Abstract
Acute corneal hydrops is a condition characterized by stromal edema due to leakage of aqueous through a tear in descemet membrane. The patient presents with sudden onset decrease in vision, photophobia, and pain. Corneal thinning and ectasias combined with trivial trauma to the eye mostly by eye rubbing is considered as the underlying cause. With conservative approach self-resolution takes around 2 to 3 months. Surgical intervention is required in cases of non-resolution of corneal edema to avoid complications and for early visual rehabilitation. Intracameral injection of air or gas such as perflouropropane is the most common surgical procedure done. Recent investigative modality such as anterior segment optical coherence tomography is an extremely useful tool for diagnosis, surgical planning, and postoperative follow up. Resolution of hydrops may improve the contact lens tolerance and visual acuity but most cases require keratoplasty for visual rehabilitation.
Keywords: Acute corneal hydrops, keratoconus, corneal edema
Acute corneal hydrops (CH) is the development of marked corneal edema due to a tear in Descemet membrane (DM) followed by leakage of aqueous into stroma. Although initially it was reported in cases of keratoconus (KC), subsequently it has also been reported in other corneal ectasias including pellucid marginal degeneration (PMD), keratoglobus, Terrien's marginal degeneration (TMD), LASIK-associated keratectasia, keratectasia after radial keratotomy (RK), deep anterior lamellar keratoplasty (DALK), and penetrating keratoplasty (PKP) for KC.[1,2] In this article we review the etiology, diagnosis, management, and outcomes in cases of acute CH in corneal ectasias.
Review of Literature
The first case of CH in KC was reported by Plaut (1900) as a sudden opacity at the apex of the cornea due to a rupture of DM which was later confirmed by Axenfeld (1906).[1,2] Acute CH occurs in approximately 2.5-3% of eyes with KC.[1,3,4] Most of the cases are seen in the second or the third decade. There is a preponderance for male gender; bilateralcases are rare.[1,3,4] No racial predisposition has been reported so far.[1,3,4]
Predisposing factors
The various risk factors which are associated with the increased risk of CH include earlier age at onset, eye rubbing, vernal keratoconjunctivitis (VKC), atopy, and Down's syndrome.[4,5,6] Amongst all eye rubbing appears to be the most important risk factor.[4]
Pathogenesis
CH occurs due to the torn DM which causes the rolling of the edges, a gap is created so that the aqueous from the anterior chamber percolates into the corneal stroma. Some sort of trauma such as vigorous eye rubbing may be the inciting factor. Continuous accumulation of the aqueous leads to the separation of the collagen lamellae and the formation of large fluid-filled stromal pockets.[1,7] Meanwhile as a part of the reparative process the adjacent endothelium grows over the defect causing a partial seal so that the seepage is prevented with subsequent resolution of stromal edema. According to various studies the resolution of corneal edema may occur any time between 5 and 36 week.[1,3,4]
Clinical Characteristics
The presenting symptoms of acute CH are markedly reduced visual acuity, intense photophobia, and pain.[1] Often a preceding history of vigorous eye rubbing or coughing may be there.[1,3,4,5] Slit-lamp examination usually reveals marked stromal and epithelial microcystic edema, intrastromal cyst/clefts and conjunctival hyperemia [Fig. 1]. The location and area of the involved cornea is variable. Corneal edema can be graded according to its extent; grade 1 within a circle of 3 mm diameter, grade 2 between circles of 3 and 5 mm diameters, and grade 3 larger than a circle of 5 mm diameter.[1,8] Time for resolution of edema and subsequent final BCVA achieved are inversely related to the area of involvement.[1,2,4,8]
Figure 1.
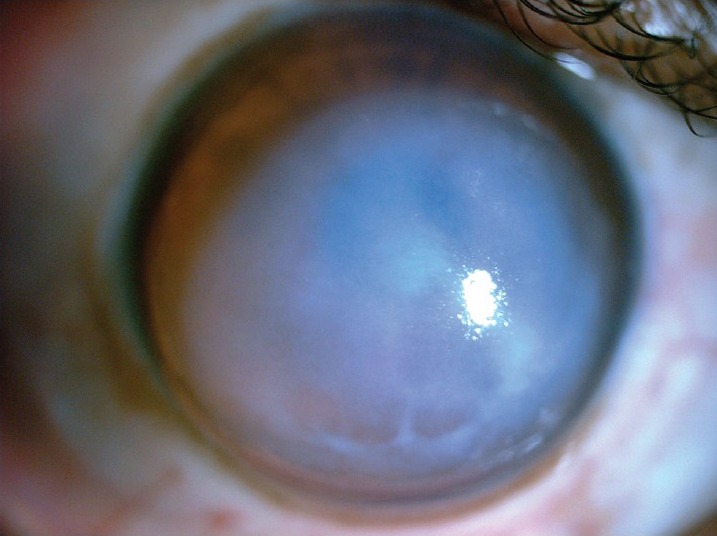
Case of acute corneal hydrops in advanced keratoconus showing marked corneal edema
Investigations
Diagnosis is usually based on history and slit-lamp findings. Investigations are required to determine the size and extent of edema and DM tear which helps in formulating the treatment plan, monitoring the response to treatment, and identifying any complication. These investigative modalities are: Ultrasound biomicroscopy (UBM) and anterior segment optical coherence tomography (ASOCT) [Fig. 2].[1,7,9]
Figure 2.
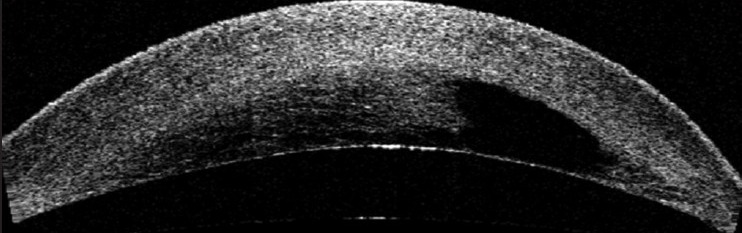
Anterior segment optical coherence tomography in a case of acute corneal hydrops showing corneal edema and the presence of a large intrastromal fluid filled cleft
Management
AcuteCH may be treated with conservative treatment or surgical intervention which commonly includes intracameral injection of air/gas.[1,2] Other surgical modalities that are helpful in special situations includes, compressive sutures along with gas injection,[10] PKP,[1] cyanoacrylate tissue adhesive with BCL,[11] and amniotic membrane transplantation (AMT) with cauterization.[12]
Conservative Approach
Medical therapy aims at providing symptomatic relief till spontaneous resolution occurs. It includes topical lubricants, antibiotics (prevent secondary infection), cycloplegics (to reduce pain and photophobia), hypertonic saline eye drops (help draw fluid), anti-glaucoma medications (to lessen the hydrodynamic force on the posterior cornea), and topical steroids or nonsteroidal anti-inflammatory drugs (NSAIDs).[1,2,3,4] The final best corrected visual acuity (BCVA) is found to be as good as to surgical intervention. Sometimes a BCL may be indicated to provide pain relief until the edema subsides or patient is comfortable.[1,2,3,4]
Intracameral Air/gas Injection
Intracameral air/gas injection shortens the period of persistence of corneal edema in CH. Various agents used include air,[13] 20% sulfur hexafluoride (SF6),[14] and 14% perflouropropane (C3F8).[3] Air stays for a shorter time, hence repeat injections may be required.[1] SF6 is long acting compared to air (around 2 weeks), however repeat injections may still be required.[1] C3F8 is the longest acting among all and usually repeat injections are not required.[1,3] These agents act by tamponade effect which prevents aqueous penetration into the stroma and also by unrolling the torn ends of ruptured DM.[1,2,3]
There are different techniques of injecting air or gas. The commonly followed procedure includes: Preoperative pupillary constriction by topical application of 2% pilocarpine nitrate eye drops at 15-20 min intervals 1 h before surgery to avoid intraoperative injury to the lens; anterior chamber (AC) paracentesis under aseptic conditions with a 26/27 gauge needle or alternately a limbalparacentesis; aspiration of 0.1 ml of aqueous humor and injection of air/gas (14% nonexpansile concentration of C3F8, 20% nonexpansile concentration of SF6, sterile air) enough to fill two-thirds of the AC.[1,2,3,13,14] Postoperatively, patient supine position is advised along with topical antibiotics, hypertonic saline, and steroids. Antiglaucoma treatment may have to be given to avoid any rise of intraocular pressure (IOP). In case of persistence of edema, repeat injections are given.[1,2,3] The postoperative complications include elevation of IOP, infection, endothelial damage, and intrastromal migration of gas.
Other Procedures
Compressive sutures along with gas injection have been tried in severe cases with wide separation of the DM edges and multiple stromal cleft.[1,10] PKP is required rarely in cases of persistent edema, perforation, large DM tear, large intrastromalcyst, and corneal neovascularisation.[1,2] Cyanoacrylate tissue adhesive with BCL can be done in cases of small perforation with fistula formation.[1,11] AMT with cauterization has been tried in persistent hydrops in mentally retarded patients as a quick and effective treatment with good results.[1,12]
Outcome
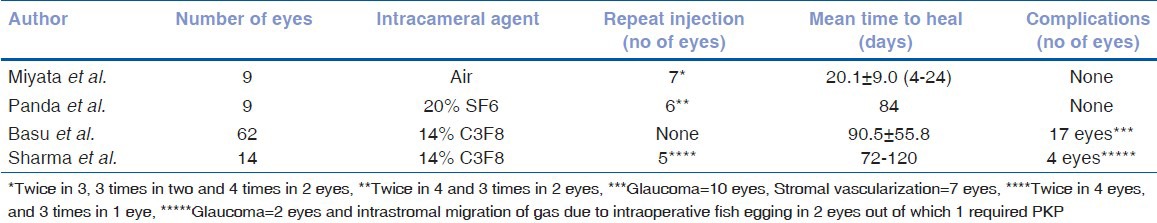
Hydrops leave a residual scar and causes flattening of the cone. In KC the cone usually does not involve the central area, hence VA shows some improvement after healing of the hydrops.[1,2,3,4] Corneal flattening may also improve the contact lens fitting.[1,2,3,4] Comparative results of various studies are given in Tables 1 and 2.
Table 1.
Outcomes of conservative treatment in acute corneal hydrops

Table 2.
Outcome of intracameral air/gas injection in acute corneal hydrops
Personnel Experience
Being a referral center CH is not a rare entity at our center. VKC associated cases often presents at an earlier age. When the extent of corneal edema is less and there are no associated stromal fluid pockets we undertake the conservative approach or intracameral air/gas injection after discussing the pros and cons of each of this procedure with the patient. For extensive corneal edema and the presence of multiple stromal fluid pockets we have standardized the new technique of intrastromal fluid drainage with air tamponade which is guided by ASOCT.[15] In this technique, multiple corneal stromal venting incisions are given depending upon the location of fluid pockets as determined from preoperative ASOCT to drain out the collected fluid along with anterior chamber air tamponade. Our technique not only reduces the duration of morbidity but also avoids the potential complications associated with repeat gas injections. Once the CH resolves the other visual rehabilitative procedures like, contact lens fitting, keratoplasties are done.
Discussion
CH is rare in KC. Among the various proposed risk factors atopy and eye rubbing account for significant proportion of cases. Newer investigation modalities such as ASOCT or UBM can be used for preoperative planning and also follow-up these cases. Conservative management may prolong the morbidity and the visual rehabilitation. The standard surgical procedure performed includes intracameral gas injection. However, in cases with extensive edema and the presence of multiple fluid pockets the new technique of ASOCT guided intrastromal fluid drainage with air tamponade is quite successful. Once the hydrops heals it may leave a residual scar. The patient may require CL or keratoplasty for visual rehabilitation subsequently.
Conclusion
CH occurs not only in KC but also in other ecstatic disorders. Although mild improvement in vision can occur but still it adversely affects the prognosis since subsequent keratoplasty is required in most of the cases. Although intracameral gas injection does not affect the final visual outcome, it reduces the duration of morbidity and also the risk of complications such as corneal neovascularization that may jeopardize the subsequent graft. Newer treatment modalities such as tissue adhesive, AMG, and compressive sutures may further widen the available options for corneal surgeons but further studies are required to validate these techniques.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tuift SJ, Gregory WM, Buckley RJ. Acute corneal hydrops in keratoconus. Ophthalmology. 1994;101:1738–44. doi: 10.1016/s0161-6420(94)31110-9. [DOI] [PubMed] [Google Scholar]
- 2.Sharma N, Maharana PK, Jhanji V, Vajpayee RB. Management of acute corneal hydrops in ectatic corneal disorders. Curr Opin Ophthalmol. 2012;23:317–23. doi: 10.1097/ICU.0b013e328354a2a8. [DOI] [PubMed] [Google Scholar]
- 3.Basu S, Vaddavalli PK, Ramappa M, Shah S, Murthy SI, Sangwan VS. Intracameral perfluoropropane gas in the treatment of acute corneal hydrops. Ophthalmology. 2011;118:934–9. doi: 10.1016/j.ophtha.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Grewal S, Laibson PR, Cohen EJ, Rapuano CJ. Acute hydrops in the corneal ectasias: Associated factors and outcomes. Trans Am Ophthalmol Soc. 1999;97:187–203. [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma R, Titiyal JS, Prakash G, Sharma N, Tandon R, Vajpayee RB. Clinical profile and risk factors for keratoplasty and development of hydrops in north Indian patients with keratoconus. Cornea. 2009;28:367–70. doi: 10.1097/ICO.0b013e31818cd077. [DOI] [PubMed] [Google Scholar]
- 6.Stoiber J, Muss W, Ruckhofer J, Grabner G. Acute keratoconus with perforation in a patient with Down's syndrome. Br J Ophthalmol. 2003;87:120. doi: 10.1136/bjo.87.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma N, Mannan R, Jhanji V, Agarwal T, Pruthi A, Titiyal JS, et al. Ultrasound biomicroscopy-guided assessment of acute corneal hydrops. Ophthalmology. 2011;118:2166–71. doi: 10.1016/j.ophtha.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 8.Akova YA, Dabil H, Kavalcioglu O, Duman S. Clinical features and keratoplasty results in keratoconus complicated by acute hydrops. Ocul Immunol Inflamm. 2000;8:101–9. [PubMed] [Google Scholar]
- 9.Basu S, Vaddavalli PK, Vemuganti GK, Ali MH, Murthy SI. Anterior segment optical coherence tomography features of acute corneal hydrops. Cornea. 2012;31:479–85. doi: 10.1097/ICO.0b013e318223988e. [DOI] [PubMed] [Google Scholar]
- 10.Rajaraman R, Singh S, Raghavan A, Karkhanis A. Efficacy and safety of intracameral perfluoropropane (C3F8) tamponade and compression sutures for the management of acute corneal hydrops. Cornea. 2009;28:317–20. doi: 10.1097/ICO.0b013e31818ada0b. [DOI] [PubMed] [Google Scholar]
- 11.Aldave AJ, Mabon M, Hollander DA, McLeod SD, Spencer WH, Abbott RL. Spontaneous corneal hydrops and perforation in keratoconus and pellucid marginal degeneration. Cornea. 2003;22:169–74. doi: 10.1097/00003226-200303000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Wylegala E, Tarnawska D. Amniotic membrane transplantation with cauterization for keratoconus complicated by persistent hydrops in mentally retarded patients. Ophthalmology. 2006;113:561–4. doi: 10.1016/j.ophtha.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Miyata K, Tsuji H, Tanabe T, Mimura Y, Amano S, Oshika T. Intracameral air injection for acute hydrops in keratoconus. Am J Ophthalmol. 2002;133:750–2. doi: 10.1016/s0002-9394(02)01437-x. [DOI] [PubMed] [Google Scholar]
- 14.Panda A, Aggarwal A, Madhavi P, Wagh VB, Dada T, Kumar A, et al. Management of acute corneal hydrops secondary to keratoconus with intracameral injection of sulfur hexafluoride (SF6) Cornea. 2007;26:1067–9. doi: 10.1097/ICO.0b013e31805444ba. [DOI] [PubMed] [Google Scholar]
- 15.Vajpayee RB, Maharana PK, Kaweri L, Sharma N, Jhanji V. Intrastromal fluid drainage with air tamponade: Anterior segment optical coherence tomography guided technique for the management of acute corneal hydrops. Br J Ophthalmol. 2013 doi: 10.1136/bjophthalmol-2013-303272. In Press. [DOI] [PubMed] [Google Scholar]


