Abstract
Advanced cases of keratoconus often require surgical intervention to restore corneal anatomy and improve eyesight. Penetrating keratoplasty (PK) although commonly performed has potential risk of immunological rejection and is now no longer automatically the first choice of surgery. DALK procedures have evolved, which allows surgical replacement of recipient's corneal stroma, leaving behind healthy descemet membrane (DM) and endothelium. This reduces the risk of allograft endothelial rejection and late graft failure. In recent times, DALK techniques have led to significant improvements in visual outcome and current results are comparable to PK. Big bubble technique of DALK has become the most popular among the various surgical techniques described. Manual near DM DALK also gives good outcome although the visual recovery is often delayed. Future integration of femtosecond laser technology along with diagnostic imaging technology is likely to further improve outcomes of DALK in keratoconus.
Keywords: Deep anterior lamellar keratoplasty, keratoconus, lamellar keratoplasty, penetrating keratoplasty
Keratoconus (Greek: kerato- horn, cornea; and konos - cone) is a degenerative, non-inflammatory corneal disorder characterized by progressive stromal thinning and ectasia. It is commonly bilateral. The disease progresses throughout the second and third decades of life. Its etiology is unknown. Management depends on the degree of ectasia. Early to moderate cases can be effectively managed with rigid gas permeable contact lenses, providing satisfactory visual rehabilitation. However, in more advanced cases, fitting of contact lens is often not satisfactory thus limiting their usage. Apical scarring in the visual axis may also affect visual function. In 10-20% of such cases, surgical intervention often becomes necessary.[1] Common surgical options include penetrating keratoplasty (PK) and deep anterior lamellar keratoplasty (DALK).
Penetrating Keratoplasty
PK is a well-studied and long established surgery for the treatment of corneal disease such as keratoconus. It is still one of the most common indications for PK accounting for 15-25% of cases.[2] Compared to other indications, PK in keratoconus generally has a very good outcome. Average reported best spectacle corrected visual acuities of 20/25-20/32 with 73-91% of eyes achieving 20/40 or better.[3] Postoperative astigmatism is a major limiting factor for visual limitation often requiring further interventions such as astigmatic keratotomies, wedge resection, and the use of excimer laser procedures.[1,3,4,5] Replacement of donor endothelium makes host rejection of the graft more likely and has been reported in 4-31% of eyes postoperatively. A 15-year study of the outcomes of PK demonstrated graft failure in 76 out of 500 eyes, with rejection accounting for 25% of those failures.[4] The overall probability of developing graft rejection was 23%. Late endothelial failure caused 29% of graft failure. Recurrence of keratoconus has been reported following PK, possibly due to undetected keratoconus in donor, or due astigmatism resulting from progression of disease in host cornea associated with thinning at graft host junction.
Deep Anterior Lamellar Keratoplasty
Deep anterior lamellar keratoplasty has been proposed as an excellent alternative to PK for corneal diseases that do not affect the endothelium. In patients with keratoconus, DALK preserves native endothelium and reduces host immune system reaction and graft rejection. Current surgical techniques of DALK involve manual dissection up to near DM or injection of air, fluid, or viscoelastic into deep stroma to create a plane of separation between DM and stromal tissue. Malbrans stromal “peeling technique” technique allows dissection of deep corneal stroma in a safe and effective manner even in cases of advanced keratoconus.[6] Intrastromal air injection to aid stromal resection was first described by Archila in 1985.[7] Price and Chau described “Air Lamellar Keratoplasty” using similar technique with deeper dissection and lyophilized full thickness donor tissue for replacement.[8] Sugita and Kondo described technique of hydro-delamination of posterior stromal fibers with an injection of saline using 27-guage cannula, which was performed after the removal of anterior three quarters depth of recipient tissue.[9] The DM was exposed in the central 5 mm zone prior to placement of full thickness donor tissue. Similar technique using viscoelastic instead of saline, i.e., “visco-delamination” was described by Morris et al.,[10] Melles described a technique of planned depth lamellar dissection in DALK. This involves injection of air into the anterior chamber following which an incision is made in peripheral corneal stroma, observing the reflex of the sharp tip against the convex mirror from air endothelium interface. At the right depth, the dark band in front of the sharp tip disappears and wrinkles appear in DM. Lamellar dissectors are then used to separate deep stromal tissue, followed by trephination and replacement with donor tissue.[11] Anwar and Teichmann described the “Big Bubble” technique, wherein air injection was performed into deep stromal tissue to achieve separation of DM from the corneal stroma.[12] A 27-guage needle is attached to an air filled syringe. Needle is inserted bevel down position, into deep stroma for 3-4 mm centrally. Forceful injection of air results in separation of DM from corneal stroma, noted as circular area with dense white border. This technique is most frequently used for DALK surgery. However, using a sharp needle, the “Big Bubble” can be achieved in only 65-69% eyes.[13,14]
Our success rates have significantly improved following some surgical modifications. Anterior segment OCT is used preoperatively to identify any involvement of DM with scar tissue. (In such cases an air injection is avoided as it carries increased risk of perforation of DM during the process). Regional pachymetry assessment is also necessary to plan depth of cut during trephination [Fig. 1].
Figure 1.
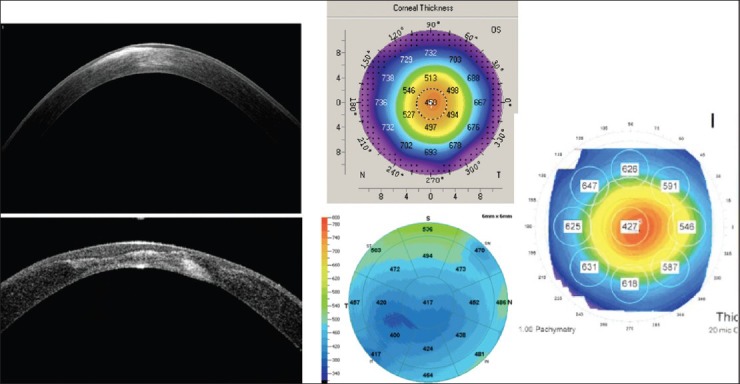
Anterior segment OCT showing stromal changes in cornea. Various methods of regional pachymetry
Suction trephine (Barron Radial Vacuum Trephine, Katena, USA) used for 80% depth cut, followed by de-bulking of 50% stroma by anterior lamellar keratectomy. Pointed dissector (Fogla Pointed Dissector, Storz Ophthalmics, USA) is used to make a track in peripheral stroma. A 27-gauge blunt tipped, bottom port, air injection cannula (Fogla 27G Air injection cannula, Storz Ophthalmics, USA) is attached to a 5cc air filled syringe. Tip of this cannula is introduced into the peripheral stromal track, and moved toward central/paracentral cornea for 3-4 mm using a wiggling motion. Air is then injected using a firm continuous pressure till the formation of big bubble is noted [Fig. 2]. Once it forms, air is gradually injected to increase the size of bubble. Once increasing resistance is felt again, air injection is discontinued. Peripheral paracentesis is performed to release aqueous and normalize intraocular pressure. Using a sharp 15/30° knife the bubble is decompressed, following which residual stromal tissue is excised exposing the DM [Fig. 3]. Donor corneal tissue (same size) is prepared and DM is removed prior to being placed on recipient and secured with 10-0 nylon sutures [Fig. 4]. Using this modified technique the success rate of big bubble is over 95% with minimal complications. Microperfration can occur intraoperatively, either due to pre-existing weak areas in DM, or instrument related. Rates vary depending on the experience of the surgeon. Conversion to PK is usually not necessary, as these can be managed in most cases with intracameral air injection. Manual DALK techniques have been described which allow deep dissection down to near DM levels.[15,16] These are useful in cases wherein big bubble is not achieved following air injection, or there is scarring process involving the DM for which air injection is contraindicated (e.g., post hydrops).
Figure 2.
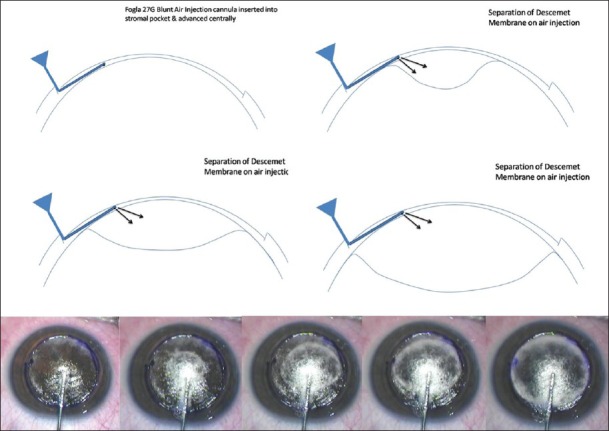
Formation of big bubble using 27G air injection cannula. Note separation of DM from corneal stroma
Figure 3.
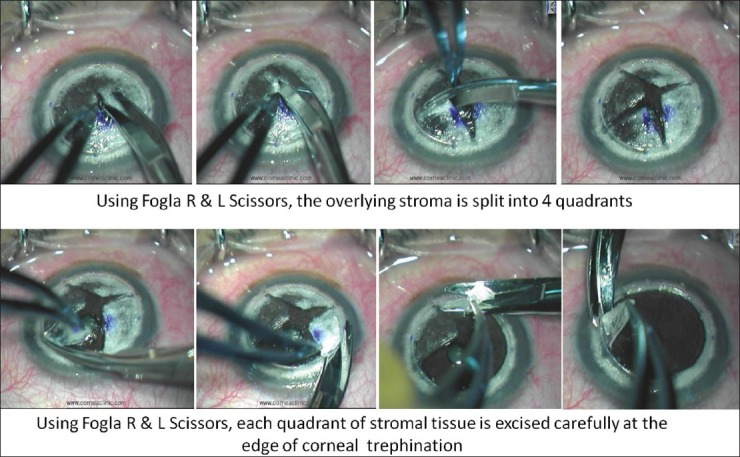
Residual stroma is split into 4 quadrants and excised
Figure 4.
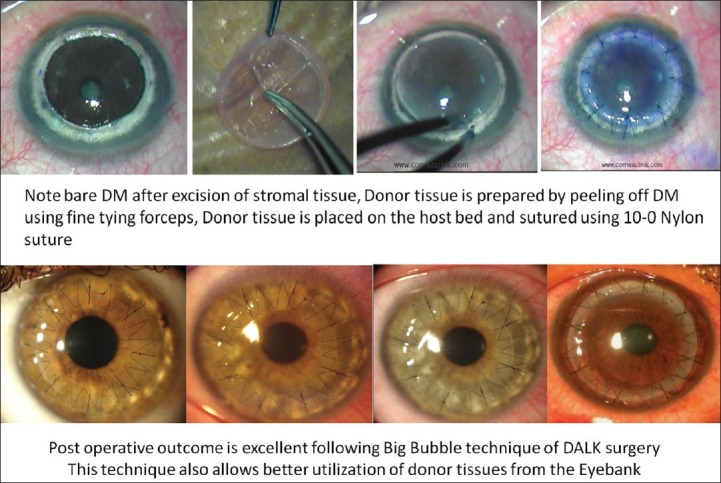
Donor tissue prepared and placed into position and secured with 10-0 nylon sutures
Femtosecond lasers have been used to create incisions with greater precision in DALK surgery. Zigzag peripheral cuts to improve graft host apposition, and also to allow proper placement of needle/cannula using femtosecond laser have been described.[17]
Suturing techniques have varied from interrupted, continuous, or a combination of both in DALK surgery. No significant differences in refractive outcome have been noted between the techniques.[18] In our experience a combination of interrupted and continuous sutures works well to minimize astigmatism. Interrupted sutures can be removed selectively to manage astigmatism from 3 months postoperatively and continuous sutures retained for a longer period (at least a year).
Visual outcome of DALK surgery is comparable to PK. In a recent retrospective study analyzing various techniques of DALK surgery, mean best spectacle corrected visual acuity (BSCVA) was noted to be 20/25 (range 20/30-20/20) at last follow up.[19] Visual outcome was not statistically different between Descemetic DALK and pre-Descemetic DALK although visual recovery was slightly delayed in second group.[19] Watson et al., compared DALK with PK.95% of PK patients achieved 20/40 vision or better compared 88% of DALK patients.[20] The encountered complications were less serious in the DALK group. Compared to PK, one of the major advantage of DALK is normal endothelial cell counts postoperatively. Following PK endothelial cell loss is up to 33% at 2 years, and 50% after 5 years.[21] Comparatively DALK has endothelial cell loss of 1.2% at 2 years.[22]
In my experience of DALK in 450 eyes of 382 patients, keratoconus was the most common indication (84.8%). Big bubble was successfully achieved in 95% eyes. Mean postoperative uncorrected visual acuity (UCVA) was noted to be 20/60 and BSCVA 20/30 at last follow up (average 36.5 + 18.2 months). Average post-operative endothelial cell count was 2849 + 305 cells/mm2 [Fig. 5]. Microperforation occurred in 8.4% eyes; none converted to PK. Stromal rejection episode in 3.6% eyes managed successfully using topical corticosteroids. 10% eyes had additional excimer laser procedures (post suture removal) for refractive correction.
Figure 5.
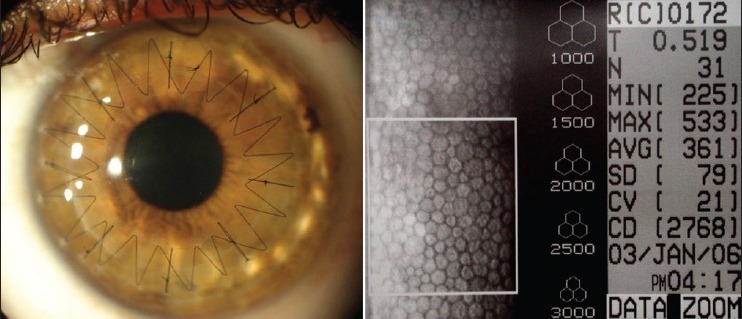
Clear corneal graft post operatively with normal endothelial cell count
DALK avoids the risk of endothelial rejection, however epithelial and stromal rejection episodes can occur which can be effectively managed using topical corticosteroids. In a recent study of outcomes using the “big bubble” technique, only 2 of 78 patients developed stromal rejection.[13] This fits well with other studies reporting rejection in 3-8% of cases.[20]
Additional refractive procedures, such as use of Excimer lasers, or phakic IOL implantation can be performed in eyes post DALK, following complete suture removal and ensuring stable topography.
Surgical management of Keratoconus has improved with advances in surgical techniques, instrumentation, and diagnostic evaluation. DALK procedures should be considered prior to PK as it helps preserves healthy host endothelium, eliminating risk of endothelial rejection, ensuring long term graft survival. Femtosecond laser technology integrated with corneal OCT is likely to improve success rates, and maximize visual outcomes of both DALK and PK in future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Al-Yousuf N, Mavrikakis I, Mavrikakis E, Daya SM. Penetrating keratoplasty: Indications over a 10 year period. Br J Ophthalmol. 2004;88:998–1001. doi: 10.1136/bjo.2003.031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javadi MA, Motlagh BF, Jafarinasab MR, Rabbanikhah Z, Anissian A, Souri H, et al. Outcomes of penetrating keratoplasty in keratoconus. Cornea. 2005;24:941–6. doi: 10.1097/01.ico.0000159730.45177.cd. [DOI] [PubMed] [Google Scholar]
- 4.Patel SV, Hodge DO, Bourne WM. Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. Am J Ophthalmol. 2005;139:311–9. doi: 10.1016/j.ajo.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 5.Barraquer CC, Rodriguez-Barraquer T. Five year results of laser in-situ keratomileusis (LASIK) after penetrating keratoplasty. Cornea. 2004;23:243–8. doi: 10.1097/00003226-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Malbran E, Stefani C. Lamellar keratoplasty in corneal ectasias. Ophthalmologica. 1972;164:50–8. doi: 10.1159/000306704. [DOI] [PubMed] [Google Scholar]
- 7.Archila E. Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea. 1985;3:217–8. [PubMed] [Google Scholar]
- 8.Price FW., Jr Air lamellar keratoplasty. Refract Corneal Surg. 1989;5:240–3. [PubMed] [Google Scholar]
- 9.Sugita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997;81:184–8. doi: 10.1136/bjo.81.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris E, Kirwan JF, Sujatha S, Rostron CK. Corneal endothelial specular microscopy following deep lamellar keratoplasty with lyophilized tissue. Eye (Lond) 1998;12:619–22. doi: 10.1038/eye.1998.155. [DOI] [PubMed] [Google Scholar]
- 11.Melles GR, Lander F, Rietveld FJ, Remeijer L, Beekhuis WH, Binder PS. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol. 1999;83:327–33. doi: 10.1136/bjo.83.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28:398–403. doi: 10.1016/s0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 13.Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007;143:117–24. doi: 10.1016/j.ajo.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Fogla R, Padmanabhan P. Results of deep lamellar keratopolasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2006;141:254–9. doi: 10.1016/j.ajo.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 15.Shi W, Li S, Gao H, Wang T, Xie L. Modified deep lamellar keratoplasty for treatment of advanced stage keratoconus with steep curvature. Ophthalmology. 2010;117:226–31. doi: 10.1016/j.ophtha.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Rama P, Knutsson KA, Razzoli G, Matuska S, Viganò M, Paganoni G. Deep anterior lamellar keratoplasty using an original manual technique. Br J Ophthalmol. 2013;97:23–7. doi: 10.1136/bjophthalmol-2011-301168. [DOI] [PubMed] [Google Scholar]
- 17.Price FW, Jr, Price MO, Grandin JC, Kwon R. Deep anterior lamellar keratoplasty with femtosecond-laser zigzag incisions. J Cataract Refract Surg. 2009;35:804–8. doi: 10.1016/j.jcrs.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Javadi MA, Naderi M, Zare M, Jenaban A, Rabei HM, Anissian A. Comparison of the effect of three suturing techniques on postkeratoplasty astigmatism in keratoconus. Cornea. 2006;25:1029–33. doi: 10.1097/01.ico.0000230498.99648.69. [DOI] [PubMed] [Google Scholar]
- 19.Sarnicola V, Toro P, Gentile D, Hannush SB. Descemetic DALK and predescemetic DALK: Outcomes of 236 cases of keratoconus. Cornea. 2010;29:53–9. doi: 10.1097/ICO.0b013e3181a31aea. [DOI] [PubMed] [Google Scholar]
- 20.Watson SL, Ramsay A, Dart JK, Bunce C, Craig E. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology. 2004;111:1676–82. doi: 10.1016/j.ophtha.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38:779–82. [PubMed] [Google Scholar]
- 22.van Dooren BT, Mulder PG, Nieuwendaal CP, Beekhuis WH, Melles GR. Endothelial cell density after deep anterior lamellar keratoplasty (Melles technique) Am J Ophthalmol. 2004;137:397–400. doi: 10.1016/j.ajo.2003.09.053. [DOI] [PubMed] [Google Scholar]


