Abstract
The microcirculation is the site of gas and nutrient exchange. Control of central or local signals acting on the myocytes, pericytes and endothelial cells within it, is essential for health. Due to technical problems of accessibility, the mechanisms controlling Ca2+ signalling and contractility of myocytes and pericytes in different sections of microvascular networks in situ have not been investigated. We aimed to investigate Ca2+ signalling and functional responses, in a microcirculatory network in situ. Using live confocal imaging of ureteric microvascular networks, we have studied the architecture, morphology, Ca2+ signalling and contractility of myocytes and pericytes. Ca2+ signals vary between distributing arcade and downstream transverse and precapillary arterioles, are modified by agonists, with sympathetic agonists being ineffective beyond transverse arterioles. In myocytes and pericytes, Ca2+ signals arise from Ca2+ release from the sarcoplasmic reticulum through inositol 1,4,5-trisphosphate-induced Ca2+ release and not via ryanodine receptors or Ca2+ entry into the cell. The responses in pericytes are less oscillatory, slower and longer-lasting than those in myocytes. Myocytes and pericytes are electrically coupled, transmitting Ca2+ signals between arteriolar and venular networks dependent on gap junctions and Ca2+ entry via L-type Ca2+ channels. Endothelial Ca2+ signalling inhibits intracellular Ca2+ oscillations in myocytes and pericytes via L-arginine/nitric oxide pathway and intercellular propagating Ca2+ signals via EDHF. Increases of Ca2+ in pericytes and myocytes constrict all vessels except capillaries. These data reveal the structural and signalling specializations allowing blood flow to be regulated by myocytes and pericytes.
Keywords: Myocytes, Pericytes, Ca2+ signalling, Endothelium, Microvascular networks
1. Introduction
The microcirculation consists of precapillary arterioles, capillaries and postcapillary venules, whose diameters range from 5 to 50 μm. The microcirculation is essential for homeostasis and tissue metabolism and has been implicated in a range of pathologies [1,2]. Attention has therefore been focussed on the role of the different cells within these microvessels; the myocytes, pericytes, endothelial cells and how they are affected by central and local agents (neuronal, hormonal and paracrine), and how their signals might be integrated to produce the appropriate functional responses, i.e. vasomotion, vasoconstriction or vasodilation [3–6]. Many techniques, particularly microscopic, have been used to address these questions, but the relative inaccessibility and small, delicate nature of the vessels has precluded comprehensive studies.
Previous studies have suggested heterogeneity in microvascular perfusion [3,4,7–9], as local factors, such as shear stress and metabolites, influence the information impinging from the autonomic and endocrine systems [9–14]. While it is clear that the vessels of the microcirculation have to integrate multiple signals to perform their physiological function [9,15], the mechanisms that regulate them are largely unknown. From recent studies it seems likely that responses to central and local vasoconstrictors will be site specific and lead to spatially modulated changes in flow through the microcirculation [16], but detailed explanations are lacking. In vivo observations support the suggestion that the microcirculation is composed of functionally distinct vessel segments arranged in series. For example, the penetrating transverse arterioles undergo extensive vasomotor activity in response to different agonists and can completely constrict thereby stopping blood flow [9].
Our knowledge of the Ca2+ signals in the individual cell types of the intact microcirculation is rudimentary. This is especially the case for pericytes, the cells that have recently been shown to affect diameter in capillaries [16–26] and venules [27–30]. Pericytes have been described differently depending upon where they have been isolated from, but there has been no systematic study of identified cells in the intact microcirculation, and thus we do not know if contraction and Ca2+ rises are a universal or a restricted function of pericytes populations [16,20,22,31–36]. There are known to be physiological differences in the responses of pericytes and myocytes to vasoactive substances [16,20], but this has not been related to their contractile activity and Ca2+ signals. Furthermore the mechanism underlying the Ca2+ signals in the two cell types remains to be established. Studies on isolated arteriolar fragments have suggested that ryanodine receptors (RyR) and L-type, voltage-gated Ca2+ channels are unimportant in arterioles less than 18 μm in diameter [37], but this is hard to relate to identified vessels in intact preparations. Nothing is known about the mechanisms producing Ca2+ rises in postcapillary venules.
Another important question is how or indeed if, signalling in myocytes and pericytes is integrated across the microvascular network. Coordination of vasomotor activity within and among microvascular networks is integral to the local control of tissue blood flow [15]. Vasoconstriction initiated at a local site can conduct along arterioles for several millimetres and manifests as the transmission of electrical signals from cell to cell through gap junction channels [8,15,38]. Recent electrophysiological data [26] demonstrated a direct electrical coupling between capillary pericytes and myocytes of upstream arterioles suggesting that the whole microvascular network could behave as a single unit (electrical syncytium) propagating electrical signals in both directions.
Many studies suggest that the endothelial cell-dependent dilatory pathways are involved in the spatial control of flow distribution at the microcirculatory level [15]. In vivo studies suggest that endogenous NO is a modulator of the regional microvascular tone but is not playing a role in the mediation of functional hyperaemia [9,11]. In contrast, endothelium derived hyperopolarizing factor (EDHF) was shown to be a key player in functional hyperaemia conducting vasodilator signal from metabolically sensitive parts of microvessels to upstream distributing arterioles and feed arteries [15]. The microvascular wall therefore appears to be a functional unit with unique physiological proprieties. In order to understand the mechanisms controlling blood flow at different levels of the microvascular network, detailed investigation is needed of the mechanisms of interplay between endothelial cell Ca2+ signalling, responsible for production of vasodilator agents, with Ca2+ signalling and contractility of myocytes and pericytes induced by different vasoconstrictors. The aim of the present work was therefore to make use of the ability to resolve all cell types of the different levels of the ureteric microvasculature and characterize and investigate the mechanisms controlling the regulation of intracellular calcium and links to contraction.
2. Methods
2.1. Animals and ureteric tissue samples
The experiments were performed on Wistar rats of both sexes (3–4 months old). All animals were treated in accordance with local institutional guidelines. Following euthanasia, whole ureters from rat of either sex were dissected and cleaned of fat and connective tissue, cut into small segments (4–5 mm long) and placed in normal Krebs solution until further use.
2.2. Calcium and diameter measurements
Ureteric tissue samples were loaded with Fluo-4 acetoxymethyl ester (Invitrogen, UK, 15 μM, dissolved in DMSO with 0.1% pluronic F-127) for 3 h at 23 °C and then transferred to indicator-free solution for ≥30 min. Fluo-4 loaded segments of ureter were transferred to a custom-made perfusion chamber mounted on the stage of inverted Olympus microscope. Superfusion of the ureteric segments in the chamber was performed by applying a positive pressure valve controlled flow of solution via a 1 mm diameter tip attached to a 3-d mechanical manipulator (Narishige, Japan) which allowed to position the superfusion tip in a desired region of the chamber. Solution was removed by suction at the other end of the chamber. All experiments were performed at 30 °C. We used a Nipkow disc, confocal microscope [39,40] (Ultraview, Perkin Elmer), connected to an iXon cooled charge-coupled device camera (Andor Technology, UK). Andor Technology iQ or iQ2 data acquisition software was used for 2- and 3-dimensional confocal imaging of ureteric microvascular networks in situ. Images were collected at 33–66 frames per second using a ×60 water objective (NA 1.20) for spatial resolution or dry (×10, NA 0.42; ×20, 0.70 NA) for a larger field of view. To measure elemental events and Ca2+ waves in myocytes and pericytes, tangential sections were used, whereas radial sections through the centre of the microvessel were used to measure Ca2+ events in myocytes, pericytes, endothelial cells and changes in vessel diameter. Mechanical activity of individual smooth muscle cells was tracked by putting the region of interest close to the edge of the contracting cell. It was possible to correlate Ca2+ signalling with contraction of individual myocytes and pericytes in both radial and tangential sections.
2.3. Solutions
Physiological saline of the following composition was used (mM): NaCl 136, KCl 5.9, MgSO4 1.2, CaCl2 2, glucose 11.5, and HEPES 11. Solutions with increased [K+] were obtained by replacing Na+ by equimolar K+. The Ca2+-free solutions contained 2 mM EGTA. 2-Aminoethoxydiphenyl borate (2-APB), ryanodine, and U-73122, were from Calbiochem (Nottingham, UK), all other chemicals were from Sigma. Phenylephrine, endothelin-1 (ET-1), ryanodine, Na nitroprusside, were dissolved in water; 2-APB, BayK 8644, S-nitroso-N-acetyl-dl-penicillamine (SNAP) in DMSO; and nifedipine in ethanol.
2.4. Statistics
A paired Student's t-test was used to test for significant differences between means. All statistical values are expressed as mean ± SEM.
3. Results
3.1. Microvascular networks in situ
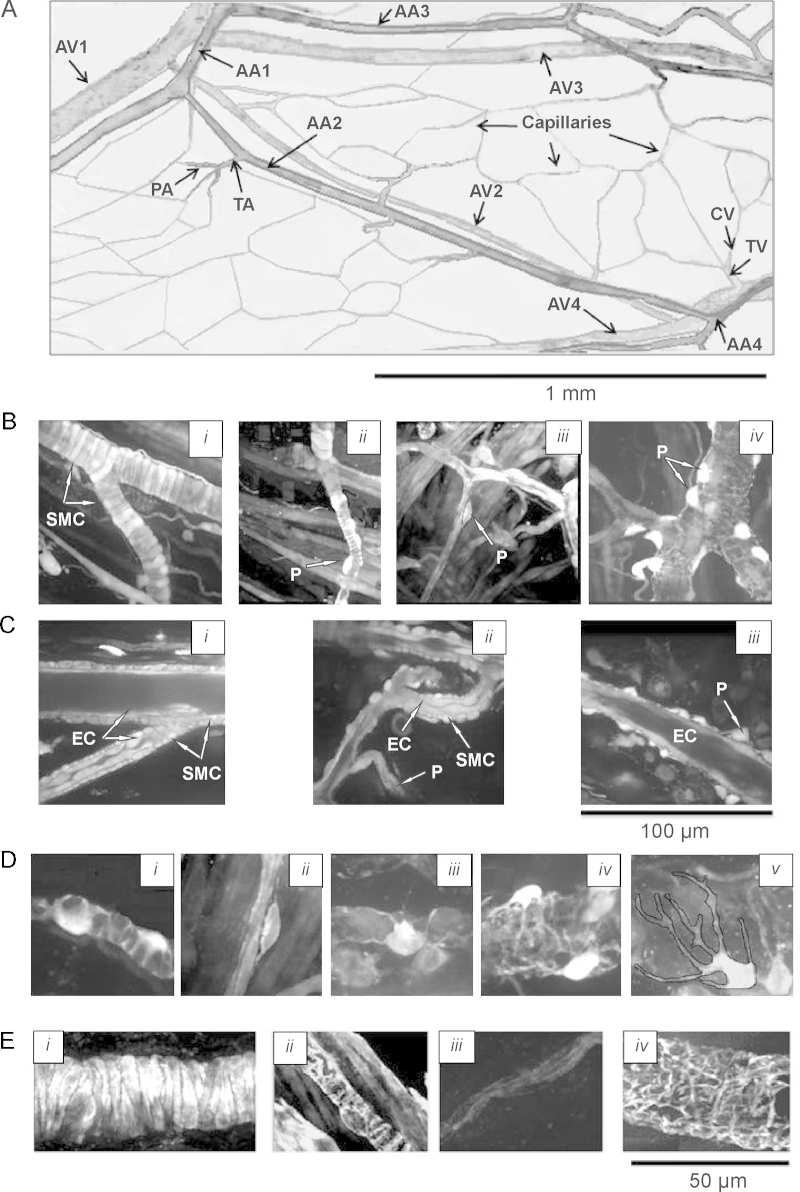
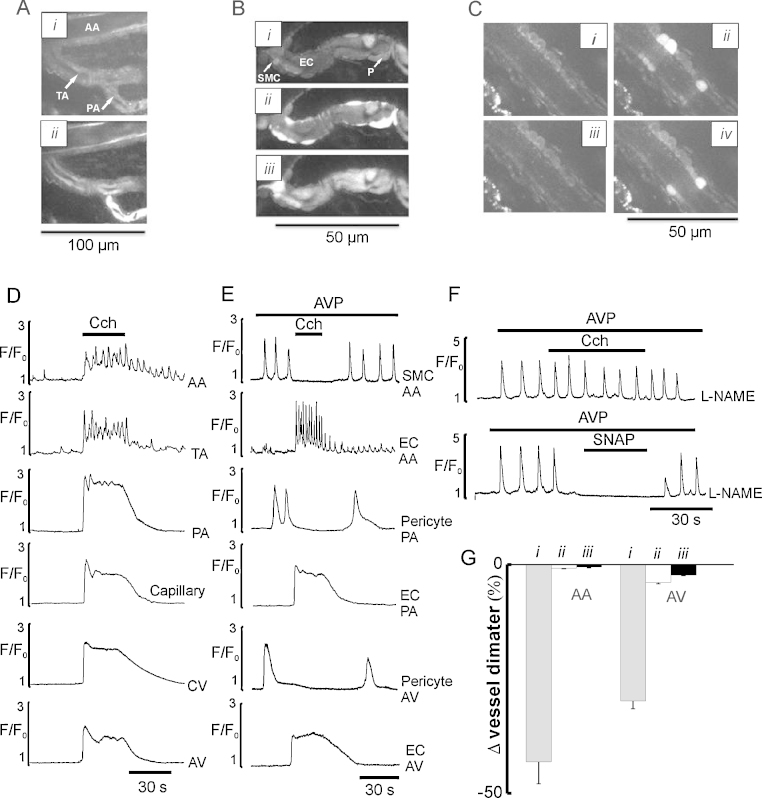
Fig. 1 shows that we can clearly identify the cell types present in the vessel walls at all levels of the microcirculation. This has enabled us to make the first comprehensive study of the in situ morphology of myocytes and pericytes, clearly identified in the different vessels of the microcirculation. We find that pericyte morphology and contractility vary specifically with their location, as discussed below.
Fig. 1.
Architecture and morphology of the ureteric microvascular network in situ. (A) The architecture of the ureteric microvascular networks in situ, obtained by taking multiple serial optical slices in x–y–z direction (Supplementary Movie 1). The network consists of the system of arcade arterioles (AA), paralleled by their counterpart arcade venules (AV) and penetrating transverse arterioles (TA), connected to capillaries via precapillary arterioles (PA). Arcade venules (AV) are connected to transverse venule (TV), which are connected to capillaries via collecting venules (CV). (B) Images of AA and TA (i), PA (ii), capillaries (iii), and postcapillary venules (iv). (C) Radial sections of AA and TA (i), PA and capillary (ii) and AV (iii), showing monolayer of endothelial cells (EC) surrounded by a monolayer of myocytes (SMC) and pericytes (P). (D) Images showing diversity of shapes of pericytes observed in PA (i), capillaries (ii), CV (iii), TV (iv) and AV (v). (E) Images of the AA (i), PA (ii), capillary (iii) and AV (iv) stained with fluorescently labelled phalloidin.
The arrangement of the microcirculatory network in the ureter, as with other tissues, [41,42] begins with feed arteries in the adventia (Fig. 1A and B; Supplementary Movie 1). These lead to interconnected arcade arterioles (AA, 18–35 μm) and penetrating transverse arterioles (TA, 12–15 μm) connected to capillaries (4–5 μm), via precapillary arterioles (PA, 8–10 μm). Collecting venules (CV, 8–10 μm) flow from the capillaries into the transverse venules (TV, 15–20 μm) and thus to the arcading venules (AV, 25–55 μm).
The walls of the largest vessels, i.e. arcade, terminal and proximal part of precapillary arterioles have a single layer of closely opposed, circularly orientated myocytes surrounding a monolayer of endothelial cells (Fig. 1Bi,ii and Ci,ii). For the rest of the microcirculatory network, from the distal part of the precapillary arterioles through to arcading venules, myocytes are absent and the vessel coat surrounding the endothelial lining is composed of pericytes morphologically different throughout the network (Fig. 1Bii–iv). In the distal precapillary arterioles, pericytes have thick cell bodies, giving an asymmetrical appearance to the wall and 2–3 pericytic finger-like processes tightly wrapping around the endothelium (Fig. 1Bii and Di; Supplementary Movie 2). Each precapillary pericyte occupies a length of 10.10 ± 0.48 μm of the vessel (n = 15). Capillaries are invested with pericytes which have more elongated cell bodies with two long slender processes and some short side branches (Fig. 1Biii and Dii; Supplementary Movie 3). Each capillary pericyte occupies a length of 30–40 μm of the vessel. The entire postcapillary venular network is surrounded by spidery pericytes, with stellate cell bodies giving slender cell projections (Fig. 1Biv and Diii-v). These projections appear to be randomly oriented with respect to the vessel axis, and overlap and/or attach to each other, forming a complicated and dense network in arcade and transverse venules (Fig. 1Biv and Div,v; Supplementary Movie 4). Our live morphological data are in good agreement with those obtained with transmitted and scanning electron microscopy [27–29,43].
As well as being morphologically distinct between vessels, the pericytes also differ in the expression of the contractile protein, α-actin. Fig. 1E shows confocal images of the arteriolar myocytes and pericytes of the precapillary arterioles, capillaries and postcapillary venules, stained in situ with fluorescently labelled phalloidin, to detect α-actin filaments in the cells. All media cells, except capillary pericytes (Fig. 1Eiii), stained for α-actin, in agreement with previous observations [44]. These observations prompt two questions: (i) what is the relationship between Ca2+ signals and contraction of pericytes and (ii) do the non-contractile capillary pericytes have agonist-stimulated Ca2+ signals?
3.2. Ca2+ signalling in myocytes
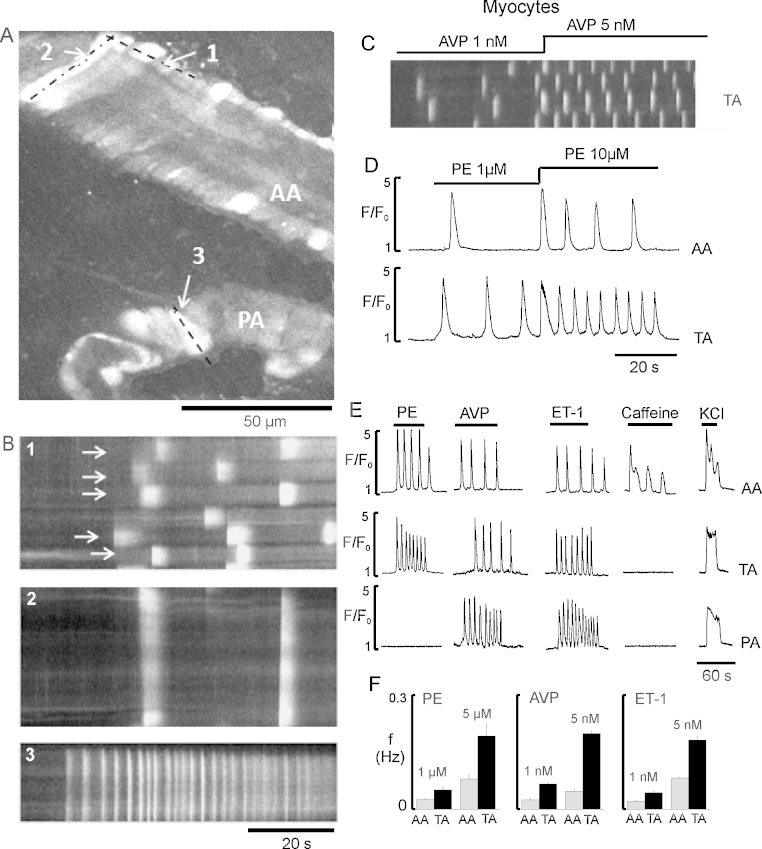
For these experiments (n = 9–30), three vasoconstrictors were selected; the central neurohormonal agonists phenylephrine (PE, 1–10 μM) and [Arg8]-Vasopressin (AVP, 1–5 nM), and the local agonist, endothelin-1 (ET-1, 1–5 nM). Fig. 2A shows rises of Ca2+ in both arcade (AA) and precapillary (PA) arterioles during application of AVP. We found that myocytes in the microcirculation have Ca2+ responses to vasoconstrictors in the form of repetitive, propagating Ca2+ waves (Fig. 2A–C). Line-scan plots, with respect to time, from several neighbouring myocytes showed that myocytes had varying delays to the onset of the Ca2+ rises, and consequently oscillations did not occur simultaneously in the vessels (Fig. 2B1, white arrows). The number of cells responding and the frequency of Ca2+ oscillations for each agonist were dependent on concentration (Fig. 2C and D). Importantly, as seen in Fig. 2E and F, the temporal characteristics of the Ca2+ oscillations in myocytes depended more on the position of the microvessel within the network rather than whether the agonist was PE, AVP or ET-1. Thus in response to agonists, Ca2+ oscillation duration at 50% peak amplitude, in arcade arterioles was ∼2.5 s but in transverse and precapillary arterioles was significantly shorter at ∼1 s, and the oscillations were almost 3-fold more frequent (Fig. 2E). Although both AVP and ET-1 increased [Ca2+]i in all myocytes, PE was only effective in arcade and transverse arterioles (n = 15; Fig. 2E, Table 1; Supplementary Movie 5). The finding that PE, a central agonist, is ineffective beyond transverse arterioles is consistent with in vivo studies of nerve stimulation of mesenteric vessels, showing constriction of the small arteries and transverse arterioles but not precapillary arterioles [7].
Fig. 2.
Heterogeneity of the Ca2+ responses, induced by different vasoconstrictors in ureteric arteriolar network in situ. (A) Image of the the AA and PA during stimulation with 5 nM AVP. (B) Line-scan plot with respect to time, from several smooth muscle cells (SMCs) (dashed line 1 indicated in A) or individual SMC of AA (dash–dotted line 2 in A) or PA (dashed line 3 in A), showing the asynchronous Ca2+ oscillations in SMCs (white lines). Arrows indicates the corresponding SMC. (C) Representative line scan plots with respect to time of the Ca2+ oscillations from several SMCs of TA, showing the effect of 1 and 5 nM AVP. (D) Graph showing Ca2+ oscillations in SMCs of TA induced by 1 and 10 μM PE. (E) Typical Ca2+ signals recorded in SMCs of AA, TA and PA, induced by 5 μM PE, 5 nM AVP, 5 nM ET-1, 2 mM caffeine and 60 mM KCl. (F) Mean data showing average frequencies of Ca2+ oscillations in myocytes of AA and TA induced by PE (1 and 5 μM), AVP (1 and 5 nM), and ET-1 (1 and 5 nM).
Table 1.
[Ca2+]i responses of myocytes and pericytes induced by different stimulants in ureteric microvascular network in situ.
| Type of cells and microvessel | PE | AVP | ET-1 | Caffeine | High-K+ |
|---|---|---|---|---|---|
| SMCs, AA | + | + | + | + | + |
| SMCs, TA | + | + | + | − | + |
| SMCs, PA | −/+ | + | + | − | + |
| Pericytes, PA | − | + | + | − | + |
| Pericytes, capillaries | − | + | + | − | + |
| Pericytes, postcapillary venules | − | + | + | − | + |
Thus we show that for myocytes in the microcirculation, the pattern of Ca2+ responses to vasoconstrictors is mainly determined by the vessel type not the agonist, which implies there will be differences in the mechanisms generating Ca2+ signals between vessels, as explored later.
3.3. Ca2+ signalling in pericytes
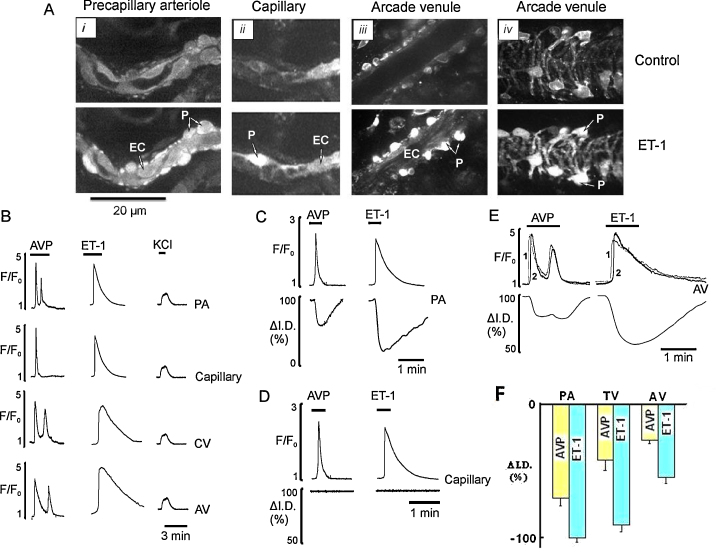
In contrast to the data from smooth muscle cells, the responses seen in pericytes did not depend on location (n = 11–28). Thus increases of Ca2+ to AVP and ET-1 were seen at all locations but no pericytes in any vessel responded to PE or caffeine (Fig. 3A and B; Table 1; Supplementary Movie 5). Pericytes responded in an agonist specific manner to AVP and ET-1 (Fig. 3B). At low concentrations (1 nM), AVP produced either no response or elicited a single Ca2+ oscillation in all sections of pericytic microvessels (Table 1). At higher concentrations of AVP (5 nM) the signal changed from one oscillation to several in most pericytes throughout the network (Fig. 3E), with frequencies of 0.02–0.03 Hz (n = 18). In contrast to AVP, in all pericytes, ET-1 (1–5 nM) caused a single Ca2+ transient which decayed very slowly (Fig. 3B–E). Examination of Figs. 2 and 3 shows that there are obvious differences between the characteristics of the Ca2+ signals in myocytes and pericytes. Next we wished to investigate if these patterns of Ca2+ oscillations, which varied between vessels, led to differing functional responses in the microcirculatory vessels.
Fig. 3.
Ca2+ signalling and pericyte mediated constriction of precapillary arterioles, capillaries and postcapillary venules. (A) Images of PA (i), capillary (ii), and AV in tangential (iii) and radial (iv) optical sections before (top) and during (bottom) exposure to 5 nM ET-1. (B) Ca2+ transients induced by 5 nM AVP, 5 nM ET-1 and 60 mM KCl in pericytes of all sections of “pericytic” microvessels: PA, capillaries, CV and AV. (C and D) Pericyte Ca2+ transients and changes in diameter at pericyte site of the PA and the capillary induced by AVP (5 nM) and ET-1 (5 nM). (E) Ca2+ transients measured from two different pericytes (1 and 2) and reduction in the diameter of the AV induced by AVP (5 nM) and ET-1 (5 nM). (F) Mean data showing vasoconstriction at pericyte site of PA, TV and AV caused by AVP (yellow bar) and ET-1 (cyan bar).
3.4. Pericyte Ca2+ signalling and venular constriction
Ca2+ transients induced by AVP in pericytes had no effect on the diameter of capillaries (n = 14; Fig. 3D) but constricted the distal part of precapillary arterioles and all sections of postcapillary venules (Fig. 3C–F). Similarly, Ca2+ transients induced by ET-1 in pericytes also had no effect on the diameter of capillaries but caused strong and long lasting constriction of precapillary and venular pericytes (n = 17; Fig. 3D). This resulted in full occlusion of the precapillary arterioles (Fig. 3Ai and C), strong constriction of transverse venules and more than 50% constriction of arcade venules (Fig. 3Aiii,iv, C, E, and F; Supplementary Movie 7).
3.5. Smooth muscle cell Ca2+ oscillations and arteriolar constriction
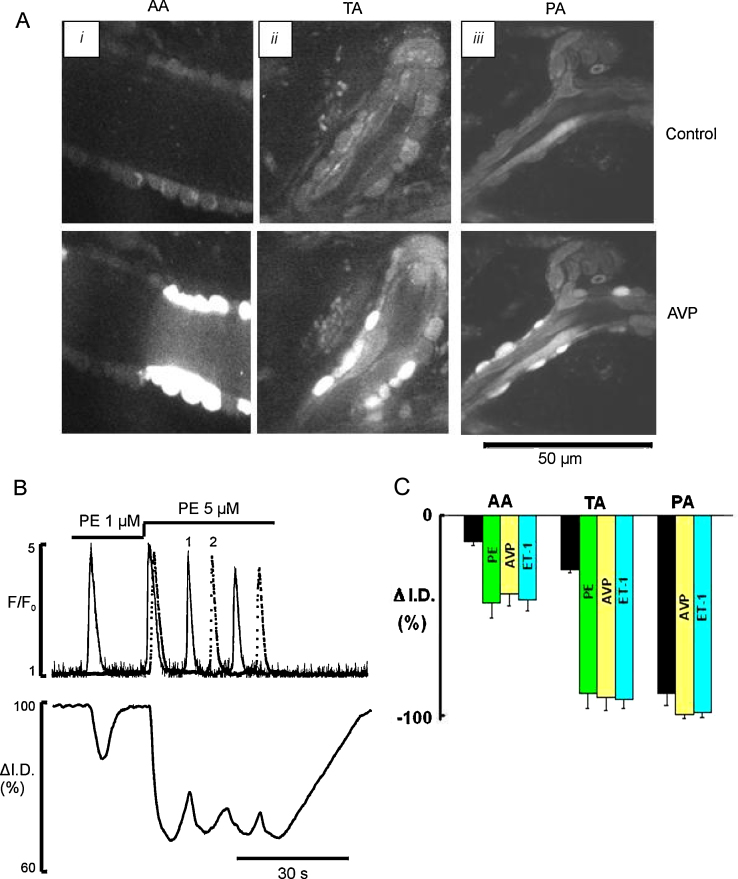
The aim of experiments such as that illustrated in Fig. 4 was to investigate whether the observed calcium signals were sufficient to close or constrict the vessels or produce vasomotion (Supplementary Movie 6). Fig. 4B shows that low frequency (<0.05 Hz) Ca2+ oscillations induced by a low concentration of PE (1 μM) resulted in only uncoordinated transient twitching of individual myocytes associated with local vasomotion. Similar results were seen with low concentrations of AVP and ET-1 throughout the arteriolar network. We found that the functional impact of single cell contraction depended upon the diameter of the microvessel and was maximal in the smallest vessels; thus in the precapillary arterioles contraction of one cell was sufficient to produce occlusion (Fig. 4C, black bar), but this was not the case in larger diameter vessels. Increasing the agonist concentration increased the frequency of oscillations (Fig. 4B and C) and thence constriction. The higher frequency of Ca2+ oscillations in the myocytes of transverse and precapillary arterioles induced a substantially greater arteriolar constriction (Fig. 4C; Supplementary Movie 6).
Fig. 4.
Ca2+ signalling and myocyte mediated constriction induced by PE, AVP and ET-1 in different sections of arteriolar network in situ. (A) Images of in situ ureteric AA (i), TA (ii), and PA (iii) in radial sections taken before (top panel) and during (bottom panel) exposure to 5 nM AVP. (B) Ca2+ oscillations and diameter changes of AA induced by 1 and 5 μM PE. Cell 1 (solid line) is responding throughout and cell 2 (dotted line) only when the concentration of PE was increased to 5 μM. (C) Mean data showing vasoconstriction produced by a single cell contraction (black bar) and high frequency Ca2+ oscillations caused by maximal concentration of PE (green bar), AVP (yellow bar), and ET-1 (cyan bar) in all sections of arteriolar network. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
These fundamental novel findings show that the tight coupling between the frequency of Ca2+ oscillation and phasic contraction of the myocytes in the vessel wall regulates arteriolar diameter in situ. Summation of the individual phasic contractions of each active cell leads to vessel constriction.
3.6. Ca2+ sources in myocytes and pericytes
To better understand the mechanisms underlying the Ca2+ changes induced by vasoconstrictor agonists in the microvascular network, we investigated the effects of (i) caffeine (2–5 mM) used to define the distribution of functional RyR, (ii) 60 mM KCl to define the distribution of voltage gated Ca2+ channels, (iii) the reliance on external Ca2+ entry and (iv) role of IP3R and RyR channels in control of Ca2+ release from internal stores (n = 9–15).
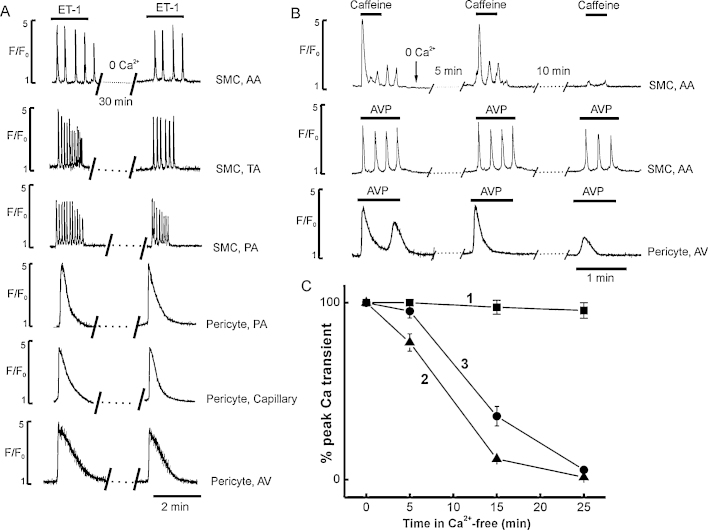
Myocytes in arcade arterioles responded to 2 mM caffeine by producing irregular Ca2+ oscillations (Fig. 2E) or a single spike followed by plateau when used at higher concentrations (5 mM). In contrast, caffeine had no effect on myocytes or pericytes in any other vessel, irrespective of the concentration used (Table 1, Fig. 2E, Supplementary Movie 5). All myocytes and pericytes throughout the network responded to high K+ depolarization with a rise in intracellular [Ca2+]i (Fig. 2E and 3B). In myocytes the high-K+ induced Ca2+ transient was about 90–100% of the peak amplitude of the Ca2+ transient induced by AVP, while in pericytes it was only ∼20–30% of the AVP response (Fig. 3B). In the absence of extracellular Ca2+, caffeine induced SR Ca2+ release was quickly abolished (Fig. 5B and C).
Fig. 5.
Ca2+ signalling induced by agonists in myocytes and pericytes in Ca2+-free media. (A) Ca2+ signalling induced by 5 nM ET-1 in myocytes and pericytes of all sections of microvascular network in normal and Ca2+ -free solution. (B and C) Original records and graphs showing Ca2+ transients induced by several consecutive applications of 2 mM caffeine (2, solid triangles) and 5 nM AVP in myocytes of AA (1, solid squares), and in pericytes of AV (3, solid circles), respectively.
The initial component of AVP or ET-1 induced Ca2+ signal in myocytes and pericytes was little affected even by prolonged exposure to Ca2+-free solution (30–60 min), throughout the arteriolar and venular network (Fig. 5A). Myocytes, but not pericytes, retained their ability to respond with repetitive Ca2+ oscillations to several consecutive applications (3–4) of 5 nM AVP in Ca2+-free media (Fig. 5B and C). Although removal of external Ca2+ had no effect on the amplitude of the initial spike component, the small sustained component was abolished (Fig. 5B, bottom trace). Also, a single application of AVP in Ca2+ free solution was sufficient to deplete the Ca2+ store in pericytes but not myocytes (Fig. 5B and C). In addition, the Ca2+ channel blocker nifedipine (10 μM) blocked high-K+ induced Ca2+ transient in myocytes and pericytes but Ca2+ transients induced by AVP or ET-1 were insensitive to nifedipine (n = 11, data not shown).
The RyR blocker ryanodine (30 μM) abolished the caffeine-induced Ca2+ transient in myocytes of arcade arterioles but had no effect on Ca2+ signalling induced by agonists in myocytes and pericytes of all other sections of the arteriolar and venular networks including arcade arterioles (n = 9, data not shown). The non–specific IP3R channel blocker 2-APB (50 μM) and the SERCA pump inhibitor CPA (20 μM) blocked Ca2+ transients induced by agonists in myocytes and pericytes in Ca2+-containing and Ca2+-free solution (n = 9, data not shown).
3.7. Effects of endothelial Ca2+ signalling
We next examined the role of the endothelium throughout the microvascular network, in the control of Ca2+ signalling and vasomotor responses induced by agonists in myocytes and pericytes. In these experiments AVP (5 nM) was used to stimulate the microvessels as it produced stable and reversible responses in both cell types. Carbachol (CCh, 1 μM) elevated intracellular Ca2+ in endothelial cells of all sections of the microvascular network (Fig. 6A and D). There were however vessel-dependent differences in the temporal and spatial characteristics of the CCh-induced Ca2+ transients in the endothelial cells (Fig. 6A and D). In arcade and transverse arterioles endothelial cells responded with high frequency Ca2+ oscillations which were asynchronous, not only among different endothelial cells but also within individual cells as we have described earlier [40]. In precapillary arterioles the Ca2+ transient was higher in amplitude and had a distinct plateau component superimposed on slow Ca2+ oscillations (Fig. 6A and D). In capillaries and all postcapillary venules, the endothelial Ca2+ transients consisted of a spike followed by a plateau component. Endothelial cells of arcade and transverse arterioles also showed spontaneous Ca2+ signals which were variable in amplitude and spatial spread [40]. Activation of endothelial Ca2+ signalling by CCh reversibly inhibited Ca2+ signals in both myocytes and pericytes and caused vasodilation in all sections of the arteriolar and venular network (n = 9; Fig. 6B, E, and G; Supplementary Movie 8). The inhibitory effects of endothelium Ca2+ signalling on IP3 mediated Ca2+ oscillations in myocytes and pericytes, could be caused by activation of Ca2+-dependent constitutive enzymes COX 1 and/or NOS-3, releasing PGI2 and NO respectively [18]. To investigate the role of PGI2 and NO, we studied the effects of the COX 1 inhibitor indomethacin (30 μM) and the eNOS inhibitor L-NAME (50 μM). L-NAME but not indomethacin blocked the inhibition of AVP-induced Ca2+ oscillations in myocytes and pericytes (n = 9; Fig. 6F). In addition the NO donor SNAP (50 μM) reversibly blocked AVP-induced Ca2+ oscillations in myocytes and pericytes, in the absence and the presence of L-NAME (n = 9; Fig. 6C and F), associated with the vasodilation of arterioles and venules (Fig. 6G) consistent with NO being the main endothelium derived relaxing factor inhibiting Ca2+ oscillations in the myocytes and pericytes.
Fig. 6.
Effects of endothelial Ca2+ signalling on Ca2+ signals in myocytes and pericytes and vasomotor activity in arteriolar and venular networks. (A) Images of the endothelial cells of the AA, TA and PA in radial section in the absence (i) and in the presence of 1 μM CCh (ii). (B) Images of PA in radial section showing endothelial cells, myocytes and pericytes at rest (i), in the presence of 5 nM AVP before (ii) and after adding 1 μM CCh (iii). (C) Images of TA in radial section of endothelial cells and myocytes at rest (i), in the presence of 5 nM AVP before (ii and iv) and after adding the NO donor SNAP (50 μM) (iii). (D) Ca2+ transients induced by 1 μM CCh in endothelial cells of different sections of arteriolar and venular networks. (E) Effects of endothelium Ca2+ signalling on AVP induced Ca2+ oscillations in myocytes and pericytes of arteriolar and venular networks. (F) Effects of CCh and SNAP on AVP-induced Ca2+ oscillations in SMC of TA in the presence of L-NAME. (G) Mean data showing vasodilation of AA and AV preconstricted with 5 nM AVP (i) induced by 1 μM CCh (ii) or 20 μM SNAP (iii), P < 0.05.
3.8. Arteriolar–venular communication
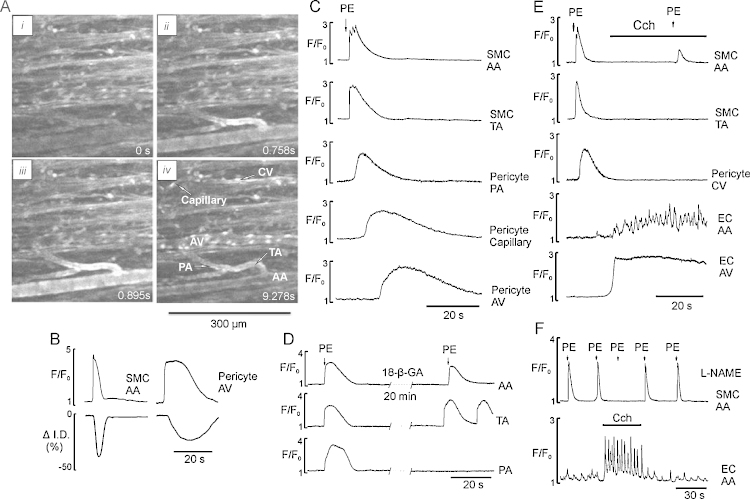
If myocytes and pericytes are electrically coupled [26] then propagating membrane depolarization should allow Ca2+ signals to propagate within and between the networks. We have examined this by pre-treating microvascular networks with the K+ channel blocker TEA and the L-type Ca2+ channel agonist BayK 8644. Under these conditions 30% of networks developed spontaneous action potentials. In the remainder, action potentials were evoked with either brief (2–3 s) regional application of PE or a high-K+ solution resulting in fast, propagating action potentials [5], which can be followed with Ca2+ sensitive indicators [40] (Fig. 7; Supplementary Movies 9 and 10). In myocytes from all sections of the arteriolar network the propagating Ca2+ transient consisted of 1–3 spikes superimposed on a plateau component (Fig. 7C). All sections of the arteriolar networks were synchronously constricted within 500 ms and the duration of the phasic constriction of arteriolar network was 6.5 ± 0.4 s (Fig. 7B). The Ca2+ transient propagated from smooth muscle cells to pericytes of precapillary arterioles from where it propagated to pericytes of capillaries followed by invasion into the arcade venules via collecting and transverse venules. This propagation at the capillary and venular networks was significantly slower (0.2–2 mm/s) than in the arteriolar (1–2 cm/s) network (Supplementary Movies 9 and 10).
Fig. 7.
Propagating Ca2+ signalling in microvascular networks pretreated with TEA and BayK 8644. (A (i–iv)) images of a fragment of arteriolar network stimulated by brief (3 s) application of PE (5 μM) showing initiation of propagating Ca2+ transient in the SMCs of TA (ii) and propagating in both directions to AA, capillaries and venules (iii and iv). (B) Ca2+ spike and changes in diameter of the AA and AV. (C) Time course of the PE-induced propagating Ca2+ transient recorded in SMCs of AA, TA and pericytes of capillaries and postcapillary venules. (D) PE-induced propagating Ca2+ transient in SMCs of AA, TA and PA in the absence and the presence of gap junction blocker 18-β-GA (50 μM), respectively. (E and F) Propagating Ca2+ transient in SMCs of different sections of arteriolar network and pericytes of CV initiated in SMCs of AA by brief (3 s) application of PE before and during stimulation of endothelium Ca2+ signalling in the absence and the presence of L-NAME, respectively.
The propagating Ca2+ transient also caused transient phasic constriction of all sections of postcapillary venules but not capillaries (Fig. 7A and B). Inhibition of gap junctions by 18-β-glycyrrhetinic acid (18-β-GA, 50 μM) within 15–20 min of exposure resulted in termination of PE-induced axially propagating Ca2+ signals from arterioles to venules. However, spontaneous Ca2+ signals could still be observed in some of the myocytes of all sections of the arteriolar but not venular networks (Fig. 7D). Nifedipine fully blocked synchronized propagating Ca2+ spikes (n = 9, data not shown). Activation of endothelial cell Ca2+ signalling by CCh resulted in fast and reversible inhibition of Ca2+ spikes in the absence and the presence of L-NAME (Fig. 7D and E; Supplementary Movie 10) suggesting involvement of EDHF but not NO, as reported earlier [40].
4. Discussion
In this study, we characterize agonist-induced Ca2+ signalling in myocytes and pericytes in different generations of an intact microvascular network in situ and explore the possibility that differences in the Ca2+ signalling within myocytes and pericytes shape the variable vasomotor responses. Live confocal imaging of intact ureteric microvessels in situ [39,40] was used as the morphologic and structural characteristics of the ureteric microvascular networks in situ were retained, making it possible to identify and follow the entire course of the network. Although our preparation and techniques are extremely powerful, we acknowledge that these observations were made in the absence of pressure and flow, although as shown in Supplementary Movie 6 erythrocytes can be seen moving through the network, and pressures in these vessels will be very low in vivo. We were able to obtain data from distributing arcade arterioles, penetrating transverse and precapillary arterioles, capillaries and all sections of postcapillary venules, which comprise microvascular networks in tissues [27–29,43]. The use of in situ confocal Ca2+ imaging brings the advantages of studying Ca2+ signalling and vasomotor responses in different parts of the microcirculation under identical experimental conditions in vitro, and thus without uncontrolled central factors, as can occur in in vivo preparations. We measured local and global changes of intracellular Ca2+ in smooth muscle cells, pericytes and endothelial cells, and correlated these with changes of vessel diameter. This is, to our knowledge, the first time that such a comparative study of Ca2+ signalling in myocytes and pericytes correlated to vasomotor responses in microvascular networks in situ has been documented.
Local paracrine and central vasoconstrictors are shown to produce different (cell- and vessel- specific) patterns of Ca2+ oscillations leading to contraction of pericytes or myocytes. In myocytes of all sections of the arteriolar network Ca2+ signals appeared as Ca2+ wave-like oscillations. These resulted from repetitive cycles of Ca2+ release and reuptake by intracellular stores, since they could be initiated and maintained for long periods in the absence of extracellular Ca2+ or in the presence of Ca2+ channel blockers as was also noted for the myocytes of intrapulmonary arterioles [45]. The Ca2+ oscillations in myocytes of the arteriolar network were asynchronous between neighbouring cells. Similar results have also been reported for intrapulmonary arterioles of lung slices [45]. The frequency of the Ca2+ oscillations and the extent of contraction induced by agonists in all sections of arterioles were concentration dependent. However, the frequency observed in transverse and precapillary arterioles with maximal concentrations of agonists was always about 2–3 times higher than those observed in arcade arterioles. The similarity of the Ca2+ oscillation frequencies within the same type of arteriole in response to different agonists suggests that the difference lies with the coupling of receptor activation to the generation of Ca2+ oscillations. The finding that RyRs are redundant throughout almost the entire microvascular network is in agreement with suggestions from studies on rat retinal arteriolar fragments [37] and hamster cremaster muscle arterioles [46]. This finding along with the lack of dependence on Ca2+ entry suggest an homogenous basic mechanism for controlling Ca2+ signalling is present in the microvasculature and this will be important to how it functions.
4.1. Ca2+ signalling and contraction in pericytes
The strength of our technique is that we can simultaneously, directly compare Ca2+ signalling between cell types under identical experimental conditions. This enabled us to not only elucidate the nature of Ca2+-signals in pericytes, which has been little studied in intact preparations, but also to compare these signals with those from neighbouring myocytes. We find that Ca2+ signalling in pericytes is dramatically different from that observed in myocytes induced by the same agonists under the same experimental conditions. In contrast to myocytes, the pericytes Ca2+ responses depend on the type of the agonist used and are similar in all sections of venular network. A number of vasoactive substances can constrict pericytes [22,24]. ET-1 induced long lasting Ca2+ transients in pericytes of precapillary arterioles, capillaries and postcapillary venules while AVP induced low frequency Ca2+ oscillations in the same pericytes when applied before or after application of ET-1 (Fig. 3E). Despite these differences in temporal characteristics, Ca2+ transients induced by ET-1 and AVP were resistant to removal of external Ca2+ or addition of Ca2+ channels blockers. As with myocytes [40] the Ca2+ signals resulted from PLC/IP3 mediated Ca2+ release from the intracellular Ca2+ stores. Ca2+ transients induced by ET-1 and AVP were associated with strong local contractions of pericytes resulting in vessel constriction. We never observed contraction of capillary pericytes. The lack of contractile response of capillary pericytes has been reported for some but not all other tissues [22]. It should be noted that the contractile responses induced by agonists in pericytes were always much longer than in myocytes, and this was especially true of the effects of ET-1, which causes contraction of pericytes lasting up to 7 min.
This novel understanding of pericyte Ca2+ signalling allows us to conclude that strong and long lasting contraction is an intrinsic property of pericytes which can now be explained by long lasting underlying Ca2+ transients, which are also slow to relax. These properties make pericytes ideal to mediate long lasting tonic vasoconstrictions. Our data suggest that pericytes regulate microvascular diameter in discrete areas of the microvasculature, and that this is likely to control regional blood flow. Pericyte mediated constriction of precapillary arterioles could be expected to reduce vascular conductance and the number of opened capillaries. This may be viewed as them performing the role of a precapillary sphincter. Pericyte constriction of postcapillary venules would be expected to increase post-capillary resistance and reduce regional vascular volume and venular leak [47]. This would be important in preventing oedema during inflammation.
4.2. Propagation of Ca2+ responses
A major conclusion from this work is that myocytes and pericytes are electrically coupled. They efficiently transmit axially, regenerative Ca2+ signals within and between arteriolar and venular networks. This passage is dependent on gap junctions and Ca2+ entry via L-type Ca2+ channels. Coordination of vasomotor activity within and among microvascular networks is integral to the local control of tissue blood flow. Vasoconstriction initiated at a local site can conduct along arterioles for several millimetres and manifests as the transmission of electrical signals from cell to cell through gap junction channels. Direct measurements of membrane potential have shown that conducted vasomotor responses in arterioles are associated with rapid propagation of an electrical signal along the vessel length which is thought to be conducted by the smooth muscle cells [8,40]. We show that in the presence of K+ channel blocker TEA and L-type Ca2+ channel agonist BayK 8644 myocytes and pericytes are able to generate axially propagating regenerative Ca2+ transients in ureteric microvessels. These data are in good agreement with recent elctrophysiological data obtained on capillary/arteriole plexuses freshly isolated from the rat retina [26]. We found that the axial transmission between myocytes in the arteriolar network was fast (1–2 cm/s) while between pericytes it was 10–20 times slower. The signal can travel in all direction and is effectively blocked by gap junction blockers and L-type Ca2+ channel blockers or removal of external Ca2+ Recently, it was suggested that capillary pericytes can generate and propagate hyperpolarizing signals and thus be involved in the mechanisms of capillary–arteriolar communication using homo-and heterocellular gap junctions [25,38]. Our data would support this suggestion.
5. Endothelial function
Consistent access to the endothelial cells of all section of the ureteric microvascular network allowed us to investigate, for the first time, the effects of endothelial Ca2+ signalling on Ca2+ signalling and vasomotor responses in myocytes and pericytes in situ. Our data revealed that endothelial Ca2+ signalling inhibits intracellular Ca2+ oscillations in myocytes and pericytes via L-arginine/nitric oxide pathway and intercellular propagating Ca2+ signals via EDHF [40]. The fact that endothelial Ca2+ signalling or NO donors inhibit Ca2+ oscillations in myocytes and pericytes in the presence or absence of external Ca2+ suggest that NO inhibits the activity of the IP3R channels. The molecular mechanism by which NO inhibited the IP3R is under current investigation. Recently the PKG substrate, IRAG, has been identified in myocytes and suggested to block IP3R activation [48,49]. Such a mechanism may contribute to our findings but needs further investigation. Our data are in agreement with other studies, which demonstrated distinct role of NO and EDHF in control of regional and conducted vasodilation [9,11,38].
In summary our data have provided much novel insight into the microvasculature, a critical part of the circulation and the part of which we know least. We have obtained direct data revealing not just the nature of the Ca2+ signals in myocytes and pericytes through an intact network, but also shown how these signals relate to functional responses in the vessels, from closure to subtler changes associated with vasomotion. Future work to perfuse the network is called for, but an enormous amount of useful data can be obtained in its absence, to provide the framework for such additional studies.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Acknowledgment
This work was supported by British Heart Foundation PG/12/62/29823.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ceca.2013.06.001.
Appendix A. Supplementary data
Live imaging of ureteric microvascular network in situ. AA, TA and PA are arcade, transverse and precapillary arterioles, respectively; AV, TV, CV are arcade, transverse and collecting venules, respectively; UMB-ureteric muscle bundles.
Morphology of ureteric precapillary arterioles in situ. 3-Dimensionlal live images of smooth muscle, pericytes and endothelial cells of all sections of arteriolar network.
Morphology of ureteric capillaries in situ. 3-Dimensionlal live images of pericytes and endothelial cells of capillaries.
Morphology of ureteric postcapillary venules in situ. 3-Dimensionlal live images of pericytes and endothelial cells of postcapillary venular network.
Ca2+ responses of arteriolar and venular networks in situ to different stimuli. Distinct Ca2+ responses of SMCs and pericytes to phenylephrine (10 μM), caffeine (5 mM) and ET-1 (5 nM).
Relationship between AVP-induced Ca2+ oscillations and vasomotor responses in arcade and transverse arteriole.
Ca2+ signals and contractility of precapillary arteriolar and postcapillary venular pericytes in situ (see text for details).
Effects of endothelium Ca2+ signalling on AVP-induced Ca2+ oscillations and vasomotor responses in arcade arteriole.
Propagating Ca2+ signalling in arteriolar and venular networks induced by brief (2–3 s) application of high-K+ pre-treated with K+ channel blocker TEA (5 mM) and L-type Ca2+ channel agonist BayK 8644 (1 μM). The intercellular Ca2+ transient in both networks appear as a regenerative Ca2+ transient travelling at a speed of 1–2 cm/s in arteriolar and 1–2 mm/s venular networks, respectively.
Inhibitory effects of endothelium Ca2+ signalling on propagating Ca2+ signalling in arteriolar and venular networks induced by brief (2–5 s) application of PE pretreated with TEA (5 mM) and BayK 8644.
References
- 1.Rosell S. Neuronal control of microvessels. Annual Review of Physiology. 1980;42:359–371. doi: 10.1146/annurev.ph.42.030180.002043. [DOI] [PubMed] [Google Scholar]
- 2.De Backer D., Ospina-Tascon G., Salgado D., Favory R., Creteur J., Vincent J.L. Monitoring the microcirculation in the critically ill patient: current methods and future approaches. Intensive Care Medicine. 2010;36:1813–1825. doi: 10.1007/s00134-010-2005-3. [DOI] [PubMed] [Google Scholar]
- 3.Colantuoni A., Bertuglia S., Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. American Journal of Physiology. 1984;246:H508–H517. doi: 10.1152/ajpheart.1984.246.4.H508. [DOI] [PubMed] [Google Scholar]
- 4.Griffith T.M. Temporal chaos in the microcirculation. Cardiovascular Research. 1996;31:342–358. [PubMed] [Google Scholar]
- 5.Bény J.L. Information networks in the arterial wall. News in Physiological Sciences. 1999;14:68–73. doi: 10.1152/physiologyonline.1999.14.2.68. [DOI] [PubMed] [Google Scholar]
- 6.Haddock R.E., Hill C.E. Rhythmicity in arterial smooth muscle. Journal of Physiology. 2005;566:645–656. doi: 10.1113/jphysiol.2005.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furness J.B., Marshall J.M. Correlation of the directly observed responses of mesenteric vessels of the rat to nerve stimulation and noradrenaline with the distribution of the adrenergic nerves. Journal of Physiology. 1974;239:75–88. doi: 10.1113/jphysiol.1974.sp010556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia J., Duling B.R. Electromechanical coupling and the conducted vasomotor response. American Journal of Physiology. 1995;38:H2022–H2030. doi: 10.1152/ajpheart.1995.269.6.H2022. [DOI] [PubMed] [Google Scholar]
- 9.Frame M.D., Sarelius I.H. Endothelial cell dilatory pathways link flow and wall shear stress in an intact arteriolar network. Journal of Applied Physiology. 1996;81:2105–2114. doi: 10.1152/jappl.1996.81.5.2105. [DOI] [PubMed] [Google Scholar]
- 10.Kelley C., D’Amore P., Hechtman H.B., Shepro D. Microvascular pericyte contractility in vitro: comparison with other cells of the vascular wall. Journal of Cell Biology. 1987;104:483–490. doi: 10.1083/jcb.104.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persson M.G., Gustafsson L.E., Wiklund N.P., Hedqvist P., Moncada S. Endogenous nitric oxide as a modulator of rabbit skeletal muscle microcirculation in vivo. British Journal of Pharmacology. 1990;100:463–466. doi: 10.1111/j.1476-5381.1990.tb15829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodge A.B., Hechtman H.B., Shepro D. Microvascular endothelial-derived autacoids regulate pericyte contractility. Cell Motility and the Cytoskeleton. 1991;18:180–188. doi: 10.1002/cm.970180304. [DOI] [PubMed] [Google Scholar]
- 13.Haefliger I.O., Zschauer A., Anderson D.R. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Investigative Ophthalmology and Visual Science. 1994;35:991–997. [PubMed] [Google Scholar]
- 14.Dehouck M.P., Vigne P., Torpier G., Breittmayer J.P., Cecchelli R., Frelin C. Endothelin-1 as a mediator of endothelial cell-pericyte interactions in bovine brain capillaries. Journal of Cerebral Blood Flow and Metabolism. 1997;17:464–469. doi: 10.1097/00004647-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Bagher P., Segal S.S. Regulation of blood flow in the microcirculation; role of conducted vasodilation. Acta Physiologica. 2011;202:271–284. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández-Klett F., Offenhauser N., Dirnagl U., Priller J., Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proceedings of the National Academy of Sciences. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakagami K., Kawamura H., Wu D.M., Puro D.G. Nitric oxide/cGMP-induced inhibition of calcium and chloride currents in retinal pericytes. Microvascular Research. 2001;62:196–203. doi: 10.1006/mvre.2001.2343. [DOI] [PubMed] [Google Scholar]
- 18.Gryglewski R.J., Chłopicki S., Uracz W., Marcinkiewicz E. Significance of endothelial prostacyclin and nitric oxide in peripheral and pulmonary circulation. Medical Science Monitor. 2001;7:1–16. [PubMed] [Google Scholar]
- 19.Kawamura H., Oku H., Li Q., Sakagami K., Puro D.G. Endothelin-induced changes in the physiology of retinal pericytes. Investigative Ophthalmology and Visual Science. 2002;43:882–888. [PubMed] [Google Scholar]
- 20.Peppiatt C.M., Howarth C., Mobbs P., Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu D.M., Miniami M., Kawamura H., Puro D.G. Electrotonic transmission within pericyte-containing retinal microvessels. Microcirculation. 2006;13:353–363. doi: 10.1080/10739680600745778. [DOI] [PubMed] [Google Scholar]
- 22.Puro D.G., Physiology Pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation. 2007;14:1–10. doi: 10.1080/10739680601072099. [DOI] [PubMed] [Google Scholar]
- 23.Kamouchi M., Kitazono T., Ago T. Hydrogen peroxide-induced Ca2+ responses in CNS pericytes. Neuroscience Letters. 2007;416:12–16. doi: 10.1016/j.neulet.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 24.Yemisci M., Gursoy-Ozdemir Y., Vural A., Can A., Topalkara K., Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nature Medicine. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 25.Mogensen C., Bergner B., Wallner S. Isolation and functional characterization of pericytes derived from hamster skeletal muscle. Acta Physiologica. 2011;201:413–426. doi: 10.1111/j.1748-1716.2010.02206.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T., Wu D.M., Xu G.Z., Puro D.G. The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. Journal of Physiology. 2011;589:2383–2399. doi: 10.1113/jphysiol.2010.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jima T., Zhang J.Q., Goto T., Kondo T., Tanaka T. A scanning electron microscopic study of the contraction of vascular wall cells in dog dental. Journal of Dental Research. 1991;70:1456–1461. doi: 10.1177/00220345910700111301. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara T., Tenkova T.I., Kondo M. Wall cytoarchitecture of the rat ciliary process microvasculature revealed with scanning electron microscopy. Anatomical Record. 1999;254:261–268. doi: 10.1002/(SICI)1097-0185(19990201)254:2<261::AID-AR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Higuchi K., Hashizume H., Aizawa Y., Ushiki T. Scanning electron microscopic studies of the vascular smooth muscle cells and pericytes in the rat heart. Archives of Histology and Cytology. 2000;63:115–126. doi: 10.1679/aohc.63.115. [DOI] [PubMed] [Google Scholar]
- 30.Hashitani H., Takano H., Fujita K., Mitsui R., Suzuki H. Functional properties of suburothelial microvessels in the rat bladder. Journal of Urology. 2011;185:2382–2391. doi: 10.1016/j.juro.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Shepro D., Morel N.M. Pericyte physiology. FASEB Journal. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 32.Hirschi K.K., D’Amore P.A. Pericytes in the microvasculature. Cardiovascular Research. 1996;32:687–698. [PubMed] [Google Scholar]
- 33.Allt G., Lawrenson J.G. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 34.Armulik A., Genove G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Peppiatt-Wildman C.M., Crawford C., Hall A.M. Fluorescence imaging of intracellular calcium signals in intact kidney tissue. Nephron Experimental Nephrology. 2012;121:49–58. doi: 10.1159/000342812. [DOI] [PubMed] [Google Scholar]
- 36.Vates G.E., Takano T., Zlokovic B., Nedergaard M. Pericyte constriction after stroke: the injury is still out. Nature Medicine. 2010;16:959. doi: 10.1038/nm0910-959. [DOI] [PubMed] [Google Scholar]
- 37.Scholfield C.N., Curtis T.M. Heterogeneity in cytosolic calcium regulation among different microvascular smooth muscle cells of the rat retina. Microvascular Research. 2000;59:233–242. doi: 10.1006/mvre.1999.2227. [DOI] [PubMed] [Google Scholar]
- 38.Budel S., Bartlett I.S., Segal S.S. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circulation Research. 2003;93:61–68. doi: 10.1161/01.RES.0000080318.81205.FD. [DOI] [PubMed] [Google Scholar]
- 39.Burdyga T., Shmygol A., Eisner D.A., Wray S. A new technique for simultaneous and in situ measurements of Ca2+ signals in arteriolar smooth muscle and endothelial cells. Cell Calcium. 2003;34:27–33. doi: 10.1016/s0143-4160(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 40.Borisova L., Wray S., Eisner D.A., Burdyga T. How structure, Ca signals, and cellular communications underlie function in precapillary arterioles. Circulation Research. 2009;105:803–810. doi: 10.1161/CIRCRESAHA.109.202960. [DOI] [PubMed] [Google Scholar]
- 41.Koller A., Dawant B., Liu A., Popel A.S., Johnson P.C. Quantitative analysis of arteriolar network architecture in cat sartorius muscle. American Journal of Physiology. 1987;22:H154–H164. doi: 10.1152/ajpheart.1987.253.1.H154. [DOI] [PubMed] [Google Scholar]
- 42.Ellsworth M.L., Liu A., Dawant B., Popel A.S., Pittman R.N. Analysis of vascular pattern and dimensions in arteriolar networks of the retractor muscle in young hamsters. Microvascular Research. 1987;34:168–183. doi: 10.1016/0026-2862(87)90051-3. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman K.W. Der feinere Bau de Bleitkapillareu. Z Anat Entwicklungench. 1923;68:29–109. [Google Scholar]
- 44.Nehls V., Drenckhahn D. Heterogeneity of microvascular pericytes for smooth musde type alpha-actin. Journal of Cell Biology. 1991;113:147–154. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez J.F., Sanderson M.J. The contraction of smooth muscle cells of intrapulmonary arterioles is determined by the frequency of Ca2+ oscillations induced by 5-HT and KCl. Journal of General Physiology. 2005;125:555–567. doi: 10.1085/jgp.200409217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westcott E.B., Jackson W.F. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. American Journal of Physiology – Heart and Circulatory Physiology. 2011;300:H1616–H1630. doi: 10.1152/ajpheart.00728.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marshall J.M., Lloyd J., Mian R. The influence of vasopressin on the arterioles and venules of skeletal muscle of the rat during systemic hypoxia. Journal of Physiology. 1993;470:473–484. doi: 10.1113/jphysiol.1993.sp019870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carvajal J.A., Germain A.M., Huidobro-Toro J.P., Weiner C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. Journal of Cellular Physiology. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 49.Ammendola A., Geiselhöringer A., Hofmann F., Schlossmann J. Molecular determinants of the interaction between the inositol 1,4,5-trisphosphate receptor-associated cGMP kinase substrate (IRAG) and cGMP kinase Ibeta. Journal of Biological Chemistry. 2001;276:24153–24159. doi: 10.1074/jbc.M101530200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Live imaging of ureteric microvascular network in situ. AA, TA and PA are arcade, transverse and precapillary arterioles, respectively; AV, TV, CV are arcade, transverse and collecting venules, respectively; UMB-ureteric muscle bundles.
Morphology of ureteric precapillary arterioles in situ. 3-Dimensionlal live images of smooth muscle, pericytes and endothelial cells of all sections of arteriolar network.
Morphology of ureteric capillaries in situ. 3-Dimensionlal live images of pericytes and endothelial cells of capillaries.
Morphology of ureteric postcapillary venules in situ. 3-Dimensionlal live images of pericytes and endothelial cells of postcapillary venular network.
Ca2+ responses of arteriolar and venular networks in situ to different stimuli. Distinct Ca2+ responses of SMCs and pericytes to phenylephrine (10 μM), caffeine (5 mM) and ET-1 (5 nM).
Relationship between AVP-induced Ca2+ oscillations and vasomotor responses in arcade and transverse arteriole.
Ca2+ signals and contractility of precapillary arteriolar and postcapillary venular pericytes in situ (see text for details).
Effects of endothelium Ca2+ signalling on AVP-induced Ca2+ oscillations and vasomotor responses in arcade arteriole.
Propagating Ca2+ signalling in arteriolar and venular networks induced by brief (2–3 s) application of high-K+ pre-treated with K+ channel blocker TEA (5 mM) and L-type Ca2+ channel agonist BayK 8644 (1 μM). The intercellular Ca2+ transient in both networks appear as a regenerative Ca2+ transient travelling at a speed of 1–2 cm/s in arteriolar and 1–2 mm/s venular networks, respectively.
Inhibitory effects of endothelium Ca2+ signalling on propagating Ca2+ signalling in arteriolar and venular networks induced by brief (2–5 s) application of PE pretreated with TEA (5 mM) and BayK 8644.