Abstract
Hemolytic conditions in preterm neonates, including Rhesus (Rh) disease, can lead to mortality and long-term impairments due to bilirubin neurotoxicity. Universal access to Rh immunoprophylaxis, coordinated perinatal-neonatal care, and effective phototherapy has virtually eliminated the risk of kernicterus in many countries. In the absence of jaundice due to isoimmunization and without access to phototherapy or exchange transfusion (in 1955), kernicterus was reported at 10.1%, 5.5%, and 1.2% in babies <30, 31-32, and 33-34 wks gestational age, respectively. Phototherapy initiated at 24±12 hr effectively prevented hyperbilirubinemia in infants <2,000 g even in the presence of hemolysis. This approach (in 1985) reduced exchange transfusions from 23.9% to 4.8%. Now with 3 decades of experience in implementing effective phototherapy, the need for exchange transfusions has virtually been eliminated. However, bilirubin neurotoxicity continues to be associated with prematurity alone. The ability to better predict this risk, other than birthweight and gestation, has been elusive. Objective tests such as total bilirubin, unbound or free bilirubin, albumin levels, and albumin-bilirubin binding, together with observations of concurrent hemolysis, sepsis, and rapid rate of bilirubin rise have been considered, but their individual or combined predictive utility has yet to be refined. The disruptive effects of immaturity, concurrent neonatal disease, cholestasis, use of total parenteral nutrition or drugs that alter bilirubin-binding abilities augment the clinical risk of neurotoxicity. Current management options rely on the “fine-tuning” of each infant's exposure to beneficial antioxidants and avoidance of silent neurotoxic properties of bilirubin navigated within the safe spectrum of operational thresholds demarcated by experts.
Keywords: Bilirubin neurotoxicity, hyperbilirubinemia, jaundice, kernicterus, preterm
CASE HISTORY
An 8-day-old preterm female Caucasian infant (birthweight: 1120 g; 29 wks post-menstrual age) had a recurrence of jaundice after a 4-day course of phototherapy. These findings were associated with the onset of intractable apneic events despite concurrent caffeine therapy. In matter of hours, she developed acute respiratory acidosis, which required intubation for respiratory support. Her prior history was reasonably uncomplicated for a very low birthweight infant (VLBW). She recovered from transient respiratory distress syndrome with a single dose of surfactant and responded to a single course of intravenous indomethacin for medical closure of a patent ductus arteriosus. Recently, enteral gavage feeds have been started. There was no respiratory distress other than use of supplemental oxygen via a nasal cannula at 0.25 L/min. Because of a recent deterioration, she was started on antibiotics and investigated for both nosocomial sepsis and intracranial bleeding. Neither condition was identified. Her total bilirubin was 11 mg/dL with an anticipated rate of bilirubin rise, due to underlying hemolysis and based on prior and subsequent data, estimated at 0.16 mg/dL/hr [Figure 1]. She became irritable, needed sedation, and was continued on ventilatory support for recurrent desaturations. Under phototherapy, her total bilirubin levels declined over the ensuing days. She was extubated from ventilator support, enteral nutrition resumed, and then subsequently discharged by term post-menstrual age. At discharge, automated auditory brainstem response (ABR) for both ears was referred. Magnetic resonance imaging at age 2 yrs indicated increased signals in the globus pallidus and signs of periventricular leukomalacia. Her neurologic examination showed signs of kernicterus and hearing impairment.
Figure 1.
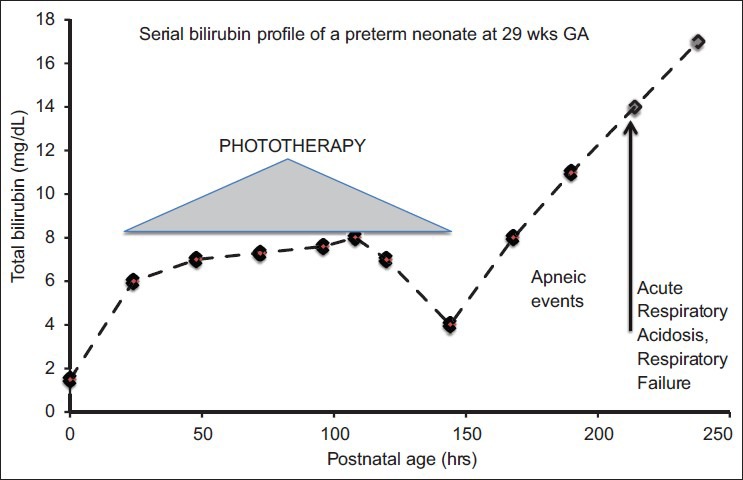
Case history
INTRODUCTION
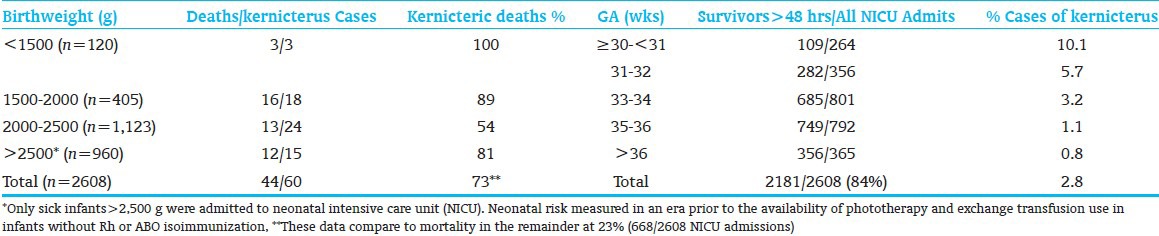
Neonatal jaundice and its correlation to hyperbilirubinemia and bilirubin neurotoxicity have been subjects of inquiry, controversy, and debate as novel therapies have become available in the care of the preterm infant.[1,2,3,4] It must be remembered that the degree of jaundice does not predict the bilirubin level. Prevention of Rh disease, starvation, prevention or early treatment of neonatal sepsis, safe use of antibiotics and drugs, and reduction of birth trauma have individually contributed to a decrease in the incidence of kernicterus in preterm infants since the 1950s.[2,3,4] In the absence of jaundice due to isoimmunization and without access to phototherapy or exchange transfusion (in 1955), kernicterus was reported at 10.1%, 5.5%, and 1.2% in babies <30, 31-32, and 33-34 wks gestational age (GA), respectively[5] [Table 1]. It is also now well established that the presence of early-onset of jaundice (age <24 hrs) is a medical emergency,[6] and that total serum/plasma bilirubin (TSB) levels measured between ages 24-60 hrs predicts severe hyperbilirubinemia and need for phototherapy.[1] Moreover, phototherapy reduces the need and/or use of exchange transfusion and that both phototherapy and exchange transfusion can individually prevent kernicterus. It is also known that the detection of jaundice or measurement of TSB has not been shown to prevent kernicterus in a randomized control trial; this kind of a study cannot be done ethically and should not be done.
Table 1.
Neonatal mortality with kernicterus among admits to neonatal nursery (by birthweight and gestational age)[5]
Natural Bilirubin Profile: TSB levels progressively increase during the first 96-120 hrs after birth. These levels usually decline depending on the maturation of the infant's liver, initiation of enteral feeds, motility of the gastrointestinal (GI) tract, and the ability of a preterm infant to clear its bilirubin load. Mostly, the decline in hyperbilirubinemia should commence by day 7 of life among those infants who have an uncomplicated clinical course and the GI clearance has been facilitated by enteral nutrition. Among most infants, jaundice should resolve by 2 wks of life. The persistence of jaundice beyond age 2 wks warrants further inquiry.
In this article, we will review the existing evidence for bilirubin-related brain injury; highlight the standard of care to prevent brain injury, and discuss the evidence for expert recommendations as well as identify existing gaps in knowledge that may be bridged through future research.
CLINICAL MANIFESTATIONS OF BILIRUBIN NEUROTOXICITY IN PRETERM NEONATES
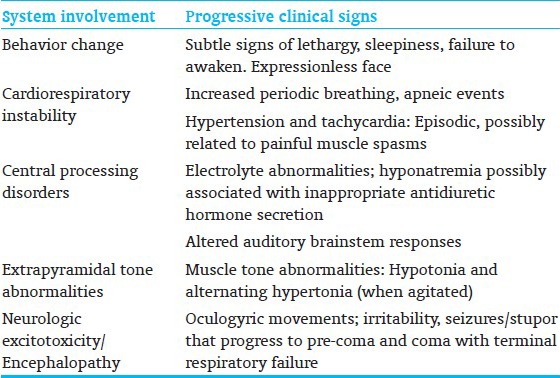
Preterm neonates who have concurrent illnesses and physiologic derangements are more vulnerable to bilirubin neurotoxicity and has been recognized and studied in clinical trials[7] [Table 2]. Bilirubin-related neurotoxicity can result in neonatal death or multisystem acute manifestations[8] [Table 3] and long-term impairments, including irreversible athetoid cerebral palsy (CP), and speech, visuomotor, auditory, and other sensori-processing disabilities. A range of TSB levels, rather than a specific or critical value, is more likely to be associated with onset of neurotoxicity in otherwise healthy preterm infants. The most frequent clinical factors include increasing immaturity, unrecognized neonatal hemolysis, array of genetic conditions [for example, glucose-6-phosphate dehydrogenase (G6PD) deficiency, congenital spherocytosis, pyruvate kinase deficiency, galactosemia, Crigler–Najjar syndrome], or concurrent conditions of dehydration, sepsis, or acidosis, hypoalbuminemia, and/or poor feeding. With current clinical practice, icteric complications are exceedingly infrequent given the liberal, prophylactic, and effective use of phototherapy. However, the risk is not zero and several recent studies have shown that even moderate or low TSB levels can lead to bilirubin-induced brain damage in sick premature infants. For the same level of hyperbilirubinemia, the risk of CP is higher in preterm and small for gestational age (SGA) infants. Risk increases both as GA decreases and as the concentration of TB rises.[9,10] Crosse et al.,[5] in 1955, best described the acute clinical signs of bilirubin neurotoxicity in preterm infants prior to the routine use of exchange transfusion and advent of phototherapy. They noted that: “The first 24-48 hours of life are the most critical. Signs develop in baby who is jaundiced… and include head retraction, an expressionless facies, usually with oculogyric movements, changes in muscle tone, cyanotic attacks, refusal to suck, vomiting and hemorrhage prior to death. In severe cases, these signs are self evident but in those less affected they are easily missed……unless specifically sought. A baby who exhibits any of these signs, especially between fourth and eighth day days of life, is re-examined at regular intervals by us for at least one year, even if (s) he appears normal at discharge. Several babies who showed minimal signs have proved to be definite cases of kernicterus”. These signs have been reiterated in more recent reports of preterm infants with acute bilirubin encephalopathy (ABE)[8] [Table 3].
Table 2.
Historic clinical risk factors for bilirubin neurotoxicity in preterm neonates[7]
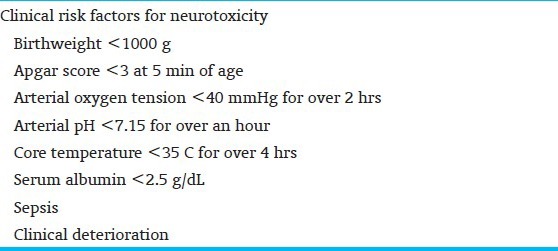
Table 3.
Multi-system signs of acute bilirubin encephalopathy in preterm neonates[8]
CLINICAL OUTCOME OF BILIRUBIN NEUROTOXICITY
The clinical outcome of bilirubin neurotoxicity usually manifests as irreversible post-icteric sequelae; the hallmark sign (usually at autopsy) is the icteric (yellow) staining of the basal ganglia, specifically of the globus pallidus.[11] The spectrum of clinical outcomes is illustrated in a modular format in Figure 2. These signs may appear even with the absence of acute neurologic signs. It can now be observed at that location as increased signals on magnetic resonance imaging. Injury occurs when the TSB level exceeds an infant's neuroprotective defenses and causes neuronal damage, primarily in the basal ganglia, central and peripheral auditory pathways, hippocampus, diencephalon, subthalamic nuclei, midbrain, pontine, brainstem nuclei for visuomotor function, respiratory, neurohumoral and electrolyte control, and in the cerebellum, most prominently in the vermis. Acute signs can present as progressive changes in an infant's mental (behavioral) status, muscle tone, cry with varying degrees of drowsiness, poor feeding, hypotonia and alternating tone followed by increasing hypertonia, especially of extensor muscles, retrocollis and opisthotonos, first intermittent and then with increasing severity, becoming constant. Acute stage mortality (about 7%) is due to respiratory failure and progressive coma or intractable seizures. Rate of progression of clinical signs depends on the rate of TSB rise, duration of hyperbilirubinemia, host susceptibility, and presence of co-morbidities. The term kernicterus is now usually reserved for irreversible classic sequelae diagnosed in infants who survive ABE and are diagnosed primarily with dystonia, athetoid CP, paralysis of upward gaze, and sensorineural hearing loss of varying degrees of severity. Cognition is usually spared to a striking degree. Bilirubin-induced neurologic dysfunction (BIND) is a wider spectrum of disorders that includes acute and classic (chronic) kernicterus, but also clinical evidence of damage confined to more narrow neural pathways, which results in isolated, less severe forms of auditory neuropathy (auditory “dys”-synchrony) defined by characteristic clinical criteria and distinctive findings on the auditory brainstem response (ABR) and normal cochlear function (normal cochlear microphonic, normal otoacoustic emissions) without severe hearing loss or by similar auditory neuropathy associated with minimal fine and/or gross motor disability. Though not yet proven, some experts believe that there may be subtle neurological manifestations of BIND,[12,13] with only signs of awkwardness, minimal fine and gross motor incoordination, gait abnormalities, fine tremors, exaggerated extrapyramidal reflexes,[13] and perhaps auditory learning and behavioral problems.[14] These subtle signs are difficult to diagnose because of delayed clinical expression and their non-specificity, but they may be signs of possible sequelae of undetected acute encephalopathy.
Figure 2.
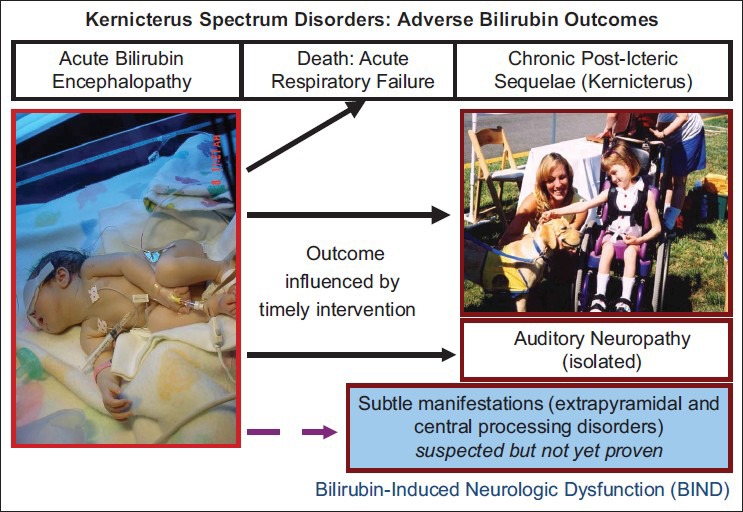
Kernicterus spectrum disorders in children of preterm birth
RISK OF NEURAL IMPAIRMENT IN VULNERABLE NEWBORNS: BENCH STUDIES
Neuronal injury may be due to increased excitotoxicity, oxidative stress, alterations in neuronal arborization, synaptotoxicity, and apoptosis that ultimately leads to cell demise. Bilirubin exposure reduces neurogenesis, and young neurons show increased susceptibility to bilirubin as compared to mature neurons, thus explaining the increased vulnerability of the preterm neonate.[15,16] Hippocampal neurons have shown a particular susceptibility to bilirubin when compared to those from the cortex and cerebellum. Responses to oxidative or neuro-inflammatory, nitrosative, or excitotoxicity stressors also lead to microglial activation and migration to site of injury and to areas undergoing astrogliosis. The microglial response is either through phagocytosis or induction of dystrophic changes. Astrocytes often show signs of increased reactivity and manifest as a disruption of synaptic plasticity. The ensuing effects can be more long-term as evidenced by decreased neurite arborization and decreased myelogenesis. In vitro studies show that hippocampal neuronal cell cultures exhibit stunted axonal elongation (needed for proper formation of neural circuits) with increasing exposure to bilirubin. The “growth cones” of axons can suffer from retraction or collapse and lead to mild to severe restriction of neuronal arborization. These growth arrests are evidenced as abnormalities in number, size, and morphology of dendritic spines, which further stunt normal neuronal maturation.
Disruption of the blood-brain barrier following inflammation occurs with increasing immaturity. Longer durations of hyperbilirubinemia compromise vascular endothelial dynamics by increasing oxidative stress, cytokine release, cell detachment,[17,18] and angiogenic sprouting. Individually, and in combination, these factors facilitate passage of bilirubin from the blood into brain and aggravate bilirubin neurotoxicity. The immature brain is more flexible in responding to injury, with possible recovery. However, the brain of a preterm infant is more vulnerable. Recovery, most likely, depends either on cellular activation, resulting in a cascade of neuroprotective or detrimental events, or from environmental influences. The full impact of these adverse exposures, maturity at time of injury, and the long-term consequences are just being elucidated[19,20] and could lead to lasting sequelae.
SYNDROME OF BILIRUBIN-INDUCED NEUROLOGIC DYSFUNCTION
BIND represents a spectrum of subtle neurologic manifestations among vulnerable infants who have experienced an exposure to bilirubin of lesser degree than generally described in previous publications.[12] Clinical neuromotor manifestations extend to a range of subtle processing disorders with objective disturbances of visuomotor, auditory, speech, cognition, and language among infants with a prior history of moderate to severe hyperbilirubinemia of varied durations. Confounding effects include prematurity, hemolysis, perinatal-neonatal complications, altered bilirubin-albumin binding, severity and duration of bilirubin exposure, and the individual vulnerability of the infant related to genetic, family, social, and educational predilections, regardless of the cause of neonatal jaundice. Tools to better assess specific domains of multisensory injury that occurs during the neonatal period may have long-term consequences. Preliminary studies have identified altered psychometric, audiologic, speech, language, and visuomotor disorders that are associated with altered bilirubin binding to albumin. The recent reanalysis of a 7-yr follow-up study are summarized in Table 4.
Table 4.
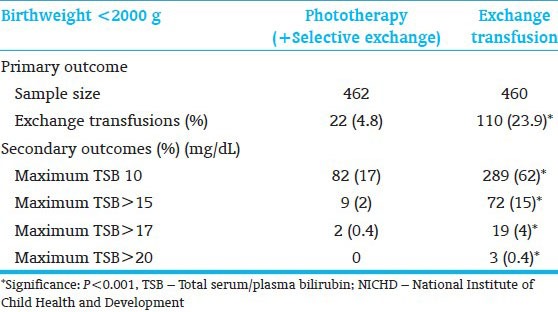
Efficacy of phototherapy to prevent exchange transfusion[7]: Outcome of NICHD phototherapy efficacy trial (1985)
RISK OF BILIRUBIN NEUROTOXICITY IN PRETERM NEONATES
In the era prior to the routine use of exchange transfusion and availability of phototherapy, Crosse et al.,[5] reported that 73.6% of preterm babies with kernicterus died as compared to 25.6% of all preterm infants [Table 1]. The highest mortality was among those of lower birthweight and earlier age of onset of clinical signs. The sequelae or outcome was dependent on maturity of infants who survived the first 2 days after birth are presented by GA stratification. These data show the risk of mortality and kernicterus for preterm infants who are not actively treated with currently proven and established with bilirubin reduction strategies. Hansen[21] and Watchko[22] have recently summarized the scientific evidence and mechanism of bilirubin neurotoxicity. These indicate that bilirubin kills specific neurons by causing necrosis; in vitro studies show that it induces apoptosis and support in vivo observations in older literature showing neuro-anatomic changes consistent with apoptosis. Evidence also suggests that bilirubin interferes with intracellular calcium homeostasis by altering function and expression of calcium/calmodulin kinase II, by selectively decreasing calcium-binding proteins in susceptible brainstem areas and increasing intracellular calcium in cultured neurons, and by sensitizing the cell to other injuries or triggering apoptosis. Bilirubin may also be cytotoxic by causing neuronal hyperexcitability, perhaps via excitatory amino acid neurotoxicity, or it may have other membrane of neurotransmitter effects. Finally, it may act by interfering with mitochondrial respiration and energy production. Thus, interventions that reduce bilirubin exposure to the neonatal brain have been shown to prevent bilirubin neurotoxicity. Time matters.
STANDARD OF CARE
Timing of interventions to reduce excessive bilirubin load
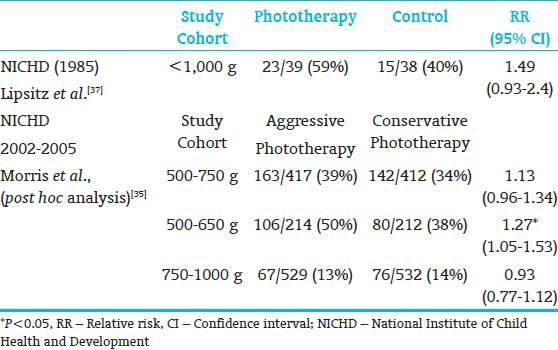
The timing of bilirubin reduction strategies impacts the outcome of preterm infants at risk for excessive hyperbilirubinemia for age. Early implementation of strategies to rapidly and effectively reduce the excessive bilirubin load prior to the onset of neurologic signs, in all likelihood, would prevent chronic post-icteric sequelae or kernicterus.[23] The initial evidence for this approach, using phototherapy, was demonstrated by a National Institute of Child Health and Development (NICHD) Neonatal Research Network clinical trial to test the efficacy of phototherapy as compared to exchange transfusion alone.[5,7,24] This study demonstrated that phototherapy initiated at 24±12 hrs effectively prevented hyperbilirubinemia in infants weighing <2,000 g even in the presence of hemolysis and reduced exchange transfusions from 23.9% to 4.8% [Table 5]. Now, with 3 decades of experience in implementing effective phototherapy, the need for exchange transfusions has virtually been eliminated. Once the clinical signs of bilirubin neurotoxicity are evident, emergent intervention to expeditiously reduce the bilirubin load is the only known recourse in clinical practice. To date, exchange transfusion coupled with a crash-cart phototherapy remains the only known clinical option. Even though there is no predictive evidence that a specific TSB level will or will not cause neurotoxic damage, the critical TSB level is influenced by postnatal age, maturity within the range of term GA, duration of hyperbilirubinemia, and rate of TSB rise.
Table 5.
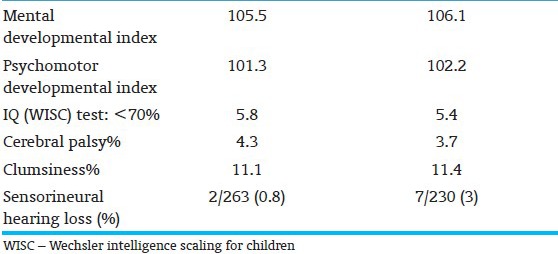
Residual long-term impairment in infants with birthweight 1000 to 2000 g treated for severe hyperbilirubinemia and tested at age 7 yrs[24] (Randomized control trial of phototherapy and selective use of exchange transfusion versus exchange transfusion alone[7])
Triage for a jaundiced preterm newborn with suspicious clinical neurologic signs.Triage process should be guided by ongoing staff education, development and sharing a local protocol and plan of action.[23,25]
Supplies: Ready access to devices, equipment, and transport isolettes to manage cardio-respiratory deterioration. Specific devices include phototherapy equipment, irradiance meters specific for the device that is being used, and protective gear for the baby including opaque eye masks and filters for exclude ultraviolet (UV) light exposure
Testing: Transcutaneous bilirubin (TcB) testing, clinical and rapid neurologic examination, and “STAT” laboratory studies (total and direct bilirubin and serum albumin measurements, blood typing, and cross-matching)
Advise parents of medical emergency
Transfer or commence treatment with intensive phototherapy as soon as possible (crash-cart approach).
EMERGENCY INTERVENTIONS FOR RAPID REDUCTION OF BILIRUBIN CONCENTRATIONS
Rapid bilirubin reduction strategies include intensive phototherapy, double volume blood exchange transfusion, occasional need for pharmacological agents often used in combination.
‘Intensive phototherapy’ is the current drug of choice to reduce the severity of neonatal unconjugated hyperbilirubinemia regardless of its etiology in a matter of 2-4 hrs.[26,27] Optimal use of phototherapy has been defined by specific ranges of TSB thresholds that have been correlated to an infant's postnatal age (in hrs) and their potential risk for bilirubin neurotoxicity (see below). Effective phototherapy implies its use as a “drug” with specific light wavelengths at a specific peak (460 nm, blue) and a range of emission spectrum (400-520 nm), preferably in a precise (narrow) bandwidth that is delivered at an irradiance (dose) of ≥30-35 μW/cm2/nm to 80% of an infant's body surface area (BSA). There are several commercial devices and delivery methods for phototherapy for use at both the hospital and home. Blue lights in the 425-475 nm range should be easily and rapidly accessible, and periodically inspected and maintained to ensure proper functioning. Avoid shadows with multiple lights. The efficacy is additionally influenced by: (a) Optimization of light administration to achieve a minimum distance between the device and the patient such that the foot print of light covers maximum BSA with minimal physical barriers; (b) infant characteristics such as the severity of jaundice, BSA proportions, as well as dermal thickness, pigmentation, and perfusion; and (c) the duration of treatment to a specific bilirubin threshold.
‘Exchange transfusion’ is a critical and invasive procedure that can significantly reduce TSB levels in a matter of 1-2 hrs.[1,28] Trained personnel in neonatal/pediatric intensive care facilities with full monitoring and resuscitation capabilities should perform this procedure. Exchange transfusion should be considered and anticipated when there are any neurologic signs even if TSB is falling, or there are significant concerns of neurotoxicity. Concerns for neurotoxicity in term infants are heightened in an asymptomatic infant when: a) The TSB level exceeds 25 mg/dL; b) intensive phototherapy fails to produce a significant TSB reduction in an infant with severe hyperbilirubinemia (a progressive TSB decline of at least >0.5 mg/dL/hr or >2 mg/dL drop in 4 hrs should be expected); or c) an infant who had an earlier successful hearing screen and fails an automated ABR screen. Before an exchange transfusion is initiated, the healthcare team should review the risks and benefits of the procedure with the parents, so parents can provide informed parental consent (see below). The adverse effects of an exchange transfusion include neonatal morbidities such as apnea, anemia, thrombocytopenia, electrolyte and calcium imbalances, risk of necrotizing enterocolitis (NEC), hemorrhage, infection, complications related to the use of blood products, and catheter-related complications. Exchange transfusion also carries the risk of neonatal mortality, especially in sick infants. Exchange transfusion is ideally performed as an isovolumic procedure, preferably with concurrent withdrawal from an arterial line and infusion through a venous line. Double volume exchange (170 mL/kg) is preferable, but in the event of technical difficulties, a single volume exchange transfusion may be adequate if supplemented with intensive phototherapy. The entire process should be accomplished within 4-6 hrs of the identification of the medical emergency.[1]
Pharmacologic options and chemoprevention strategies have been reviewed in recent articles.[1,29] Pharmacologic interventions have a limited role in the emergency room management of a sick infant and their usefulness and limitations are discussed in these reviews.
Albumin infusion
At times, an albumin infusion (1 g/kg) has been suggested prior to an exchange transfusion, especially if serum albumin is low (<3.0 g/dL). However, there is current no evidence to support this practice. In the preterm infant, there is concern for increased intravascular volume, increased alveolar leak, and cardiopulmonary compromise.
Intravenous gamma immunoglobulin
Intravenous immunoglobulin (IVIG) may be administered when the hyperbilirubinemia is attributed to isoimmunization. IVIG has been shown, anecdotally, to reduce the need for exchange transfusions in Rh and ABO hemolytic diseases.[1] Although data are limited, there is no evidentiary basis for its use and there are concerns for significant side effects.
Phenobarbital
Phenobarbital can accelerate bilirubin excretion by increasing hepatic clearance. However, this drug is no longer recommended, as it has no clinical effect when administered to infants <32 wks GA and is ineffective when given prior to 12 hrs of age. The adverse effects of this therapy include sedation, risk of hemorrhagic disease, and the potentially addictive nature of phenobarbital. This drug has a slow onset of effect (usually several days) and a long duration of action (1-2 wks) after its discontinuation. For all of these reasons, the use of phenobarbital is no longer recommended.[1]
Tin mesoporphyrin (SnMP)
Stannic porphyrins, in particular tin mesoporphyrin, inhibit the enzyme heme oxygenase (HO) and block the transformation of heme to biliverdin and bilirubin.[1,30] These have also been noted to cause photosensitization (especially with intense white light phototherapy). SnMP has been investigated in clinical pharmacological and toxicological studies and is effective in reducing TSB levels in infants at risk for severe hyperbilirubinemia with no significant side effects. Most infants do not need further phototherapy after SnMP is administered. SnMP is currently being evaluated for safety, and the Food and Drug Administration has not yet approved its use in the United States.
Other strategies that warrant further investigations and clinical trials are use of agents that interrupt the enterohepatic circulation and bilirubin accumulation from the continued action of β-glucuronidase.[31] Chemoprevention with use of casein supplements or other agents such as L-aspartic acid could decrease intestinal reabsorption of bilirubin and may play a potential preventive or adjunctive clinical role.
FOLLOW-UP OF PRETERM INFANTS AT RISK FOR BIND
Post-icteric sequelae are often unrecognized, mislabeled, or misdiagnosed in preterm infants. These errors have led prolonged diagnostic and health-seeking odysseys for families. Follow-up studies of infants enrolled in the NICHD trial of 1979-85 demonstrated the challenges of follow-up in this population as well as the residual morbidities identified at age 7 yrs[24] [Table 6]. Oh et al.,[32] through a retrospective observational analysis in babies with BW <1,000 g, noted that TSB concentrations during the first 14 days of birth are directly correlated with death, neurodevelopmental impairment sensorineural hearing and other physical impairments. Confounding effects of modest hyperbilirubinemia or potential toxic effects of phototherapy could not be excluded. These have been supplemented by similar concerns for adverse outcomes at age 16-22 months for preterm infants <1,000 g[33,34,35,36,37] [Tables 7 and 8]. Infants with TSB levels that approach thresholds for an exchange transfusion should be followed through infancy until school age for awkwardness, gait abnormality, failure of fine stereognosis, gaze abnormalities, poor coordination, and exaggerated extra pyramidal reflexes. Follow-up should include neurologic and neurodevelopmental evaluation, neuroimaging with magnetic resonance, and ABRs.
Table 6.
Outcome at age 7 yrs in preterm infants with neonatal jaundice: Percent infants with suspiciously abnormal findings for specific psychometric tests and association with prematurity and disordered bilirubin-albumin binding[12]
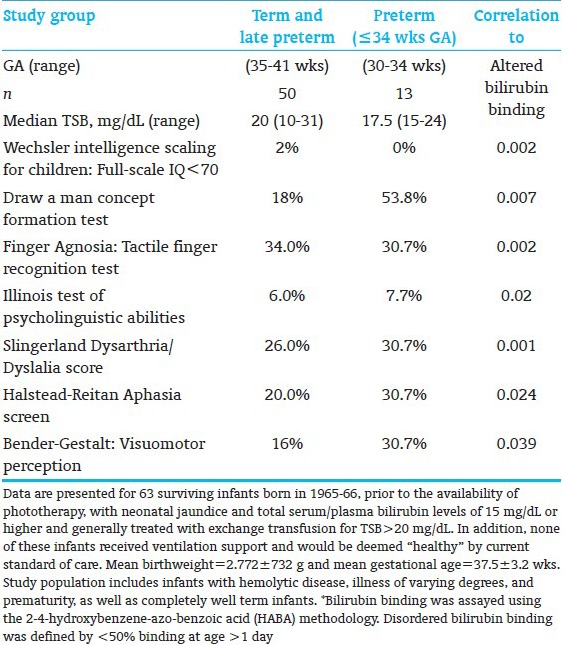
Table 7.
Irreversible impairment at age 16-22 months in infants <1,000 g[35]
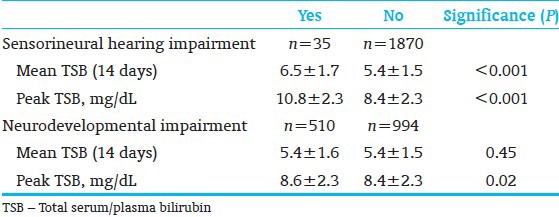
Table 8.
Concerns for over-treatment with phototherapy in extremely low birthweight infants
CURRENT OPERATIONAL THRESHOLDS
The TSB thresholds for exchange transfusion have been established[7] and also adjusted for possible application of bilirubin: Albumin ratio[38] [Tables 9 and 10]. Maisels et al.,[4] have recommended an approach to institute and develop practice patterns that minimize variations in clinical use phototherapy and exchange transfusion in the care of preterm infants <35 wks GA [Figure 3]. The data to develop a formal evidence-based recommendations are confound by small sample sizes, incremental introductions of life-saving technologies that these approaches need to consensus-based with reliance on expert experiences and know-how of technology limitations. The validity of such an approach can only by tested with long-range outcomes of (at least 6-9 yrs of age) for bilirubin-related adverse outcomes.
Table 9.
Operational thresholds for exchange transfusion in preterm infants[7]
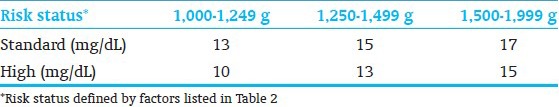
Table 10.
Bilirubin-albumin ratio: Theoretical criteria for exchange transfusion in BW<2000 g
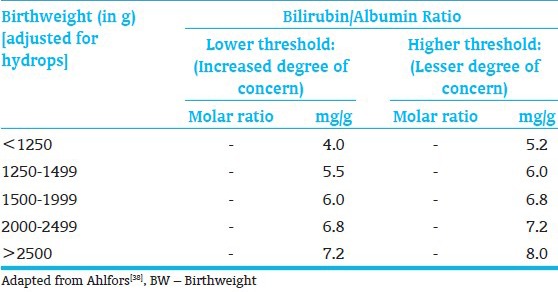
Figure 3.
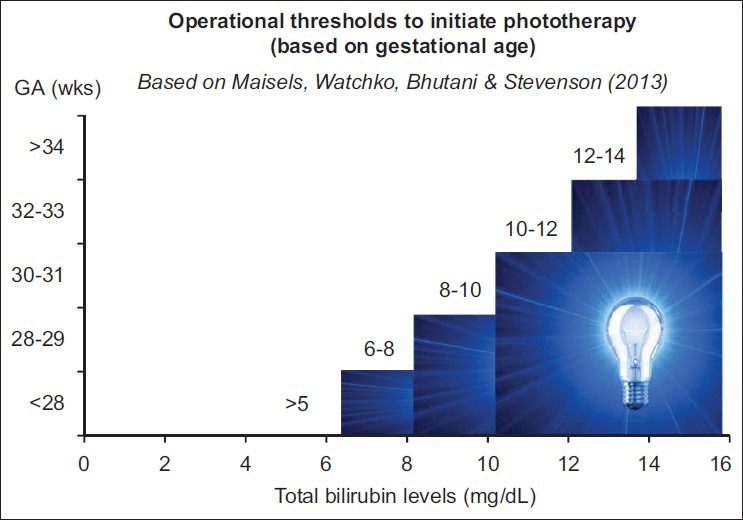
Operationalization of phototherapy-based expert recommendations
CONCLUSION
Once, kernicterus in preterm infants was associated with Rh isoimmunization. Universal access to Rh immunoprophylaxis, coordinated perinatal care, newborn screening, and effective phototherapy can virtually eliminate this condition worldwide. However, the vulnerable preterm infant is also inherently and biologic at risk for subtle to moderate bilirubin neurotoxicity as well as yet unrecognized side effects of intervention. There is continued to better refine our risk-assessment abilities for both under- and overtreatment of babies vulnerable to BIND.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.American Academy of Pediatrics. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 2.Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: An update with clarifications. Pediatrics. 2009;124:1193–8. doi: 10.1542/peds.2009-0329. [DOI] [PubMed] [Google Scholar]
- 3.Ip S, Lau J, Chung M, Kulig J, Sege R, Glicken S, et al. Hyperbilirubinemia and kernicterus: 50 years later. Pediatrics. 2004;114:263–4. doi: 10.1542/peds.114.1.263. [DOI] [PubMed] [Google Scholar]
- 4.Maisels MJ, Watchko JF, Bhutani VK, Stevenson DK. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J Perinatol. 2012;32:660–4. doi: 10.1038/jp.2012.71. [DOI] [PubMed] [Google Scholar]
- 5.Crosse VM, Meyer TC, Gerrard JW. Kernicterus and prematurity. Arch Dis Child. 1955;30:501–8. doi: 10.1136/adc.30.154.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman TB, Liljestrand P, Escobar GJ. Jaundice noted in the first 24 hours after birth in a managed care organization. Arch Pediatr Adolesc Med. 2002;156:1244–50. doi: 10.1001/archpedi.156.12.1244. [DOI] [PubMed] [Google Scholar]
- 7.Brown AK, Kim MH, Wu PY, Bryla DA. Efficacy of phototherapy in prevention and management of neonatal hyperbilirubinemia. Pediatrics. 1985;75:393–400. [PubMed] [Google Scholar]
- 8.Bhutani VK, Johnson LH, Shapiro SM. Kernicterus in sick and preterm infants (1999-2002): A need for an effective preventive approach. Semin Perinatol. 2004;28:319–25. doi: 10.1053/j.semperi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 9.van de Bor M, van Zeben-van der Aa TM, Verloove-Vanhorick SP, Brand R, Ruys JH. Hyperbilirubinemia in preterm infants and neurodevelopmental outcome at 2 years of age: results of a national collaborative survey. Pediatrics. 1989;83:915–20. [PubMed] [Google Scholar]
- 10.Airede AI. Relation of peak total serum bilirubin concentrations to neurodevelopmental outcome at 2 years of age in premature African neonates. Ann Trop Paediatr. 1992;12:249–54. doi: 10.1080/02724936.1992.11747580. [DOI] [PubMed] [Google Scholar]
- 11.Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004. J Perinatol. 2009;29(Suppl 1):S25–45. doi: 10.1038/jp.2008.211. [DOI] [PubMed] [Google Scholar]
- 12.Johnson L, Bhutani VK. The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Perinatol. 2011;35:101–13. doi: 10.1053/j.semperi.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Newman TB, Klebanoff MA. Neonatal hyperbilirubinemia and long-term outcome: Another look at the Collaborative Perinatal Project. Pediatrics. 1993;92:651–7. [PubMed] [Google Scholar]
- 14.Shapiro SM. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND) J Perinatol. 2005;25:54–9. doi: 10.1038/sj.jp.7211157. [DOI] [PubMed] [Google Scholar]
- 15.Brites D. The evolving landscape of neurotoxicity by unconjugated bilirubin: Role of glial cells and inflammation. Front Pharmacol. 2012;3:88. doi: 10.3389/fphar.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brites D, Brito MA. Bilirubin toxicity. In: Stevenson DK, Maisels MJ, Watchko JF, editors. Care of the Jaundiced Neonate. 1st edn. New York: McGraw Hill; 2012. pp. 115–43. [Google Scholar]
- 17.Palmela I, Cardoso FL, Bernas M, Correia L, Vaz AR, Silva RF, et al. Elevated levels of bilirubin and long-term exposure impair human brain microvascular endothelial cell integrity. Curr Neurovasc Res. 2011;8:153–69. doi: 10.2174/156720211795495358. [DOI] [PubMed] [Google Scholar]
- 18.Palmela I, Sasaki H, Cardoso FL, Moutinho M, Kim KS, Brites D, et al. Time-dependent dual effects of high levels of unconjugated bilirubin on the human blood-brain barrier lining. Front Cell Neurosci. 2012;6:22. doi: 10.3389/fncel.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, et al. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011;70:550–65. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- 20.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–57. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 21.Hansen TW. Kernicterus: An international perspective. Semin Neonatol. 2002;7:103–9. doi: 10.1053/siny.2002.0118. [DOI] [PubMed] [Google Scholar]
- 22.Watchko JF. Kernicterus and the molecular mechanisms of bilirubin-induced CNS injury in newborns. Neuromolecular Med. 2006;8:513–29. doi: 10.1385/NMM:8:4:513. [DOI] [PubMed] [Google Scholar]
- 23.Smitherman H, Stark AR, Bhutani VK. Early recognition of neonatal hyperbilirubinemia and its emergent management. Semin Fetal Neonatal Med. 2006;11:214–24. doi: 10.1016/j.siny.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Scheidt PC, Graubard BI, Nelson KB, Hirtz DG, Hoffman HJ, Gartner LM, et al. Intelligence at six years in relation to neonatal bilirubin levels: Follow-up of the National Institute of Child Health and Human Development Clinical Trial of Phototherapy. Pediatrics. 1991;87:797–805. [PubMed] [Google Scholar]
- 25.Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30(Suppl):S6–15. doi: 10.1038/jp.2010.98. [DOI] [PubMed] [Google Scholar]
- 26.Bhutani VK. Committee on Fetus and Newborn, American Academy of Pediatrics. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2011;128:e1046–52. doi: 10.1542/peds.2011-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maisels MJ, McDonagh AF. Phototherapy for neonatal jaundice. N Engl J Med. 2008;358:920–8. doi: 10.1056/NEJMct0708376. [DOI] [PubMed] [Google Scholar]
- 28.Murki S, Kumar P. Blood exchange transfusion for infants with severe neonatal hyperbilirubinemia. Semin Perinatol. 2011;35:175–84. doi: 10.1053/j.semperi.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med. 2001;344:581–90. doi: 10.1056/NEJM200102223440807. [DOI] [PubMed] [Google Scholar]
- 30.Wong RJ, Bhutani VK, Vreman HJ, Stevenson DK. Tin mesoporphyrin for the prevention of severe neonatal hyperbilirubinemia. NeoReviews. 2007;8:e77–84. [Google Scholar]
- 31.Gourley GR, Li Z, Kreamer BL, Kosorok MR. A controlled, randomized, double-blind trial of prophylaxis against jaundice among breastfed newborns. Pediatrics. 2005;116:385–91. doi: 10.1542/peds.2004-1807. [DOI] [PubMed] [Google Scholar]
- 32.Oh W, Tyson JE, Fanaroff AA, Vohr BR, Perritt R, Stoll BJ, et al. Association between peak serum bilirubin and neurodevelopmental outcomes in extremely low birth weight infants. Pediatrics. 2003;112:773–9. doi: 10.1542/peds.112.4.773. [DOI] [PubMed] [Google Scholar]
- 33.Hintz SR, Stevenson DK, Yao Q, Wong RJ, Das A, Van Meurs KP, et al. Is phototherapy exposure associated with better or worse outcomes in 501- to 1000-g-birth-weight infants? Acta Paediatr. 2011;100:960–5. doi: 10.1111/j.1651-2227.2011.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris BH, Oh W, Tyson JE, Stevenson DK, Phelps DL, O’Shea TM, et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N Engl J Med. 2008;359:1885–96. doi: 10.1056/NEJMoa0803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris BH, Tyson JE, Stevenson DK, Oh W, Phelps DL, O’Shea TM, et al. Efficacy of phototherapy devices and outcomes among extremely low birth weight infants: Multi-center observational study. J Perinatol. 2013;33:126–33. doi: 10.1038/jp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyson JE, Pedroza C, Langer J, Green C, Morris B, Stevenson D, et al. Does aggressive phototherapy increase mortality while decreasing profound impairment among the smallest and sickest newborns? J Perinatol. 2012;32:677–84. doi: 10.1038/jp.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipsitz PJ, Gartner LM, Bryla DA. Neonatal and infant mortality in relation to phototherapy. Pediatrics. 1985;75:422–6. [PubMed] [Google Scholar]
- 38.Ahlfors CE. Criteria for exchange transfusion in jaundiced newborns. Pediatrics. 1994;93:488–94. [PubMed] [Google Scholar]


