/1081597X-20121011-02. [DOI] [PubMed] [Google Scholar]
- 12.Bakshi E, Barkana Y, Goldich Y, Avni I, Zadok D. Corneal Cross-Linking for Progressive Keratoconus in Children: Our Experience. Int J Keratoco Ectatic Corneal Dis. 2012;1:53–6. [Google Scholar]
- 13.Vinciguerra P, Albé E, Frueh BE, Trazza S, Epstein D. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol. 2012;154:520–6. doi: 10.1016/j.ajo.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Denaro R, Balestrazzi A. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 2012;31:227–31. doi: 10.1097/ico.0b013e31822159f6. [DOI] [PubMed] [Google Scholar]
- 15.Chatzis N, Hafezi F. Progression of keratoconus and efficacy of pediatric [corrected] corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012;28:753–8. doi: 10.3928/1081597X-20121011-01. [DOI] [PubMed] [Google Scholar]
- 16.Magli A, Forte R, Tortori A, Capasso L, Marsico G, Piozzi E. Epithelium-off corneal collagen cross-linking versus transepithelial cross-linking for pediatric keratoconus. Cornea. 2013;32:597–601. doi: 10.1097/ICO.0b013e31826cf32d. [DOI] [PubMed] [Google Scholar]
- 17.Buzzonetti L, Petrocelli G. Transepithelial corneal cross-linking in pediatric patients: Early results. J Refract Surg. 2012;28:763–7. doi: 10.3928/1081597X-20121011-03. [DOI] [PubMed] [Google Scholar]
- 18.Zotta PG, Moschou KA, Diakonis VF, Kymionis GD, Almaliotis DD, Karamitsos AP, et al. Corneal collagen cross-linking for progressive keratoconus in pediatric patients: A feasibility study. J Refract Surg. 2012;28:793–9. doi: 10.3928/1081597X-20121011-08. [DOI] [PubMed] [Google Scholar]
- 19.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin ultraviolet- A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–5. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 20.Tanter M, Touboul D, Bercoff J, Fink M. High Resolution quantitative imaging of cornea elasticity using supersonic shear imaging. IEEE Trans Med Imaging. 2009;5:5. doi: 10.1109/TMI.2009.2021471. [DOI] [PubMed] [Google Scholar]
- 21.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-Ainduced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–27. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 22.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet A corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149:585–93. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 24.Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: Preliminary results. J Refract Surg. 2008;24:S720–5. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 25.Gkika M, Labiris G, Kozobolis V. Corneal collagen cross-linking using riboflavin and ultraviolet-A irradiation: A review of clinical and experimental studies. Int Ophthalmol. 2011;31:309–19. doi: 10.1007/s10792-011-9460-x. [DOI] [PubMed] [Google Scholar]
- 26.Snibson GR. Collagen cross-linking: A new treatment paradigm in corneal disease-A review. Clin Exp Ophthalmol. 2010;38:141–53. doi: 10.1111/j.1442-9071.2010.02228.x. [DOI] [PubMed] [Google Scholar]
- 27.Touboul D, Efron N, Smadja D, Praud D, Malet F, Colin J. Corneal confocal microscopy following conventional, transepithelial, and accelerated corneal collagen cross-linking procedures for keratoconus. J Refract Surg. 2012;28:769–76. doi: 10.3928/1081597X-20121016-01. [DOI] [PubMed] [Google Scholar]
- 28.Léoni-Mesplié S, Mortemousque B, Touboul D, Malet F, Praud D, Mesplié N, et al. Scalability and severity of keratoconus in children. Am J Ophthalmol. 2012;154:56–62. doi: 10.1016/j.ajo.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Al Suhaibani AH, Al-Rajhi AA, Al-Motowa S, Wagoner MD. Inverse relationship between age and severity and sequelae of acute corneal hydrops associated with keratoconus. Br J Ophthalmol. 2007;91:984–5. [Google Scholar]
- 30.Li X, Yang H, Rabinowitz YS. Longitudinal study of keratoconus progression. Exp Eye Res. 2007;85:502–7. doi: 10.1016/j.exer.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ertan A, Muftuoglu O. Keratoconus clinical findings according to different age and gender groups. Cornea. 2008;27:1109–13. doi: 10.1097/ICO.0b013e31817f815a. [DOI] [PubMed] [Google Scholar]
- 32.Reeves SW, Stinnett S, Adelman RA, Afshari NA. Risk factors for progression to penetrating keratoplasty in patients with keratoconus. Am J Ophthalmol. 2005;140:607–11. doi: 10.1016/j.ajo.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Kamiya K, Shimizu K, Ohmoto F. Effect of aging on corneal biomechanical parameters using the ocular response analyzer. J Refract Surg. 2009;25:888–93. doi: 10.3928/1081597X-20090917-10. [DOI] [PubMed] [Google Scholar]
- 34.Vanathi M, Panda A, Vengayil S, Chaudhuri Z, Dada T. Pediatric keratoplasty. Surv Ophthalmol. 2009;54:245–71. doi: 10.1016/j.survophthal.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Sangwan VS, Jain V, Vemuganti GK, Murthy SI. Vernal keratoconjunctivitis with limbal stem cell deficiency. Cornea. 2011;30:491–6. doi: 10.1097/ico.0b013e3181cbf9d3. [DOI] [PubMed] [Google Scholar]
- 36.Baiocchi S, Mazzotta C, Cerretani D, Caporossi T, Caporossi A. Corneal crosslinking: Riboflavin concentration in corneal stroma exposed with and without epithelium. J Cataract Refract Surg. 2009;35:893–9. doi: 10.1016/j.jcrs.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Leccisotti A, Islam T. Transepithelial corneal collagen cross-linking in keratoconus. J Refract Surg. 2010;26:942–8. doi: 10.3928/1081597X-20100212-09. [DOI] [PubMed] [Google Scholar]
- 38.Touboul D, Efron N, Smadja D, Praud D, Malet F, Colin J. Corneal confocal microscopy following conventional, transepithelial, and accelerated corneal collagen cross-linking procedures for keratoconus. J Refract Surg. 2012;28:769–76. doi: 10.3928/1081597X-20121016-01. [DOI] [PubMed] [Google Scholar]
- 39.Kymionis GD, Grentzelos MA, Kounis GA, Diakonis VF, Limnopoulou AN, Panagopoulou SI. Combined transepithelial phototherapeutic keratectomy and corneal collagen cross-linking for progressive keratoconus. Ophthalmology. 2012;119:1777–84. doi: 10.1016/j.ophtha.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 40.Pinero D, Alio L. Intracorneal ring segments in ectatic corneal disease: A review. Clin Exp Ophthalmol. 2010;38:154–67. doi: 10.1111/j.1442-9071.2010.02197.x. [DOI] [PubMed] [Google Scholar]
- 41.Rabinowitz YS, Li X, Ignacio TS, Maguen E. Intacs inserts using the femtosecond laser compared to the mechanical spreader in the treatment of keratoconus. J Refract Surg. 2006;22:764–71. doi: 10.3928/1081-597X-20061001-06. [DOI] [PubMed] [Google Scholar]
- 42.Jensen LB, Hjortdal J, Ehlers N. Longterm follow-up of penetrating keratoplasty for keratoconus. Acta Ophthalmol. 2010;88:347–51. doi: 10.1111/j.1755-3768.2009.01525.x. [DOI] [PubMed] [Google Scholar]
- 43.Pramanik S, Musch DC, Sutphin JE, Farjo AA. Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology. 2006;113:1633–8. doi: 10.1016/j.ophtha.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 44.Reinhart WJ, Musch DC, Jacobs DS, Lee WB, Kaufman SC, Shtein RM. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty a report by the american academy of ophthalmology. Ophthalmology. 2011;118:209–18. doi: 10.1016/j.ophtha.2010.11.002. [DOI] [PubMed] [Google Scholar]


