Abstract
In the past five decades or so evidence has been accumulating about an environmental factor, which appears to be influencing mortality, in particular, cardiovascular mortality, and this is the hardness of the drinking water. In addition, several epidemiological investigations have demonstrated the relation between risk for cardiovascular disease, growth retardation, reproductive failure, and other health problems and hardness of drinking water or its content of magnesium and calcium. In addition, the acidity of the water influences the reabsorption of calcium and magnesium in the renal tubule. Not only, calcium and magnesium, but other constituents also affect different health aspects. Thus, the present review attempts to explore the health effects of hard water and its constituents.
Keywords: Alzheimer's disease, calcium, cancer, cardiovascular disease, diabetes, hard water, magnesium, reproductive health
INTRODUCTION
Water is essential for hydration and therefore, for life. It is also very important in food preparation and cooking, sanitation and hygiene, and a wide range of other uses. The drinking water supply has a primary objective of protecting human health, including ensuring access to adequate quantities of safe water. It is estimated that approximately 17% of the world's population uses water from the unprotected and remote sources, 32% from some form of protected sources and 51% from some sort of centralized (piped) system to the dwelling or a plot. Of the latter, a small but increasing proportion applies some form of treatment within the home. Individual water consumption occurs both at home and elsewhere, such as at schools and workplaces. Drinking-water is consumed not only as water per se but also in beverages and incorporated in food-stuffs. In response to increasing global and local water scarcity, there is an increasing use of sources such as recovered/recycled water, harvested rainwater, and desalinated water. 884 million people lack access to safe water supplies; approximately one in eight people.[1] Among them a good percentage consumes hard water, which is considered to be a significant etiological factor around the globe causing many diseases such as cardiovascular problems, diabetes, reproductive failure, neural diseases, and renal dysfunction and so on.
Hard water is usually defined as water, which contains a high concentration of calcium and magnesium ions. However, hardness can be caused by several other dissolved metals; those forms divalent or multivalent cations, including aluminum, barium, strontium, iron, zinc, and manganese. Normally, monovalent ions such as sodium and potassium do not cause hardness. These divalent cations have a propensity to come together with anions in the water to form stable salts. The type of anion found in these salts distinguishes between the two types of hardness-carbonate and non-carbonate hardness [Table 1].
Table 1.
Carbonate and non-carbonate hardness compounds
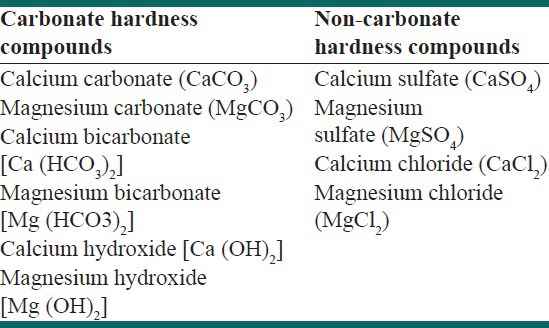
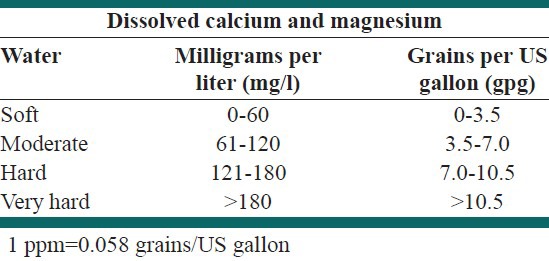
Carbonate hardness is caused by the metals combined with a form of alkalinity. Alkalinity is the capacity of water to neutralize acids and is attributed to compounds such as carbonate, bicarbonate, hydroxide, and sometimes borate, silicate, and phosphate. In contrast, non-carbonate hardness forms when metals combine with anything other than alkalinity. Carbonate hardness is sometimes called temporary hardness because it can be removed by boiling water. Non-carbonate hardness cannot be broken down by boiling the water, so it is also known as permanent hardness. In general, it is necessary to distinguish between the two types of hardness because the removal method differs for the two. Total hardness includes both temporary and permanent hardness caused by the calcium and magnesium, on the basis of which water is categorized as soft or hard and very hard [Table 2]. The ratio of calcium and magnesium in water is also a crucial factor indicating the hardness and in the causation of several hard water health problems. Hardness generally enters groundwater as the water percolates through minerals containing calcium or magnesium. The most common sources of hardness are limestone (which introduces calcium into the water) and dolomite (which introduces magnesium.) Since, hardness enters the water in this manner groundwater generally has a greater hardness than surface water.[2]
Table 2.
Concentrations of dissolved calcium and magnesium in soft and hard water
POTENTIAL HEALTH EFFECTS
Hard water has no known adverse health effect, WHO says at its Geneva Conference. In addition, hard water, particularly very hard water, could provide an important supplementary contribution to total calcium and magnesium intake.[3] The health effects of hard water are mainly due to the effects of the salts dissolved in it, primarily calcium and magnesium. To a large extent, individuals are protected from excess intakes of calcium by a tightly regulated intestinal absorption mechanism through the action of 1, 25-dihydroxy-vitamin D, the hormonally active form of vitamin D. Although, calcium can interact with iron, zinc, magnesium, and phosphorus within the intestine, thereby reducing the absorption of these minerals. On the other hand, the major cause of hypermagnesemia is renal insufficiency associated with a significantly decreased ability to excrete magnesium.[4] Increased intake of magnesium salts may cause a change in bowel habits (diarrhea). Drinking-water in which both magnesium and sulfate are present in high concentrations (~250 mg/l each) can have a laxative effect. Laxative effects have also been associated with excess intake of magnesium taken in the form of supplements, but not with magnesium in the diet.
Cardiovascular disease
In most large-scale studies, an inverse relationship between the hardness of drinking-water and cardiovascular disease has been reported.[5,6,7,8,9,10,11] However, no such association has been found in some other studies,[12,13] particularly in those involving small geographical areas a clear association is often not found.[14] The extent to which confounding variables, such as climatic, socioeconomic, or major risk factors, may account for the inverse relationship is unclear. Nevertheless, in a number of studies, a weak inverse relationship was reported after allowance was made for climatic and socioeconomic factors[15] and after major risk factors such as hypertension, smoking habits, and elevated serum lipids were taken into account.[16,17] An inverse relationship between hardness and cardiovascular disease has been reported in men after allowing for climatic and certain social factors, but only up to about 170 mg of calcium carbonate/l.[18] A variety of hypotheses have been proposed to explain the possible inverse association.[19,20,21,22,23,24] However, none has been fully substantiated, nor has a particular element been found to be conclusively associated with the cardiovascular disease. It may be correlated with a high level of magnesium in hard water, which has some anti-stress actions against coronary heart disease. In a study of regional differences in mortality in cardiovascular diseases in 76 municipalities in mid-Sweden a considerable gradient was found between the western areas with a high mortality and soft water and the eastern areas with a low mortality and hard water. The hardness of water defined as the sum of the content of calcium and magnesium, was shown to be of considerable influence of the differences in mortality compared to major risk factors.[16] The incidence of coronary heart disease varies widely in different geographical regions over the world and serious epidemiological studies have been carried out to identify variables that could explain this fact. The role of water hardness has been widely investigated and evaluated for many years in several studies where regional differences in cardiovascular disease have been discussed.[16,25,26,27,28,29] Earlier studies have found positive correlations between water and dietary magnesium and calcium and blood pressure.[30,31,32,33] In Finland and South Africa it was found that the incidence of death ascribed to ischemic heart disease is inversely correlated with the concentration of magnesium in drinking water[29] and also in a Swedish case control study magnesium, and calcium in drinking water were associated with a lower mortality from acute myocardial infarction in women but not with the total incidence.[26,34] However, other studies could not confirm these findings,[31,32] conversely, in a study of magnesium in drinking water supplies and mortality from acute myocardial infarction in North-West England, there was likewise evidence of an association between magnesium and cardiovascular mortality.[25] In a Swedish study, the skeletal muscle magnesium levels were a significantly higher in persons living in an area with a higher water magnesium.[35] The concentration of magnesium in striated muscle has been used as a marker to evaluate the ion content in the soft tissue.
Cancer
Important findings in this field were provided recently by Taiwanese scientists. In most of their studies, the authors indicated a negative statistical association of various types of cancer morbidity/mortality with the hardness of water and calcium. In a review of these publications, it is worth noting the results concerning the possible association between the risk of gastric cancer and the levels of calcium and magnesium.[36] Some studies suggest there was a significant protective effect of calcium intake from drinking water on the risk of gastric cancer. Magnesium also exerted a protective effect against gastric cancer, but only for the group with the highest levels of magnesium exposure.[37] In another matched case-control study, the authors found a possible association between the risk of colon cancer and hardness levels in drinking water from municipal supplies in Japan (obtained trend analyses showed an increasing odd ratio for the cancer with decreasing hardness in drinking water).[38,39] Similar epidemiological trends were also achieved for the relations between hardness levels in drinking water, and the risk of rectal cancer and pancreatic cancer mortality, however, the researchers did not find any association with magnesium levels (the adjusted odds ratios were not statistically significant for the relationship between magnesium concentrations in drinking water and rectal cancer).[40] One of the strongest epidemiological evidences of significant protective effect of magnesium intake from the drinking water was that gave for the risk for esophageal cancer and ovarian cancer.[41,42] Unfortunately, these authors did not find any results pertaining to the similar trend between drinking-water magnesium and liver cancer. The first strong evidence concerning the possible ecological relation between exposure to water magnesium and hepatic cancer was reported in Eastern Europe.[43]
Cerebrovascular mortality
Some reports suggest there is a significant protective effect of magnesium intake from the drinking water on the risk of cerebrovascular disease.[44] Despite their inherent limitations, studies on the ecological correlation between mortality and environmental exposures have been used widely to generate or discredit epidemiological hypotheses. Dietary calcium is the main source of calcium intake. Epidemiological studies have shown that dietary calcium is inversely associated with the blood pressure. With much of the epidemiological literature suggesting a relationship between the dietary calcium and blood pressure, it would seem reasonable to expect that intake of dietary calcium could reduce the risk of cardiovascular events, such as stroke that are commonly associated with hypertension.[45] However, controlling for magnesium levels eliminates the perceived effect of calcium levels on cerebrovascular mortality. In the general population, the major proportion of magnesium intake is through food, and a smaller proportion is through drinking water. For individuals with the borderline magnesium deficiency, waterborne magnesium can make an important contribution to their total intake. In addition, the loss of magnesium from food is lower when the food is cooked in magnesium-rich water. Magnesium in water can also play a critical role because of its high bioavailability. Magnesium in water appears as hydrated ions, which are more easily absorbed than magnesium in food. The contribution of water magnesium among persons who drink water with a high magnesium levels could be crucial in the prevention of magnesium deficiency. The significant association between mortality from the cerebrovascular disease and the levels of magnesium in drinking water is supported by knowledge of the functions of magnesium. Magnesium is an enzyme (Na+/K+ ATPase) activator and regulates cellular energy metabolism, vascular tone, and the cell membrane ion transport. A lack of magnesium leads to a decrease in the concentration of intracellular potassium and an increase in calcium levels. Magnesium deficiency may increase the contractility of blood vessels. Magnesium causes vasodilation by stimulation of endothelial prostacyclin release and in vivo, prevents vasoconstriction of the intracranial vessels after experimental subarachnoid hemorrhage. In addition, fear of cerebrovascular disease should not deter anyone from drinking water with a low magnesium levels. In conclusion, the results of the present study show that there is a significant protective effect of magnesium intake from drinking water on the risk of cerebrovascular disease. This is an important finding for the Taiwan water industry and human health risk assessment.[46]
Malformations of central nervous system
There is good evidence that environmental influences must play some part, possibly a major part, in the etiology of neural tube malformations in the human embryo. Almost all that evidence, however, relates to non-specific and uncertain markers of as yet unidentified specific teratogens. For example, the frequency of malformations of the central nervous system varies greatly from country to country.[47,48] It also varies from area to area within countries: In the United States for the period 1950-59, mortality from spina bifida (myelomeningocele) was 2-3 times greater on the Atlantic coast than on the Pacific coast;[49] in South Wales the frequency of central nervous system malformations in the coal-mining valleys is almost twice as high as in the coastal plain;[50] in England and Wales as a whole the frequency is highest in the north, the north-west, and Wales and lowest in the East, Southeast, and South.[51,52] It is higher in first-born than in later born infants and in infants born to younger and older mothers than to mothers in mid-reproductive life.[51,53] It is higher among infants born in the poorer than in the well-to-do strata of society.[54,55,56] It tends to be higher among winter than among summer births.[51,53,55,57,58,59] Striking secular swings in frequency have been reported in the Birmingham, Scotland, Dublin, and Boston. 70-71 Penrose[48] seems to have been the first to speculate “the geographical variations observed in the incidence (of anencephalus) might suggest a… causal agent, such as the presence or absence of trace elements in the water supply.” This suggestion has been taken up by Fedrick.[60] She related data on anencephalus for 10 different areas in the United Kingdom (from 10 different studies and relating to 10 different time-periods) to information about the water supplies of those areas obtained from various sources. Despite such manifestly unsatisfactory data, she found that the frequency of anencephalus was a significantly related to measurements of the total hardness, calcium content, and pH of the local water supplies. Stocks[52] examined the mean annual death rates (still-births plus infant deaths) for congenital malformations in the 15 hospital regions of England and Wales. Mortality rates were highest in the north and the west and lowest in the Southeast. Having observed that mortality from cardiovascular disease followed much the same regional pattern, he proceeded to correlate death rates from congenital malformations in the 15 regions with death rates of women aged 25-54 from the certain causes in the corresponding regions. He found that mortality from malformations of the neural tube correlated very closely with mortality from the cardiovascular diseases, whereas other malformations produced insignificant negative correlations. He concluded that because mortality from cardiovascular disease in the county boroughs of England and Wales has been shown to be strongly an associated with softness of their water supplies,[61] a water factor might be responsible for the regional variations of mortality from the central nervous system malformations. In this paper, and against this background, we relate area differences in mortality from malformations of the central nervous system in South Wales to estimates of the hardness of the water supplies in these areas. We also present new data on perinatal mortality from anencephalus in the county boroughs of England and Wales and relate them to estimates of the hardness of their water supplies.
Alzheimer's disease
The issue of aluminum as a cause for Alzheimer's disease has been contentious. In special circumstances such as renal failure and massive exposure to aluminum in certain occupations aluminum may cause brain pathology similar to Alzheimer Disease. However, there is no definite evidence of the role of this metal in the causation or development of Alzheimer disease. In a survey of 88 county districts within England and Wales, rates of Alzheimer's disease in people under the age of 70 years were estimated from the records of the computerized tomographic (CT) scanning units that served these districts. Rates were adjusted to compensate for differences in the distance from the nearest CT scanning unit and for differences in the size of the population served by the units. Aluminum concentrations in water over the past 10 years were obtained from water authorities and water companies. The risk of Alzheimer's disease was 1.5 times higher in districts where the mean aluminum concentration exceeded 0.11 mg/l than in districts where concentrations were less than 0.01 mg/l.[62]
Diabetes
Hard water is indicative of the presence of higher levels of magnesium. In certain areas, drinking water actually contains 100% or more of the recommended daily allowance about magnesium, which is around 300-400 mg daily with levels varying according to gender and age. Because, all kinases and other ATP-related enzymes and channels regulating insulin action are dependent on magnesium, it is not surprising that serum magnesium concentrations have been found to be decreased in non-diabetic subjects with metabolic syndrome and that hypomagnesaemia is a common feature in subjects with type-2 diabetes. Whether the low intracellular magnesium content is secondary to or precedes insulin resistance is unclear; however, recent evidence suggests that sub-clinical magnesium deficiency may precipitate a diabetic state. Studies are needed to determine the role of sub-clinical magnesium status in diabetes risk. This should include measures of glycosylated hemoglobin, an indicator of glycemic control that has been found to respond to oral magnesium supplementation and to correlate negatively with serum ionized magnesium or serum total magnesium in type 2 diabetics.[63]
Childhood atopic dermatitis
Atopic dermatitis (or eczema) is an inflammatory, chronically relapsing, non-contagious and pruritic skin disorder. The environment plays an important part in the etiology of atopic eczema, but the specific causes are unknown. Exposure to hard water is thought to be a risk factor for eczema. The prevalence of symptoms of atopic eczema among Japanese, Nottinghamshire and Spanish children is the most. The reasons for such a high prevalence are unknown. The study used data on water hardness and chlorine content of the water supply; prevalence of atopic dermatitis diagnosed by physicians and episodes of wheezing reported by the parents; and potential confounding factors by socioeconomic and health-care status per municipality. The prevalence of atopic eczema was significantly higher in the highest water hardness category than that in the lowest respectively. A significant relationship between the chlorine content of the water supply and the prevalence of atopic dermatitis was observed after adjustment for confounding factors. Water hardness may increase the risk of atopic dermatitis among elementary-school children in Japan, as well as in the United Kingdom.[64]
Kidney stones
The hardness of water is due to the presence of carbonate and sulfate salts of calcium and magnesium. More than 3/4th of kidney stones are generally composed of calcium salt and usually occur as calcium oxalate and less commonly as calcium phosphate. The remaining 20% of stones are composed of uric acid, struvite and cystine stone. Stones form in urine that is supersaturated and this saturation is dependent on chemical free ion activity, which makes the urine under-saturated. In this situation, the stone will not grow and may even dissolve. Increased urinary ion excretion and decreased urine volume will both an increase free ion activity and favor stone formation and growth. Formation of kidney stones (nephrolithiasis) is based on genetic, metabolic, nutritional and environmental factors. Metabolic factors involved in stone formation include hypercalciuria, hypocitraturia (due to renal disease), hyperuricosuria, hyperoxalaturia, cystinuria and infections. Environmental and nutritional factors include dehydration, high salt intake, a diet rich in animal proteins and calcium rich diet when oxalate intake is restricted. The impact of water hardness of urinary stone formation remains unclear, despite a weak correlation between water hardness and urinary calcium, magnesium, and citrate excretion. Several studies have shown no association between water hardness and the incidence of urinary stone formation. A correlation between water hardness and urinary calcium, citrate, and magnesium levels has been observed although the significance of this is not known. Some studies suggest that in the preventive approach to calcium nephrolithiasis, intake of soft water has been preferable to hard water since it is associated with a lower risk for recurrence of calcium stones.[65]
Reproductive health
There are few reports of the effect of water hardness over reproductive health of men, most of them emphasized on the effect of its constituents, calcium, and magnesium,[66] while others on some other constituents like fluoride.[67] However, some reports show the occurrence of reproductive failure and stillbirth in India in hard water regions of India.[68,69] Some of these are showing the effect of excess calcium on the reproductive system and its negative influence on fertility.[68,70,71] These reports demonstrated the oxidative stress induced infertility in men by calcium,[72] but showed beneficial effects of magnesium.[4] There are also some reports of effects of waterborne fluoride on growth, reproduction and survival which showed long-term exposure of fluoride causes a progressive decline in reproduction.[67] On the contrary, in female, magnesium sulfate present in hard water is indicated to prevent eclampsia in patients with pre-eclampsia. Magnesium sulfate decreases the risk of developing eclampsia around 50% and also decreases maternal mortality.[73] The WHO considers that magnesium sulfate is the elective drug for the prevention of eclampsia in patients suffering from pre-eclampsia. Magnesium sulfate has also been demonstrated to prevent preterm labor.[74]
Digestive health and constipation
Even GI health is also reported being benefited from hard water since it provides potentially alleviating effects on the onset of constipation in the 85% cases. A rich union of calcium and magnesium in hard water, in a right combination, helps to combat constipation. The calcium in hard water results in teaming up with excess bile and its resident fats to lather up the soap like insoluble substance, which is emitted from the body during bowel movements. Indeed, many renowned scientists have considered hard water as a boon as it has some fantastic health benefits that seem to encourage longer life expectancy and improved health. Magnesium salt represents with a laxative effect. This provides a rapid evacuation of intestine. Magnesium citrate, magnesium phosphate, and magnesium hydroxide are also used. The American Gastroenterology Association recommends milk of magnesia for the management of constipation as one of the therapeutic options; however, the Rehabilitation Nursing Foundation discourages the routine use of saline magnesium laxatives due to possible side effects such as abdominal cramping, watery stools, and potential for dehydration and hypermagnesium. They only indicate the use of these laxatives in end-stage patients when other options have failed, and with and adequate prospective evaluation of magnesium levels.
Bone mineral density
The correlation between calcium and magnesium in drinking water and its impact on bone health are unidentified. There is some evidence that high-calcium water is beneficial to bone.[75] It has been reported in a study that spine mineral density was significantly higher in women aged 30-70 years living in Sangemini, a region of central Italy, who drank the local high-calcium water (318 mg/l), compared with women in the same region who drank low calcium water (<60 mg/l). The estimated difference in calcium intake from an assessment of diet and water was 258 mg/day on average.[76] In an evaluation of calcium ingested from water and hip bone mineral density in French women aged 75 years or older, an increase in calcium of 100 mg/day from drinking-water was found to be associated with a 0.5% increase in femoral bone density).[77]
Other health effects
The results of several studies have suggested that a variety of other diseases are also inversely correlated with the hardness of water, including anencephaly[78,79] and various types of cancer.[80,81,82,83,84,85,86] However, the significance of these results is unclear, and it has been suggested that the associations may reflect disease patterns that can be explained by social, climatological, and environmental factors, rather than by the hardness of the water.
CONCLUSIONS
Hardness is important for drinking-water from the point of view of both aesthetic acceptability and operational considerations. Although, there is some evidence from epidemiological studies for a protective effect of magnesium or hardness on cardiovascular mortality, the evidence is being debated and does not prove causality. Further studies are being conducted. In spite of this, drinking-water may be a source of calcium and magnesium in the diet and could be important for those who are marginal for calcium and magnesium intake. Where drinking-water supplies are supplemented with or replaced by dematerialized water that requires conditioning, consideration should be given to adding calcium and magnesium salts to achieve concentrations similar to those that the population received from the original supply. Consumers should be informed of the mineral composition of their water when it has been altered by piped suppliers or treatment device manufacturers and by means for supplementing if desired. The contribution of drinking-water minerals for mineral nutrition should be considered where changes in supply are proposed or where novel sources, such as seawater or brackish water, are exploited for drinking-water. There are insufficient data to suggest either minimum or maximum concentrations of minerals at this time, and so no guideline values are proposed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.UNICEF/WHO. Progress on Drinking Water and Sanitation: Special Focus on Sanitation. 2008 [Google Scholar]
- 2.Prepas EE, Pinel-Alloul B, Chambers PA, Murphy TP, Reedyk S, Sandland G, et al. Lime treatment and its effects on the chemistry and biota of hardwater eutrophic lakes. Freshw Biol. 2001;46:1049–60. [Google Scholar]
- 3.Galan P, Arnaud MJ, Czernichow S, Delabroise AM, Preziosi P, Bertrais S, et al. Contribution of mineral waters to dietary calcium and magnesium intake in a French adult population. J Am Diet Assoc. 2002;102:1658–62. doi: 10.1016/s0002-8223(02)90353-6. [DOI] [PubMed] [Google Scholar]
- 4.Chandra AK, Sengupta P, Goswami H, Sarkar M. Effects of dietary magnesium on testicular histology, steroidogenesis, spermatogenesis and oxidative stress markers in adult rats. Indian J Exp Biol. 2013;51:37–47. [PubMed] [Google Scholar]
- 5.Anderson TW, Neri LC, Schreiber GB, Talbot FD, Zdrojewski A. Letter: Ischemic heart disease, water hardness and myocardial magnesium. Can Med Assoc J. 1975;113:199–203. [PMC free article] [PubMed] [Google Scholar]
- 6.Masironi R, Pisa Z, Clayton D. Myocardial infarction and water hardness in the WHO myocardial infarction registry network. Bull World Health Organ. 1979;57:291–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Leoni V, Fabiani L, Ticchiarelli L. Water hardness and cardiovascular mortality rate in Abruzzo, Italy. Arch Environ Health. 1985;40:274–8. doi: 10.1080/00039896.1985.10545931. [DOI] [PubMed] [Google Scholar]
- 8.Sengupta P, Chaudhuri P, Bhattacharya K. Screening obesity by direct and derived anthropometric indices with evaluation of physical efficiency among female college students of Kolkata. Annals Med Health Sci Res. doi: 10.4103/2141-9248.122066. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sengupta P, Sahoo S. A cross sectional study to evaluate the fitness pattern among the young fishermen of Coastal Orissa. Indian J Public Health Research and Development. 2013;4:171–175. [Google Scholar]
- 10.Kubis M. Relation of water hardness to the occurrence of acute myocardial infarct. Acta Univ Palacki Olomuc Fac Med. 1985;111:321–4. [PubMed] [Google Scholar]
- 11.Sengupta P. Challenge of infertility: How protective the yoga therapy is? Ancient Sci Life. 2012;32:61–62. doi: 10.4103/0257-7941.113796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackinnon AU, Taylor SH. Relationship between ‘sudden’ coronary deaths and drinking water hardness in five Yorkshire cities and towns. Int J Epidemiol. 1980;9:247–9. doi: 10.1093/ije/9.3.247. [DOI] [PubMed] [Google Scholar]
- 13.Sonneborn M, Mandelkow J, Schon D, Hoffmeister H, Zoeteman BCJ. Health effects of inorganic drinking water constituents, including hardness, iodide and fluoride. CRC Crit Rev Environ Control. 1983;13:1–22. [Google Scholar]
- 14.Meyers DH, Williams G. Mortality from all causes, and from ischaemic heart disease, in the Australian capital cities. Med J Aust. 1977;2:504–5. doi: 10.5694/j.1326-5377.1977.tb117758.x. [DOI] [PubMed] [Google Scholar]
- 15.Pocock SJ, Shaper AG, Cook DG, Packham RF, Lacey RF, Powell P, et al. British Regional Heart Study: Geographic variations in cardiovascular mortality, and the role of water quality. Br Med J. 1980;280:1243–9. doi: 10.1136/bmj.280.6226.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nerbrand C, Svärdsudd K, Ek J, Tibblin G. Cardiovascular mortality and morbidity in seven counties in Sweden in relation to water hardness and geological settings. The project: Myocardial infarction in mid-Sweden. Eur Heart J. 1992;13:721–7. doi: 10.1093/oxfordjournals.eurheartj.a060246. [DOI] [PubMed] [Google Scholar]
- 17.Shaper AG, Pocock SJ, Walker M, Cohen NM, Wale CJ, Thomson AG. British regional heart study: Cardiovascular risk factors in middle-aged men in 24 towns. Br Med J (Clin Res Ed) 1981;283:179–86. doi: 10.1136/bmj.283.6285.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacey RF, Shaper AG. Changes in water hardness and cardiovascular death rates. Int J Epidemiol. 1984;13:18–24. doi: 10.1093/ije/13.1.18. [DOI] [PubMed] [Google Scholar]
- 19.Pomrehn PR. Softened water usage and blood pressure. In: Calabrese EJ, Tuthill RW, Condie L, editors. Advances in modern toxicology-inorganics in drinking water and cardiovascular disease. Princeton, NJ: Princeton Scientific Publishing; 1985. [Google Scholar]
- 20.Marier JR, Neri LC. Quantifying the role of magnesium in the interrelationship between human mortality/morbidity and water hardness. Magnesium. 1985;4:53–9. [PubMed] [Google Scholar]
- 21.Sengupta P, chaudhuri P, Bhattacharya K. A small-scale cross-sectional study for the assessment of cardiorespiratory fitness in relation to body composition and morphometric characters in fishermen of Araku valley, Andhra Pradesh, India. Int J Prev Med. In Press. [PMC free article] [PubMed] [Google Scholar]
- 22.Alexa L. An assessment of minerals in drinking water from the Iasi County and the incidence of cardiovascular disease. Revista de igiena bacteriologie, virusologie, parazitologie, epidemiologie, pneumoftiziologie, Seria bacteriologie, virusologie, parazitologie. Epidemiol. 1988;37:35–43. [Google Scholar]
- 23.Derry CW, Bourne DE, Sayed AR. The relationship between the hardness of treated water and cardiovascular disease mortality in South African urban areas. S Afr Med J. 1990;77:522–4. [PubMed] [Google Scholar]
- 24.Singh RB. Effect of dietary magnesium supplementation in the prevention of coronary heart disease and sudden cardiac death. Magnes Trace Elem. 1990;9:143–51. [PubMed] [Google Scholar]
- 25.Maheswaran R, Morris S, Falconer S, Grossinho A, Perry I, Wakefield J, et al. Magnesium in drinking water supplies and mortality from acute myocardial infarction in north west England. Heart. 1999;82:455–60. doi: 10.1136/hrt.82.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubenowitz E, Axelsson G, Rylander R. Magnesium and calcium in drinking water and death from acute myocardial infarction in women. Epidemiology. 1999;10:31–6. [PubMed] [Google Scholar]
- 27.Reunanen A, Knekt P, Marniemi J, Mäki J, Maatela J, Aromaa A. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr. 1996;50:431–7. [PubMed] [Google Scholar]
- 28.Comstock GW. Water hardness and cardiovascular diseases. Am J Epidemiol. 1979;110:375–400. doi: 10.1093/oxfordjournals.aje.a112823. [DOI] [PubMed] [Google Scholar]
- 29.Luoma H, Aromaa A, Helminen S, Murtomaa H, Kiviluoto L, Punsar S, et al. Risk of myocardial infarction in Finnish men in relation to fluoride, magnesium and calcium concentration in drinking water. Acta Med Scand. 1983;213:171–6. doi: 10.1111/j.0954-6820.1983.tb03712.x. [DOI] [PubMed] [Google Scholar]
- 30.Sauvant MP, Pepin D. Drinking water and cardiovascular disease. Food Chem Toxicol. 2002;40:1311–25. doi: 10.1016/s0278-6915(02)00081-9. [DOI] [PubMed] [Google Scholar]
- 31.Kesteloot H, Joossens JV. Relationship of dietary sodium, potassium, calcium, and magnesium with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension. 1988;12:594–9. doi: 10.1161/01.hyp.12.6.594. [DOI] [PubMed] [Google Scholar]
- 32.Joffres MR, Reed DM, Yano K. Relationship of magnesium intake and other dietary factors to blood pressure: The Honolulu heart study. Am J Clin Nutr. 1987;45:469–75. doi: 10.1093/ajcn/45.2.469. [DOI] [PubMed] [Google Scholar]
- 33.Kesteloot H. Handbook of hypertension. Australia: Elsevier; 1985. Blood pressure, calcium and water-hardness. [Google Scholar]
- 34.Rubenowitz E, Axelsson G, Rylander R. Magnesium in drinking water and death from acute myocardial infarction. Am J Epidemiol. 1996;143:456–62. doi: 10.1093/oxfordjournals.aje.a008765. [DOI] [PubMed] [Google Scholar]
- 35.Dyckner T, Wester PO. Effect of magnesium on blood pressure. Br Med J (Clin Res Ed) 1983;286:1847–9. doi: 10.1136/bmj.286.6381.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang CY. Calcium and magnesium in drinking water and risk of death from cerebrovascular disease. Stroke. 1998;29:411–4. doi: 10.1161/01.str.29.2.411. [DOI] [PubMed] [Google Scholar]
- 37.Kneller RW, McLaughlin JK, Bjelke E, Schuman LM, Blot WJ, Wacholder S, et al. A cohort study of stomach cancer in a high-risk American population. Cancer. 1991;68:672–8. doi: 10.1002/1097-0142(19910801)68:3<672::aid-cncr2820680339>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 38.Kuriki K, Tajima K. The increasing incidence of colorectal cancer and the preventive strategy in Japan. Asian Pac J Cancer Prev. 2006;7:495–501. [PubMed] [Google Scholar]
- 39.Hara N, Sakata K, Nagai M, Fujita Y, Hashimoto T, Yanagawa H. Statistical analyses on the pattern of food consumption and digestive-tract cancers in Japan. Nutr Cancer. 1984;6:220–8. doi: 10.1080/01635588509513828. [DOI] [PubMed] [Google Scholar]
- 40.Ghadirian P, Thouez JP, PetitClerc C. International comparisons of nutrition and mortality from pancreatic cancer. Cancer Detect Prev. 1991;15:357–62. [PubMed] [Google Scholar]
- 41.Larsson SC, Bergkvist L, Wolk A. Milk and lactose intakes and ovarian cancer risk in the Swedish Mammography Cohort. Am J Clin Nutr. 2004;80:1353–7. doi: 10.1093/ajcn/80.5.1353. [DOI] [PubMed] [Google Scholar]
- 42.Thouez JP, Ghadirian P, Petitclerc C, Hamelin P. International comparisons of nutrition and mortality from cancers of the oesophagus, stomach and pancreas. Geogr Med. 1990;20:39–50. [PubMed] [Google Scholar]
- 43.Tukiendorf A. Magnesium in drinking water and liver cancer morbidity: A possible relation? Cent Eur J Public Health. 2002;10:157–62. [PubMed] [Google Scholar]
- 44.Yang CY, Cheng MF, Tsai SS, Hsieh YL. Calcium, magnesium, and nitrate in drinking water and gastric cancer mortality. Jpn J Cancer Res. 1998;89:124–30. doi: 10.1111/j.1349-7006.1998.tb00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allender PS, Cutler JA, Follmann D, Cappuccio FP, Pryer J, Elliott P. Dietary calcium and blood pressure: A meta-analysis of randomized clinical trials. Ann Intern Med. 1996;124:825–31. doi: 10.7326/0003-4819-124-9-199605010-00007. [DOI] [PubMed] [Google Scholar]
- 46.Yang CY, Chiu HF, Chiu JF, Wang TN, Cheng MF. Magnesium and calcium in drinking water and cerebrovascular mortality in Taiwan. Magnes Res. 1997;10:51–7. [PubMed] [Google Scholar]
- 47.Stevenson AC, Johnston HA, Stewart MI, Golding DR. Congenital malformations. A report of a study of series of consecutive births in 24 centres. Bull World Health Organ. 1966;(34 Suppl):9–127. [PMC free article] [PubMed] [Google Scholar]
- 48.Penrose LS. Genetics of anencephaly. J Ment Defic Res. 1957;1:4–15. doi: 10.1111/j.1365-2788.1957.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 49.Hewitt D. Geographical variations in the mortality attributed to spina bifida and other congenital malformations. Br J Prev Soc Med. 1963;17:13–22. doi: 10.1136/jech.17.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laurence KM, Carter CO, David PA. Br J Prev Soc Med. 1967;21:146. doi: 10.1136/jech.22.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogers SC. Epidemilogy of stillbirths from congenital abnormalities in England and Wales, 1961-1966. Dev Med Child Neurol. 1969;11:617–29. doi: 10.1111/j.1469-8749.1969.tb01492.x. [DOI] [PubMed] [Google Scholar]
- 52.Stocks P. Incidence of congenital malformations in the regions of England and Wales. Br J Prev Soc Med. 1970;24:67–7. doi: 10.1136/jech.24.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Record RG. Anencephalus in Scotland. Br J Prev Soc Med. 1961;15:93–105. doi: 10.1136/jech.15.3.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson WJ, Baird D, Thomson AM. Epidemiology of stillbirths and infant deaths due to congenital malformation. Lancet. 1958;1:1304–6. doi: 10.1016/s0140-6736(58)92062-2. [DOI] [PubMed] [Google Scholar]
- 55.Edwards JH. Congenital malformations of the central nervous system in Scotland. Br J Prev Soc Med. 1958;12:115–30. doi: 10.1136/jech.12.3.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laurence KM, Carter CO, David PA. Major central nervous system malformations in South Wales. II. Pregnancy factors, seasonal variation, and social class effects. Br J Prev Soc Med. 1968;22:212–2. doi: 10.1136/jech.22.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mckeown T, Record RG. Seasonal incidence of congenital malformations of the central nervous system. Lancet. 1951;1:192–6. doi: 10.1016/s0140-6736(51)93354-5. [DOI] [PubMed] [Google Scholar]
- 58.Slater BC, Watson GI, Mcdonald JC. Seasonal variation in congenital abnormalities. Preliminary report of a survey conducted by the research committee of council of the college of general practitioners. Br J Prev Soc Med. 1964;18:1–7. doi: 10.1136/jech.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leck I, Record RG. Seasonal incidence of anencephalus. Br J Prev Soc Med. 1966;20:67–75. doi: 10.1136/jech.20.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fedrick J. Anencephalus and the local water supply. Nature. 1970;227:176–7. doi: 10.1038/227176a0. [DOI] [PubMed] [Google Scholar]
- 61.Crawford MD, Gardner MJ, Morris JN. Mortality and hardness of local water-supplies. Lancet. 1968;1:827–31. doi: 10.1016/s0140-6736(68)90297-3. [DOI] [PubMed] [Google Scholar]
- 62.Martyn CN, Barker DJ, Osmond C, Harris EC, Edwardson JA, Lacey RF. Geographical relation between Alzheimer's disease and aluminum in drinking water. Lancet. 1989;1:59–62. [PubMed] [Google Scholar]
- 63.Lopez-Ridaura R, Willett WC, Rimm EB, Liu S, Stampfer MJ, Manson JE, et al. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care. 2004;27:134–40. doi: 10.2337/diacare.27.1.134. [DOI] [PubMed] [Google Scholar]
- 64.Miyake Y, Yokoyama T, Yura A, Iki M, Shimizu T. Ecological association of water hardness with prevalence of childhood atopic dermatitis in a Japanese urban area. Environ Res. 2004;94:33–7. doi: 10.1016/s0013-9351(03)00068-9. [DOI] [PubMed] [Google Scholar]
- 65.Bellizzi V, De Nicola L, Minutolo R, Russo D, Cianciaruso B, Andreucci M, et al. Effects of water hardness on urinary risk factors for kidney stones in patients with idiopathic nephrolithiasis. Nephron. 1999;81:66–70. doi: 10.1159/000046301. [DOI] [PubMed] [Google Scholar]
- 66.Sengupta P, Sarkar M, Chandra A. 18th West Bengal State Science and Technology Congress; 2011. Hard water intake and its consequence on male reproductive physiology; pp. 113–4. [Google Scholar]
- 67.Dave G. Effects of fluoride on growth, reproduction and survival in Daphnia magna. Comp Biochem Physiol C. 1984;78:425–31. doi: 10.1016/0742-8413(84)90110-5. [DOI] [PubMed] [Google Scholar]
- 68.Dutta S, Joshi KR, Sengupta P, Bhattacharya K. Unilateral and bilateral cryptorchidism and its effect on the testicular morphology, histology, accessory sex organs and sperm count in Laboratory Mice. J Hum Repro Sci. doi: 10.4103/0974-1208.117172. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sengupta P, Sarkar M, Chandra A. abstract of national conference by vidyasagar university; 2010. Effect of consumption of excess hard water salts on male gonadal status in adult albino rats; p. 277. [Google Scholar]
- 70.Chandra AK, Sengupta P, Goswami H, Sarkar M. Excessive dietary calcium in the disruption of structural and functional status of adult male reproductive system in rat with possible mechanism. Mol Cell Biochem. 2012;364:181–191. doi: 10.1007/s11010-011-1217-3. [DOI] [PubMed] [Google Scholar]
- 71.Sengupta P. The laboratory rat: Relating its age with human's. Int J Prev Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 72.Sengupta P, Chaudhuri P, Bhattacharya K. Male Reproductive Health and Yoga. Int J Yoga. 2013;6:87–95. doi: 10.4103/0973-6131.113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sengupta P. Health impacts of yoga and pranayama: a state-of-the-art review. Int J Prev Med. 2012;3:444–58. [PMC free article] [PubMed] [Google Scholar]
- 74.Sengupta P. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem Toxicol. 2013;36:353–368. doi: 10.3109/01480545.2012.710631. [DOI] [PubMed] [Google Scholar]
- 75.Chandra AK, Goswami H, Sengupta P. Dietary calcium induced cytological and biochemical changes in thyroid. Environ Toxicol Pharmacol. 2012;34:454–65. doi: 10.1016/j.etap.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 76.Costi D, Calcaterra PG, Iori N, Vourna S, Nappi G, Passeri M. Importance of bioavailable calcium drinking water for the maintenance of bone mass in post-menopausal women. J Endocrinol Invest. 1999;22:852–6. doi: 10.1007/BF03343658. [DOI] [PubMed] [Google Scholar]
- 77.Aptel I, Cance-Rouzaud A, Grandjean H. Association between calcium ingested from drinking water and femoral bone density in elderly women: Evidence from the EPIDOS cohort. J Bone Miner Res. 1999;14:829–33. doi: 10.1359/jbmr.1999.14.5.829. [DOI] [PubMed] [Google Scholar]
- 78.Crawford MD, Gardner MJ, Sedgwick PA. Infant mortality and hardness of local water supplies. Lancet. 1972;1:988–2. doi: 10.1016/s0140-6736(72)91157-9. [DOI] [PubMed] [Google Scholar]
- 79.Bound JP, Harvey PW, Brookes DM, Sayers BM. The incidence of anencephalus in the Fylde peninsula 1956-76 and changes in water hardness. J Epidemiol Community Health. 1981;35:102–5. doi: 10.1136/jech.35.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zemła B. Geography of the incidence of stomach cancer in relation to hardness of drinking water and water supply. Wiad Lek. 1980;33:1027–31. [PubMed] [Google Scholar]
- 81.Wigle DT, Mao Y, Semenciw R, Smith MH, Toft P. Contaminants in drinking water and cancer risks in Canadian cities. Can J Public Health. 1986;77:335–42. [PubMed] [Google Scholar]
- 82.Sengupta P. Chemosterilization: Spermatogenesis, steroidogenesis, reproductive functions, and behavior from historical perspective to contemporary practice. J Basic Clin Repro Sci. 2013;2:1–2. [Google Scholar]
- 83.Sengupta P, Sahoo S. Evaluation of health status of fishers: prediction of cardiovascular fitness and anaerobic power. World J Life Sci Med Res. 2011;1:25–30. [Google Scholar]
- 84.Sengupta P, Goswami H, Chandra AK. Environmental threat to male fertility by hard water metals: How protective are the citrus foods? 100th Indian Science Congress. 2013:201–202. [Google Scholar]
- 85.Sengupta P. A Scientific review of age determination for a laboratory rat: How old is it in comparison with human age? Biomed Int. 2011;2:81–89. [Google Scholar]
- 86.Sengupta P, Goswami H, Chandra AK. 19th West Bengal State Science & Technology Congress; 2013. Which one is more potent in ameliorating the impacts of chronic hard water induced male infertility, citric acid or EDTA? p. 247. [Google Scholar]


