Abstract
Background: Cognitive behavioural therapy for psychosis (CBTp) is currently a recommended form of psychosocial treatment for persons suffering from persistent psychotic symptoms. It has been argued that effect sizes from efficacy studies cannot be generalized to real clinical settings. Aims: Our aim was to evaluate whether the positive results from randomized controlled trials conducted by experts could be replicated in clinical setting with a heterogeneous sample of patients with psychotic disorder. Method: Patients referred to the study were either randomized to CBTp + TAU (the treatment group) or to a waiting-list group, only receiving TAU. The patients were assessed on different outcome measures such as the Brief Psychiatric Rating Scale (BPRS), the Scale for the Assessment of Negative Symptoms (SANS), and the Psychotic Symptom Rating Scales (PSYRATS), at pretreatment, at posttreatment (6 months), and at 12 months follow-up. In total, 45 patients participated in the study. Results: The results showed that 20 sessions of CBTp performed significantly better than the waiting list controls with respect to the global score on the BPRS, the delusional scale on the PSYRATS, and the GAF symptom score at posttreatment. At 12 months follow-up only the GAF symptom score remained significantly changed for the total sample. Conclusions: The study revealed that CBTp delivered by non-experts in routine clinical settings can produce improvements in positive psychotic symptoms, and also that some of these improvements can be maintained at one year follow-up.
Keywords: Cognitive behaviour therapy, psychosis, schizophrenia
Introduction
Psychotic symptoms within the schizophrenia spectrum disorders have traditionally been assumed to be resistant to psychotherapy (Mueser and Berenbaum, 1990). More recently, several reviews and meta-analyses have demonstrated acceptable effect sizes. For example, Wykes, Steel, Everitt and Tarrier's (2008) meta-analysis revealed that cognitive behaviour therapy for psychosis (CBTp) shows significant effect for positive symptoms, negative symptoms, functioning, mood and social anxiety at posttreatment, with effect size range of 0.35–0.44. Based on the results of several reviews and meta-analyses CBTp has been recommended as a psychosocial treatment for persons suffering from persistent psychotic symptoms (Dickerson, 2004; Gaudiano, 2005; Jones, Cormac, Silveira and Campbell, 2010; Pfammatter, Junghan and Brenner, 2006; Pilling et al., 2002; Rathod, Kingdon, Weiden and Turkington, 2008; Rector and Beck, 2001; Wykes et al., 2008; Zimmermann, Favrod, Trieu and Pomini, 2005).
Although empirical support for CBTp has been established, there still have not been any well-controlled trials that have attempted to identify the components of CBT for psychosis, or to identify the specific mechanisms responsible for treatment effectiveness (Gaudiano, 2005). There have, however, been some attempts to develop theories to explain particular elements of change in CBT for psychosis (Beck, 2004; Beck and Rector, 2002, 2003; Rector, Beck and Stolar, 2005), but we still lack a detailed theoretical and psychological understanding of the psychopathological processes in psychosis that could specify these changes observed in therapy (Tarrier, 2006). An interesting unanswered question is whether the process of change is best described through change in thought content or whether it is more a function of metacognitive awareness (Gaudiano, 2005).
Even though many studies have shown promising results, Wykes et al. (2008) have drawn attention to the important fact that in efficacy trials most treatment studies applying CBT in the treatment of psychotic symptoms have been carried out by highly trained clinicians or experts from academic settings. It has been argued that effect sizes from efficacy studies cannot be generalized to other settings, populations, and treatment providers (Morrison et al., 2004). In clinical practice the treatment of psychotic symptoms is provided by professionals with different levels of experience and training, and it may be difficult to implement manualized treatment approaches as this presupposes basic skills, time-consuming training, and highly motivated therapists. Hence, there is a need to evaluate results from effect studies in real settings, to determine whether positive results from such trials could be replicated in such settings. Although some CBTp studies have been carried out by non-experts in routine clinical settings (Morrison et al., 2004; Peters et al., 2010; Turkington, Kingdon and Turner, 2002), far too few studies have been conducted independent of the developers of the treatment models (Durham et al., 2003; Farhall, Freeman, Shawyer and Trauer, 2009).
Effectiveness research in routine settings is difficult due to the need to adapt to everyday practice with respect to patient selection. In addition, therapists included in effectiveness research may not have the necessary essential therapeutic skills for providing CBTp. CBTp is a complex method applied to patients with chronic symptoms and therefore more knowledge is needed to demonstrate the treatment capability of CBTp interventions outside academic research settings and model developer settings.
Our study tested the effectiveness of CBTp provided by supervised routine care clinicians in a clinical setting with a heterogeneous sample of patients with psychotic disorders. As more knowledge about the long-term effect of CBTp in routine clinical settings was needed, we designed a 12-month follow-up assessment.
Method
Design
A randomized controlled comparative trial was conducted with pre-, post-, and 12-month follow-up assessments. Half of the patients were randomized to receive CBTp in addition to treatment as usual (TAU) (hereafter referred to as the treatment group). The remaining patients were randomized to a waiting list (WL) control group receiving TAU (hereafter referred to as the waiting list group). The waiting list group waited 6 months from randomization before they received individual CBTp in addition to TAU.
Participants
The participants were referred by consultant psychiatrists, clinical psychologists and psychiatric nurses from outpatient and inpatient mental health clinics in Mid-Norway. They were recruited over a period of 4 years (2002–2005). The inclusion criteria for the study were: i) suffering from schizophrenia, schizoaffective disorder, or persistent delusional disorder according to ICD-10 (WHO, 1992); ii) residual auditory hallucinations and delusions experienced in the last 6 months, which had caused distress despite the use of neuroleptics; iii) in the age group 18–60 years; and iv) ability to give informed consent to participate in the study. The exclusion criteria were: i) no perceived distress produced by delusions or hearing voices; and ii) no substance use diagnosis. The diagnostic assessment was conducted by the treating psychiatrist in the clinic before a patient was referred to the study. In addition, the medication dosages during the trial were left to the decision of the treating psychiatrist in the clinic. In order to optimize the external validity of the effectiveness trial, the inclusion and exclusion criteria were kept to a minimum and patients who were ambivalent about consenting to participate in the study were given repeated information about the study and a generous amount of time to consider whether they wanted to participate.
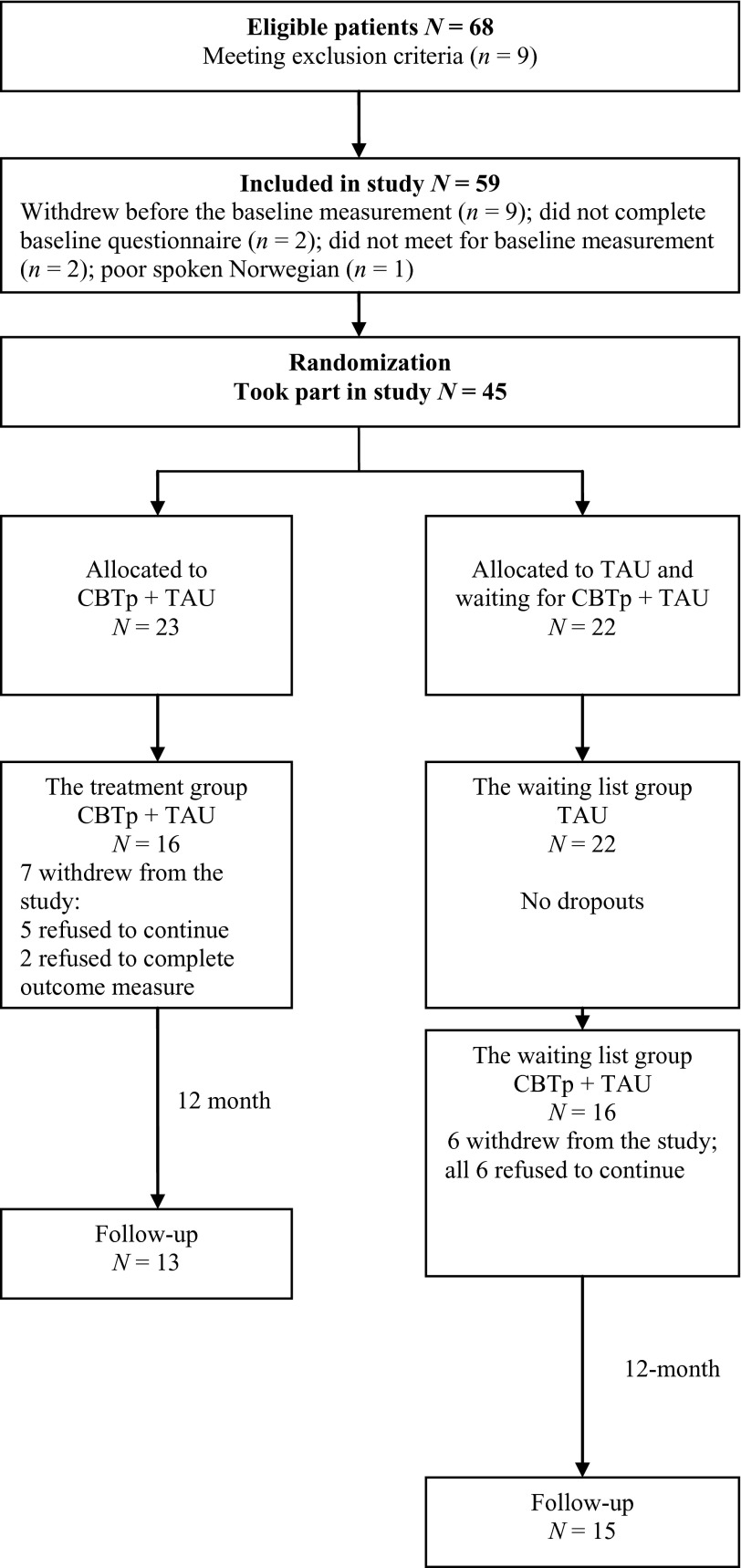
A total of 68 patients were referred to the study; 9 patients did not meet the inclusion criteria, 2 patients did not satisfy the diagnostic criteria, 2 patients reported no delusions or auditory hallucinations, and 5 patients reported no distress due to delusions and auditory hallucinations (see Figure 1). The remaining 59 patients were included in the study. Of these, 9 patients withdrew before the baseline measurement, 2 patients did not meet for baseline measurement, 2 patients did not complete baseline questionnaires, and one patient was unable to speak Norwegian sufficiently well to participate. A total of 45 patients were randomly allocated to either the treatment group (n = 23) or the waiting list group (n = 22).
Figure 1.
CONSORT diagram for participants throughout the study
Of the patients allocated to the treatment group, 5 refused to continue the therapy, and 2 patients did not meet for post-treatment. No patients in the waiting list group dropped out during the waiting period. However, when the waiting list group received CBTp + TAU after waiting for 6 months, 6 patients refused to continue the therapy and hence a total of 32 patients received the complete intervention. This indicates that the dropout rate was 30.43% from the immediate group and 27.27% from the delayed group, which is in line with Peters et al. (2010). At the 12-month follow-up, a further 3 patients in the treatment group dropped out of the trial and one patient dropped out of the waiting list group; therefore 28 patients completed the follow-up. Patients who did not complete the treatment or the assessments at post-treatment and follow-up in our trial were defined as dropouts.
Procedure
The randomization was administrated by an independent office not involved in the study. In order to avoid potential group bias in the distribution of auditory hallucinations, the participants were stratified with respect to whether or not they had auditory hallucinations. The block design was arranged with different inter-block probabilities of group allocation, which were blind to the assessors.
All assessments were carried out by three psychologists and one psychiatric nurse, none of which was involved in the patients’ therapy. All four professionals were trained in the use of assessment measures, but it was not possible to keep them blind to the treatment condition. Meetings between the raters were held in order to prevent drift in accuracy of ratings across the study. The patients in the treatment group were assessed at baseline (before randomization), at posttreatment (6 months after baseline), and at follow-up (12 months after therapy). The waiting list group was assessed at four times: at baseline (before randomization), after waiting 6 months for CBTp, at post-treatment (12 months after baseline), and at follow-up (12 months after therapy).
Measures
The Brief Psychiatric Rating Scale (BPRS; Ventura, Green, Shaner and Liberman, 1993) was used to measure general psychiatric symptoms. The BPRS measures the severity of 24 different psychiatric symptoms on one of seven ordinal intensity descriptors ranging from low severity (not present or not observed) to maximum severity (extremely severe). The BPRS also provides overall sum scores and severity ratings on schizophrenia and psychosis subscales.
The Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1989) was used to assess negative symptoms. The SANS is a 6-point (0–5) rating instrument and consists of five subscales: affective flattening or blunting; alogia; avolition-apathy; anhedonia-asociality; and attentional impairment.
The Psychotic Symptom Rating Scales (PSYRATS; Haddock, McCarron, Tarrier and Faragher, 1999), measures the severity of a number of dimensions of auditory hallucinations and delusions (two separate scales), including the amount and intensity of distress associated with these symptoms. A 5-point ordinal scale is used to rate symptom scores (0–4). The auditory hallucinations subscale (PSYRATS H) is an 11-item scale, that clusters on three different factors (Valmaggia, van der Gaag, Tarrier, Pijnenborg and Slooff, 2005). One factor relates to physical characteristics (frequency, duration, location, and loudness), the second factor relates to emotional characteristics (amount and degree of negative content and of distress), and the third factor is a cognitive interpretation factor (disruption, belief about origin, and attribution of control). The delusions subscale (PSYRATS D) is a 6-item scale that clusters on two factors: a cognitive interpretation factor (amount and duration of preoccupation, conviction, and disruption) and an emotional characteristics factor (amount and intensity of distress) (Valmaggia et al., 2005). Both the auditory hallucination scale and the delusion scale have acceptable inter-rater reliability and validity (Haddock et al., 1999).
The Global Assessment of Functioning Scale (GAF; Endicott, Spitzer, Fleiss and Cohen, 1979) was used to assess psychological functioning in the patients. The split version of GAF provides single measures of symptoms and functioning ranging from 0 to100. The instrument has shown to be reliable and valid in a sample of severely mentally ill patients (Jones, Thornicroft, Coffey and Dunn, 1995).
Treatment
Cognitive Behaviour Therapy for Psychosis (CBTp)
Participants received 20 sessions of individual cognitive therapy based on a simplified version of the treatment model developed by Chadwick, Birchwood and Trower (1996). The purpose of the therapy was to reduce the distress that accompanies delusional beliefs and auditory hallucinations by challenging the dysfunctional beliefs of voices and delusions within a cognitive restructuring framework. Particularly for auditory hallucinations, the aim was to challenge beliefs about the power of the voices. The duration and frequency of the sessions were somewhat flexible in order to accommodate the needs of individual patients. As a rule, each patient was offered 45 minutes of therapy. There were weekly sessions during the first 8 weeks of treatment. Thereafter, the patients received fortnightly sessions over a period lasting between 4 and 6 months.
Treatment as Usual (TAU)
Patients randomly assigned to the waiting list group continued to receive treatment as directed by the referring practitioner. The nature of the TAU interventions included contact with a community case manager, supportive psychosocial interventions delivered from the patients’ local therapists, and neuroleptic medication. None of the patients in the waiting list group received systematic and individualized CBTp in the 6-month waiting period.
Therapists
The therapy was provided by five clinical psychologists and three nurse therapists. All therapists were employed in routine clinical settings and had clinical experience in treating people with psychosis. To ensure treatment fidelity, all therapists received training in the model used in the study. The therapists received fortnightly client-specific supervision in CBT by a national supervisor (JAH). In cases when consent was given, the treatment sessions were video recorded and discussed in the supervision group. In addition, the therapists attended two 2-day workshops on CBTp held by Professor Max Birchwood from All Saints Hospital in Birmingham. None of the therapists had specific training in CBTp when recruited to the study. The mean years of clinical experience for the psychologists (n = 4) were 1.26 years (SD = 0.5–2.0), and the mean years of clinical experiences for the nurses (n = 3) were 12.0 years (SD = 2.0).
Ethical considerations
The study was approved by the Regional Committees of Medical and Health Research Ethics in Mid-Norway and by the Norwegian Social Science Data Services.
Statistical analyses
The objective of the statistical analyses was to compare outcomes between the treatment group and the waiting list group, and thereafter to explore the effect on the total sample at posttreatment and at 12-month follow-up. The main statistical analyses of the outcome data were based on both intention to treat and treatment completers. Pretreatment group differences between dropouts and completers and between the treatment group and the waiting list group were tested by independent t-tests and chi-squared tests. To explore the differences between the treatment group and the waiting list group from pre- to posttreatment, analysis of covariance (ANCOVA) were computed at posttreatment using pretreatment scores for the corresponding dependent variables and the summary score for BPRS as covariates.
Outcome data from the total sample of CBTp were calculated in several ways. Mean scores were used in the analysis of variance (ANOVA) with repeated measures (full factorial model) using pretreatment scores as reference point. Subsequently, scores at posttreatment and follow-up were compared with pretreatment scores (simple contrast for repeated measures).
The power to detect a group difference of d = 0.050 with N = 32 was low. In order to balance the risk of making Type I and Type II errors by having a small sample size and several outcome measures a significance level of .05 was chosen. Uncontrolled effect sizes (ESs) were calculated for each measurement at posttreatment and 12-month follow-up for the total sample, using Cohen's formula (Cohen, 1992). ESs are calculated by subtracting mean scores at posttreatment and follow-up from the pretreatment scores and thereafter dividing by the pooled standard deviation (Cohen, 1988). In our study, inter-rater reliability was assessed using BPRS. Two videos from the assessment were randomly selected and scored independently by the raters in the study. The intraclass correlation coefficient for the first video was 0.82, and 0.94 for the second video. Missing data were given the Last Observation Carried Forward (LOCF).
Results
The demographic and clinical characteristics of the patients in the treatment group and the waiting list group are listed in Table 1. Independent t-tests indicated no group differences with respect to age, age at first time contact with the health service, or age at first hospitalization. Moreover, chi-square analyses revealed no differences between the two groups in terms of sex or diagnostic status.
Table 1.
Patients’ age, sex, diagnoses, and contact with the health service
| Waiting list group (n = 22) | Treatment group (n = 23) | Total (n = 55) | |
|---|---|---|---|
| Age (M, SD) | 37.50 (11.15) | 35.26 (8.89) | 36.36 (10.0) |
| Sex (n) | |||
| Females | 8 | 8 | 16 |
| Males | 14 | 15 | 29 |
| Diagnosis (n) | |||
| Schizophrenia | 18 | 16 | 34 |
| Delusional disorder | 3 | 6 | 9 |
| Schizo-affective disorder | 1 | 1 | 2 |
| Age at first-time contact with the health service | 21.38 (6.22) | 22.09 (7.69) | 21.75 (6.95) |
| Age at first hospitalization | 23.9 (5.45) | 27.0 (6.64) | 25.49 (6.21) |
Intention-to-treat analyses
One-way analysis of covariance indicated that patients in the treatment group exhibited significantly lower levels of cognitive and emotional delusions as assessed on the PSYRATS at post-treatment compared to patients in the waiting list group. No other significant group differences were found between the two groups at posttreatment related to the intention-to-treat analyses (Table 2).
Table 2.
ANCOVAs for waiting list group compared to treatment group (intention to treat)
| Waiting list group | Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||||||
| Instrument: | N | M | SD | M | SD | N | M | SD | M | SD | F | p |
| BPRS | ||||||||||||
| Overall | 22 | 52.62 | 10.54 | 52.39 | 9.32 | 23 | 46.35 | 8.63 | 44.07 | 9.57 | 3.43 | .07 |
| SANS | ||||||||||||
| Global | 22 | 8.09 | 4.26 | 8.14 | 3.54 | 23 | 6.48 | 3.00 | 6.30 | 3.32 | .75 | .38 |
| GAF | ||||||||||||
| Symptom | 22 | 35.73 | 6.14 | 38.36 | 4.78 | 23 | 37.52 | 5.66 | 42.70 | 8.62 | 2.63 | .11 |
| Function | 22 | 37.68 | 8.28 | 39.55 | 7.21 | 23 | 42.57 | 8.25 | 44.09 | 9.85 | 1.46 | .23 |
| PSYRATS AH | ||||||||||||
| Physical | 22 | 7.95 | 5.51 | 6.14 | 4.64 | 23 | 6.17 | 4.90 | 6.04 | 5.30 | 2.12 | .15 |
| Emotional | 22 | 9.05 | 5.99 | 7.32 | 5.61 | 23 | 5.65 | 5.57 | 5.52 | 5.73 | .41 | .53 |
| Cognitive | 22 | 5.50 | 3.69 | 5.32 | 4.02 | 23 | 4.30 | 3.66 | 4.13 | 4.05 | .13 | .72 |
| PSYRATS D | ||||||||||||
| Cognitive | 22 | 9.00 | 5.45 | 8.32 | 4.87 | 22 | 6.36 | 4.97 | 3.91 | 5.20 | 7.01 | .01 |
| Emotional | 22 | 3.41 | 2.72 | 3.86 | 2.42 | 23 | 3.13 | 3.17 | 1.35 | 2.42 | 10.91 | .00 |
Treatment completers
The treatment group was compared with the waiting list group after 6 months: seven patients in the treatment group dropped out during the active treatment phase, but no patients in the waiting list group dropped out in the period when they were waiting for CBTp + TAU. The difference in dropout rate was statistically significant between the groups. Treatment completers in the treatment group did not differ from the waiting list group at pretest on any of the outcome measures, except BPRS.
The ANCOVA indicated that, compared to the waiting list group, the treatment group exhibited significantly lower scores on the subscales cognitive and emotional delusions as assessed on the PSYRATS at posttest. Further results showed that, compared to patients in the waiting list group, the treatment group also exhibited significantly less psychiatric symptoms at posttest as measured on the BPRS and a significant increase on the GAF symptom scale (Table 3).
Table 3.
ANCOVAs for waiting list group compared to treatment group (treatment completers)
| Waiting list group | Treatment group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||||||
| Instrument: | N | M | SD | M | SD | N | M | SD | M | SD | F | p |
| BPRS | ||||||||||||
| Overall | 22 | 52.62 | 10.54 | 52.39 | 9.32 | 16 | 44.87 | 8.83 | 41.73 | 9.60 | 5.25 | .03 |
| SANS | ||||||||||||
| Global | 22 | 8.09 | 4.26 | 8.14 | 3.54 | 16 | 5.75 | 3.09 | 5.50 | 3.48 | 1.44 | .24 |
| GAF | ||||||||||||
| Symptom | 22 | 35.73 | 6.14 | 38.36 | 4.78 | 16 | 36.81 | 5.91 | 44.25* | 9.49 | 4.82 | .04 |
| Function | 22 | 37.68 | 8.28 | 39.55 | 7.21 | 16 | 44.50 | 9.02 | 46.69 | 10.65 | 3.68 | .06 |
| PSYRATS AH | ||||||||||||
| Physical | 22 | 7.95 | 5.51 | 6.14 | 4.64 | 16 | 6.75 | 4.96 | 6.56 | 5.55 | 0.94 | .34 |
| Emotional | 22 | 9.05 | 5.99 | 7.32 | 5.61 | 15 | 6.44 | 5.25 | 6.00 | 5.62 | 0.11 | .74 |
| Cognitive | 22 | 5.50 | 3.69 | 5.32 | 4.02 | 16 | 4.69 | 3.65 | 4.10 | 4.13 | 0.26 | .61 |
| PSYRATS D | ||||||||||||
| Cognitive | 20 | 9.00 | 5.45 | 8.15 | 5.08 | 16 | 5.19 | 4.52 | 1.63 | 3.50 | 16.33 | .00 |
| Emotional | 20 | 3.41 | 2.72 | 3.75 | 2.49 | 16 | 2.88 | 3.22 | 0.31 | 0.87 | 23.14 | .00 |
Effect of CBTp related to the total sample
Mean scores for the different measures on the total sample were used in ANOVAs with repeated measures (full factorial model) to assess the effect of the CBT on the whole sample. The pretreatment scores served as a reference point and were compared to the scores at posttreatment and 12-month follow-up. The results are shown in Table 4.
Table 4.
ANOVA with repeated measures within subjects at posttreatment and 12-month follow-up (the total sample)
| Instrument | Assessment | N | M | SD | df | F |
|---|---|---|---|---|---|---|
| BPRS overall | Pre | 27 | 50.06 | 9.67 | ||
| Post | 27 | 44.91 | 9.36 | (1,26) | 11.77** | |
| 12-month | 27 | 46.36 | 11.02 | (1,26) | 3.72 | |
| SANS global | Pre | 27 | 7.48 | 3.59 | ||
| Post | 27 | 6.19 | 3.41 | (1,26) | 3.21 | |
| 12-month | 27 | 7.59 | 3.63 | (1,26) | 0.03 | |
| GAF symptom | Pre | 26 | 36.62 | 4.89 | ||
| Post | 26 | 42.92 | 8.48 | (1,25) | 17.32*** | |
| 12-month | 26 | 41.42 | 6.96 | (1,25) | 9.50** | |
| GAF function | Pre | 26 | 42.23 | 9.47 | ||
| Post | 26 | 44.58 | 9.57 | (1,25) | 1.13 | |
| 12-month | 26 | 45.88 | 9.99 | (1,25) | 3.06 | |
| PSYRATS AH | ||||||
| Physical | Pre | 28 | 5.82 | 4.71 | ||
| Post | 28 | 5.21 | 4.92 | (1,27) | 2.31 | |
| 12-month | 28 | 5.44 | 4.82 | (1,27) | 0.43 | |
| Emotional | Pre | 28 | 6.61 | 5.51 | ||
| Post | 28 | 5.21 | 5.13 | (1,27) | 6.53* | |
| 12-month | 28 | 5.54 | 5.36 | (1,27) | 2.58 | |
| Cognitive | Pre | 28 | 4.84 | 3.99 | ||
| Post | 28 | 4.05 | 3.96 | (1,27) | 2.31 | |
| 12-month | 28 | 4.25 | 3.82 | (1,27) | 1.38 | |
| PSYRATS D | ||||||
| Cognitive | Pre | 26 | 6.85 | 5.00 | (1,25) | |
| Post | 26 | 4.38 | 4.67 | (1,25) | 8.41** | |
| 12-month | 26 | 5.50 | 4.70 | (1,25) | 2.62 | |
| Emotional | Pre | 26 | 3.27 | 2.88 | ||
| Post | 26 | 1.85 | 2.34 | (1,25) | 5.10* | |
| 12-month | 26 | 2.58 | 2.66 | (1,25) | 1.63 |
Notes: *** = Significant level 0.001; ** = Significant level 0.01; * = Significant level 0.05.
Scores on the BPRS, the factor related to emotions of auditory hallucinations on the PSYRATS, and both of the factors related to the delusions subscale of the PSYRATS had a significant reduction from pre- to posttreatment. The scores on the GAF symptom scale had a significant increase from pre-assessment to post-assessment (higher scores indicating a decrease in symptoms). At follow-up 12 months later, only the scores on the GAF symptom scale were still significant.
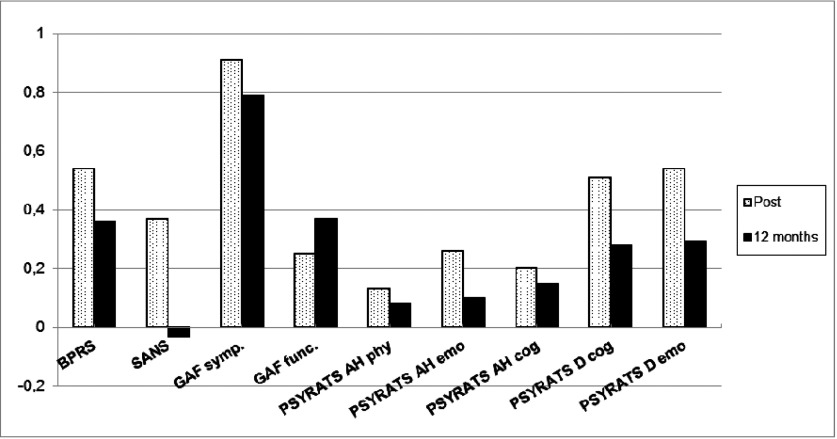
All measurements showed an increase in ES from pretreatment scores to posttreatment, where the BPRS, the GAF symptom scale, and the factors related to the delusions subscale on the PSYRATS had the largest effect sizes. The ES on all measurements decreased from posttreatment to 12-month follow-up (see Figure 2).
Figure 2.
Uncontrolled effect sizes (Cohen's d) for CBTp (total sample) at posttreatment and 12-month follow-up. Notes: Significant level 0.001; GAF symptom at posttreatment; Significant level 0.01: BPRS, PSYRATS D (cognitive) at posttreatment, GAF symptoms at 12-month follow-up; Significant level 0.05: PSYRATS AH (emotional), PSYRATS D (emotional) at posttreatment.
Discussion
The results showed that patients in the treatment group receiving 20 sessions CBTp + TAU performed significantly better than the waiting list group receiving TAU with respect to the global score on the BPRS, the delusional scale on the PSYRATS, and the GAF symptom score. Only the GAF symptom score remained significantly changed at 12-month follow-up for the total sample. The treatment gains for the total sample at follow-up were limited. It is well known that most psychotic disorders are chronic and fluctuate in symptom severity over time. Our results suggest that adding CBTp to TAU has a short-lived effect. Further studies are needed to assess to what extent more long-term CBTp or adding CBT booster sessions may lead to more lasting treatment effects. The effect sizes calculated for the CBTp in the study could be considered from moderate to large on psychotic symptoms (except for auditory hallucinations) at posttreatment. With the exception of the GAF-symptoms score, these dropped to a smaller range at 12-month follow-up.
Compared to other effect studies and meta-analyses (see NICE, 2009), our study could not replicate reported favourable outcomes for auditory hallucinations and negative symptoms. However, the study showed the same trend of success reported by the National Institute for Clinical Excellence (NICE; 2009) in treating general psychiatric symptoms and delusions. When compared to other effectiveness studies of CBTp, our study had more favourable results than those conducted by Farhall et al. (2011) and Peters et al. (2010), and we also replicated the positive findings from the studies by Morrison et al. (2004) and Durham et al. (2003). Hence, the results show that CBTp delivered by non-experts in routine clinical settings can produce improvements in positive psychotic symptoms, and that some of these improvements can be maintained at one-year follow-up.
Only the difference between CBTp + TAU and TAU was examined in the study. Recent reviews have revealed no significant difference in treatment outcomes between CBTp and less complex active treatments (Jones, Hacker, Cormac, Meaden and Irving, 2012). Hence, it is not possible to determine whether the superior effect of CBTp + TAU was due to treatment-specific ingredients in CBTp or merely to simpler, non-specific attentional effects, which could have been demonstrated with a less complex treatment alternative. Moreover, although including TAU in a treatment condition is clinically relevant, it makes it difficult to assess and control for what kind of treatment patients actually received.
The study showed that the therapy was not effective for voices. One possible explanation may be that the therapy was not tailored specifically for patients suffering from auditory hallucinations. By focusing primarily on cognitive restructuring and beliefs about the power of the voices and the lack of focus on the relationship between the voice and the voice-hearer (Hayward, Berry and Ashton, 2011; Paulik, 2011) might have resulted in the loss of a possible necessary factor for change. Another possible explanation may be that there was insufficient focus on replacing maladaptive coping strategies with more adaptive coping strategies (Turkington et al., 2008).
The study had some limitations. Most importantly, the high rate of dropouts could have influenced our results, even though such a rate is quite common in clinical trials exploring the effect of CBTp. A further weakness is that the assessors were not completely blind to the allocation of CBTp and the waiting list condition at posttreatment. This could have influenced their ratings, although there were small changes during the waiting list period. Further, the statistical power to detect a significant difference between groups was low. The risk of making Type II errors was therefore rather high. On the other hand, the study included several outcome variables increasing the risk of making Type I errors. The treatment results should thus be treated with caution, and replication using a larger sample size is recommended.
This study supports the findings from other effectiveness studies that suggest that if therapists in routine clinical care receive adequate training and some supervision in performing CBTp, results from efficacy studies could be replicated in ordinary clinical settings. More research is therefore warranted, both to determine the level of qualifications required to deliver CBTp in an efficacious way and to explore whether CBTp should be offered as a component of routine care for patients with psychotic disorders (Morrison et al., 2004).
Acknowledgements
We thank all participants who took part in the study, and all members of staff who helped in the recruitment. We also thank Kyrre Svarva at the Norwegian University of Science and Technology (NTNU) for statistical assistance. The study was supported by the Department of Research and Development at St Olavs University Hospital, Trondheim, Norway.
References
- Andreasen N. C. (1989). The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundation. British Journal of Psychiatry, 155(suppl. 7), 49–52 [PubMed] [Google Scholar]
- Beck A. T. (2004). A cognitive model of schizophrenia. Journal of Cognitive Psychotherapy, 18, 281–288 [Google Scholar]
- Beck A. T. and Rector N. A. (2002). Delusions: a cognitive perspective. Journal of Cognitive Psychotherapy, 16, 455–468 [Google Scholar]
- Beck A. T. and Rector N. A. (2003). A cognitive model of hallucinations. Cognitive Therapy and Research, 27, 19–52 [Google Scholar]
- Chadwick P., Birchwood M. and Trower P. (1996). Cognitive Therapy of Voices, Delusions and Paranoia., Chichester, UK: Wiley [Google Scholar]
- Cohen J. (1988). Statistical Power Analysis for the Behavioural Sciences (2nd ed.). Hillsdale: NJ: Lawrence Erlbaum Associates [Google Scholar]
- Cohen J. (1992). A power primer. Psychological Bulletin, 112, 155–159 [DOI] [PubMed] [Google Scholar]
- Dickerson F. B. (2004). Update on cognitive behavorial psychotherapy for schizophrenia: review of recent studies. Journal of Cognitive Psychotherapy, 18, 189–203 [Google Scholar]
- Durham R. C. R., Guthrie M. M., Morton R. V. M., Reid D. A. D., Treliving L. R. L., Fowler D. D., et al. (2003). Tayside-Fife clinical trial of cognitive-behavioural therapy for medication-resistant psychotic symptoms: results to 3-month follow-up. The British Journal of Psychiatry, 182, 303–311 [DOI] [PubMed] [Google Scholar]
- Endicott J., Spitzer R. L., Fleiss J. L. and Cohen J. (1979). The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry, 33, 766–771 [DOI] [PubMed] [Google Scholar]
- Farhall J. J., Freeman N. C. N., Shawyer F. F. and Trauer T. F. (2009). An effectiveness trial of cognitive behaviour therapy in a representative sample of outpatients with psychosis. The British Journal of Clinical Psychology, 48, 47–62 [DOI] [PubMed] [Google Scholar]
- Gaudiano B. A. (2005). Cognitive behavior therapies for psychotic disorders: current empirical status and future directions. Clinical Psychology: Science and Practice, 12, 33–50 [Google Scholar]
- Haddock G., McCarron J., Tarrier N. and Faragher E. B. (1999). Scales to measure dimensions of hallucinations and delusions: the Psychotic Symptom Rating Scales (PSYRATS). Psychological Medicine, 29, 879–889 [DOI] [PubMed] [Google Scholar]
- Hayward M., Berry K. and Ashton A. (2011). Applying interpersonal theories to the understanding of and therapy for auditory hallucinations: a review of the literature and directions for further research. Clinical Psychology Review, 31, 1313–1323 [DOI] [PubMed] [Google Scholar]
- Jones C., Cormac I., Silveira D. M. N. J. and Campbell C. (2010). Cognitive behaviour therapy for schizophrenia (Review). The Cochrane Library [DOI] [PubMed]
- Jones C., Hacker D., Cormac I., Meaden A. and Irving C. B. (2012). Cognitive behaviour therapy versus other psychosocial treatments for schizophrenia (Review). The Cochrane Library, 1–155. [DOI] [PMC free article] [PubMed]
- Jones S. H., Thornicroft G., Coffey M. and Dunn G. (1995). A brief mental health outcome scale: reliability and validity of the Global Assessment of Functioning (GAF). British Journal of Psychiatry, 166, 654–659 [DOI] [PubMed] [Google Scholar]
- Morrison A. P., Renton J. C., Williams S., Dunn H., Knight A., Kreutz M., et al. (2004). Delivering cognitive therapy to people with psychosis in a community mental health setting: an effectiveness study. Acta Psychiatrica Scandinavica, 110, 36–44 [DOI] [PubMed] [Google Scholar]
- Mueser K. T. and Berenbaum H. (1990). Psychodynamic treatment of schizophrenia: is there a future? Psychological Medicine, 20, 253–262 [DOI] [PubMed] [Google Scholar]
- NICE (2009). Schizophrenia: core interventions in the treatment and management of schizophrenia in adults in primary and secondary care. London: National Institute for Clinical Excellence [Google Scholar]
- Paulik G. (2011). The role of social schema in the experience of auditory hallucinations: a systematic review and a proposal for the inclusion of social schema in a cognitive behavioural model of voice hearing. Clinical Psychology and Psychotherapy, 19, 459–472 [DOI] [PubMed] [Google Scholar]
- Peters E., Landau S., McCrone P., Cooke M., Fisher P., Steel C., et al. (2010). A randomised controlled trial of cognitive behaviour therapy for psychosis in a routine clinical service. Acta Psychiatrica Scandinavica, 122, 302–318 [DOI] [PubMed] [Google Scholar]
- Pfammatter M., Junghan U. M. and Brenner H. D. (2006). Efficacy of psychological therapy in schizophrenia: conclusions from meta-analyses. Schizophrenia Bulletin, 32, 64–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling S., Bebbington P., Kuipers E., Garety P., Geddes J., Orbach G., et al. (2002). Psychological treatments in schizophrenia: I. meta-analysis of family intervention and cognitive behaviour therapy. Psychological Medicine, 32, 763–782 [DOI] [PubMed] [Google Scholar]
- Rathod S. S., Kingdon D. D., Weiden P. P. and Turkington D. D. (2008). Cognitive-behavioral therapy for medication-resistant schizophrenia: a review. Journal of Psychiatric Practice, 14, 22–33 [DOI] [PubMed] [Google Scholar]
- Rector N. A. and Beck A. T. (2001). Cognitive behavioral therapy for schizophrenia: an empirical review. Journal of Nervous and Mental Disease, 189, 278–287 [DOI] [PubMed] [Google Scholar]
- Rector N. A., Beck A. T. and Stolar N. (2005). The negative symptoms of schizophrenia: a cognitive perspective. Canadian Journal of Psychiatry, 50, 247–257 [DOI] [PubMed] [Google Scholar]
- Tarrier N. (2006). Cognitive behavior therapy for psychotic disorders: current issues and future developments. Clinical Psychology: Science and Practice, 12, 51–56 [Google Scholar]
- Turkington D., Kingdon D. and Turner T. (2002). Effectiveness of a brief cognitive-behavioural therapy intervention in the treatment of schizophrenia. British Journal of Psychiatry, 180, 523–527 [DOI] [PubMed] [Google Scholar]
- Turkington D., Sensky T., Scott J., Barnes T. R., Nur U., Siddle R., et al. (2008). A randomized controlled trial of cognitive-behavior therapy for persistent symptoms in schizophrenia: a five-year follow-up. Schizophrenia Research, 98, 1–7 [DOI] [PubMed] [Google Scholar]
- Valmaggia L. R., Van Der Gaag M., Tarrier N., Pijnenborg M. and Slooff C. J. (2005). Cognitive-behavioural therapy for refractory psychotic symptoms of schizophrenia resistant to atypical antipsychotic medication: randomised controlled trial. British Journal of Psychiatry, 186, 324–330 [DOI] [PubMed] [Google Scholar]
- Ventura J., Green M. F., Shaner A. and Liberman R. P. (1993). Training and quality assurance with the Brief Psychiatric Rating Scale: “The Drift Busters”. International Journal of Methods in Psychiatric Research, 3, 221–244 [Google Scholar]
- WHO (1992). The ICD-10 Classification of Mental and Behavioral Disorder: clinical descriptions and diagnosis guidelines. Geneva: World Health Organization [Google Scholar]
- Wykes T., Steel C., Everitt B. and Tarrier N. (2008). Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophrenia Bulletin, 34, 523–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G., Favrod J., Trieu V. H. and Pomini V. (2005). The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta-analysis. Schizophrenia Research, 77, 1–9 [DOI] [PubMed] [Google Scholar]