Abstract
Background:
There is conflicting evidence regarding the relationship between iron stores and cardiovascular disease (CVD). The present study aimed to investigate the association between body iron indices and some cardiovascular risk factors.
Methods:
In a case–control study conducted in the south of Shiraz, Iran, we determined ferritin, iron, total iron binding capacity (TIBC), metabolic risk factors, C-reactive protein (CRP), and anthropometric measurements in 100 men aged 45 years and higher with newly diagnosed CVD and 100 adjusted controls without evidence for CVD.
Results:
The mean of low density lipoprotein (LDL-c), CRP, and ferritin concentrations were significantly higher in cases than controls, and high density lipoprotein (HDL-c) was significantly lower in cases than controls. Pearson correlation coefficient between CRP and the other risk factors in case group showed that only ferritin, serum iron, waist circumference, and LDL-c significantly correlated with CRP (r = 0.32 with P < 0.001, r = 0.29 with P < 0.05, r = 0.41 with P < 0.01, and r = 0.36 with P < 0.001, respectively).
Conclusions:
This study indicated an association between a positive balance of body iron and CVD. Hence, caution should be exercised in administration of iron supplements to patients with CVD and in consumption of food rich in iron by them.
Keywords: C-reactive protein, cardiovascular disease, ferritin
INTRODUCTION
Although researchers have made tremendous gains in understanding cardiovascular disease (CVD) over the past several decades, traditionally recognized risk factors such as cholesterol levels, blood pressure, smoking, obesity, and sedentary lifestyle only account for 50% of the incidence of heart disease. It is clear that aggressive approaches to correct elevated cholesterol levels and recommendations for modifying other coronary risk factors are not enough. With this in mind, recent researches have taken a sharp turn to identify other casual factors such as chronic infections and elevated iron levels. Iron is a key component in catalyzing the production of reactive radicals and causing oxidative stress and lipid peroxidation.[1,2,3,4] Oxidative stress and lipid peroxidation have been linked to several pathologies, including atherosclerosis.[5,6,7]
Excessive iron has been proposed to be a potent risk factor for coronary heart disease (CHD), especially for acute myocardial infarction (AMI).[8,9,10] Supporting evidence comes from in vitro lipid peroxidation and lipoprotein modification studies,[11,12] cholesterol-fed iron-overloaded animal models,[13,14] and analyses of the composition of human atherosclerotic lesions,[15,16] although there is conflicting evidence regarding the relationship between iron and CVD.
Of these, increased estimated body iron stores have been associated with increased risk of CHD death or AMI in some,[17,18] but not in all studies.[19,20,21] On the other hand, chronic inflammation has been hypothesized to promote the development and progression of atherosclerosis. Higher levels of inflammatory markers such as C-reactive protein (CRP) have indicated increased CVD risk.[22,23] Therefore, the purpose of the present study was to evaluate the relationship between body iron situation with cardiovascular risk factors and inflammation in patients with CVD.
METHODS
Participants and design
The present study is a case-control study. The study sample comprised 883 men, older than 45 years, who referred to Al-zahra Heart Hospital of Shiraz University of Medical Sciences between 2007 and 2009 for coronary angiography. Coronary angiograms were assessed by an experienced cardiologist. Finally, 100 participants who fulfilled all the inclusion criteria were chosen. Criteria for case inclusion were: (a) age older than 45 years; (b) no prevalent CHD (prevalent CHD was defined as either a history of AMI or angina pectoris, positive angina pectoris on effort, or use of nitroglycerin tablets); (c) absence of any systematic disease; (d) no vitamin or mineral supplements, antihypertensive drugs, and antilipidemic medications taken regularly during previous years; (e) no smoking; and f) significant artery disease (was defined by the presence of coronary stenosis). One hundred control participants, matched according to age, sex, coronary angiogram examination, and place of residence, were chosen.
Participants were given an oral and written explanation of the study, including its procedures, and were asked to read and sign an informed consent document. The study protocol and ethical aspects were approved by the ethics committee of the Research Council of the Dean of Research Affairs of Shiraz University of Medical Sciences.
Background characteristics
Demographic data, any concurrent illness history, and information on medication, smoking, and vitamin and mineral supplementation were collected by interviews. Anthropometric assessment was done, and food consumption data were collected. Anthropometric assessments included measurement of weight and height. Body weight was measured to the nearest 0.1 kg using the Seca 713 scales while the participants were minimally clothed. Height of the participants without shoes was determined using measuring tape, and subsequently body mass index (BMI) was calculated by dividing weight (kg) by squared height (m2). Waist circumference (WC) was measured using an inelastic tape over light clothing at the point midway between the iliac crest and the last floating rib at the end of a normal inhalation. Hip circumference (HC) was measured at the maximal gluteal protrusion or at the most prominent area of the buttocks at the level of symphysis pubis in a horizontal plane.
Biochemical measurements
After the patients underwent coronary angiography, 5 ml fasting venous blood samples were drawn from the arm. Blood was collected for measurement of serum ferritin, total iron binding capacity (TIBC), serum iron, total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL-c), high-density lipoprotein (HDL-c), and C-reactive protein (CRP). Ferritin was measured using the enzyme-linked immunosorbent assay (ELISA) method [the interassay coefficient of variation (CV) ranged from 3.8% to 5.1%]; serum iron (CVs ranged from 3.1% to 3.6%) and TIBC (CVs ranged from 3.3% to 5.1%) were measured by the colorimetric method. TC (CVs ranged from 3.3% to 4.1%), TG (CVs ranged from 2.9% to 3.6%), and HDL-c (CVs ranged from 3.6% to 4.2%) were measured using enzymatic colorimetric assay. CRP (CVs ranged from 4.3% to 6.9%) was measured using immunoturbidometric assay. The LDL-c was estimated using the Friedewald formula: LDL-c = TC – HDL-c – (TG/5).[24]
Statistical analysis
Data processing and statistical analyses were done using SPSS version 11 for Windows (SPSS Inc., Chicago, IL, USA, 2001). Normally distributed data were expressed as mean (±SDs) and were compared by independent Student's t-test. The simple linear regression model was used to test for possible association (s), and multiple linear regression analysis using stepwise methods was performed to determine the most significant predictors of changes, i.e., CRP. Significance was set at P < 0.05.
RESULTS
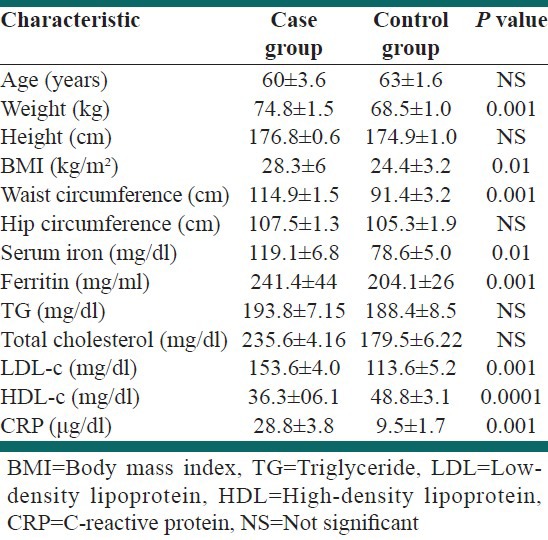
The main risk factor characteristics among the case and control participants are shown in Table 1. The mean serum ferritin, serum iron, CRP, weight, BMI, WC, and LDL-c were significantly higher and HDL-c was significantly lower among the cases than the controls.
Table 1.
Demographic and laboratory characteristics of the study population
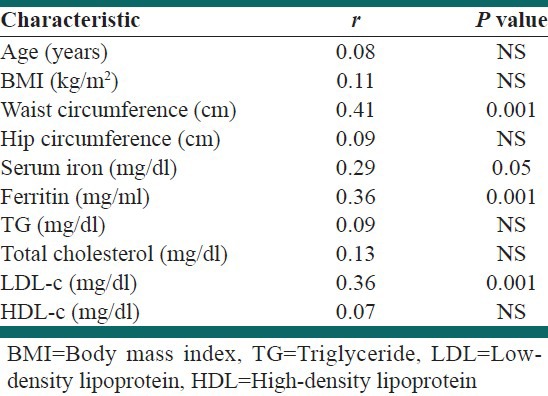
It is observed in Table 2 that the Pearson correlation coefficients between CRP and the other risk factors in case group showed that only ferritin, serum iron, WC, and LDL-c significantly correlated with CRP (r = 0.32 with P < 0.001, r = 0.29 with P < 0.05, r = 0.41 with P < 0.01, and r = 0.36 with P < 0.001, respectively). Further investigation into the changes in CRP in the case group was carried out using multiple regression analysis in which the independent variables included were: Ferritin, serum iron, BMI, WC, HC, LDL, and HDL. Using regression procedure, only WC contributed significantly to the CRP (r = 0.58, P < 0.001).
Table 2.
Correlation of risk factors with C-reactive protein in case group
DISCUSSION
The result of our study showed that the concentration of ferritin as an indicator of iron status was significantly higher in patients with CVD. More than two decades ago, it was proposed that differences in body iron stores may account for differential heart disease prevalence in men and women.[25] The iron-heart disease hypothesis rests on the supposition that high body iron burdens are a risk factor for increased oxidative stress, and oxidative stress is a risk factor for chronic diseases such as heart disease. Initial support for the iron hypothesis came from a prospective study on middle-aged eastern Finnish men,[26] which showed that men with serum ferritin concentration higher than 200 μg/l had a 2.2-fold factor-adjusted risk of AMI compared with those with serum ferritin concentration lower than 200 μg/l. Also, in a cohort study, Tuomanieu and co-workers[27] showed that voluntary blood donors had a relative AMI risk of 0.14 compared with non-donors. Short-term changes in blood constituents take place after blood donation, and the reduction in serum ferritin concentration, indicating loss of iron, is the marked consequence. But based on in vivo and in vitro findings, this hypothesis is inconsistent. While the results of some studies have been in favor of iron being a risk factor, others have not.[28]
A possible explanation for the controversy concerning ferritin levels and CAD may be the fact that ferritin is an acute phase reactant. Although serum ferritin concentration is the best noninvasively measurable indicator of body iron stores, ferritin is an acute phase protein that may become elevated in inflammation. Therefore, studies that used myocardial infarction as atherosclerosis indicator may be confounded by the inflammatory response associated with these conditions. However, to rule out potential confounding, we assessed serum iron and TIBC also. But the best indicator of iron status could be measurement of serum soluble transferrin receptor concentration. On the other hand, we excluded subjects with any other disease that may be the cause of elevated ferritin.
Several lines of evidence have suggested an important role of inflammation in the development of atherosclerosis and CVDs. Some studies showed that increases in inflammatory indices such as CRP are very responsive to iron stores. In our study, the statistical model showed that when the serum ferritin concentration was used as an independent variable, participants with higher level of ferritin had a higher concentration of CRP (r = 0.39, P < 0.001). On the other hand, in our study, CRP has a significant correlation with LDL-c. An explanation for the correlation between ferritin and CRP in this study may be the fact that lipid peroxidation via the interaction of lipids with iron may play a role in increasing oxidative stress, thereby promoting chronic inflammation. However, the association between ferritin and CRP may be explained by infection.
CRP is made by the liver in response to inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNFα).[29] Adipose tissue is a major source of these inflammatory cytokines.[30] Consequently, a strong positive association has been found between measures of obesity, such as WC and BMI, and CRP.[31,32] Our study did not show a significant correlation between obesity and CRP, whereas WC as an indicator of central obesity showed positive correlation with CRP (r = 0.41, P < 0.001). This finding suggests that BMI is an indicator of heaviness rather than fatness, and cannot distinguish body fat from fat-free mass. Further investigation into the changes in CRP concentration in these participants was carried out using multiple regression analysis in which the independent variables included were: age, BMI, WC, HC, serum iron, ferritin, TG, TC, LDL-c, and HDL-c. Using a stepwise regression procedure, only WC contributed significantly to the CRP level (r = −0.39, P < 0.001); thus, participants with higher WC also had a higher CRP concentration. An excess of visceral fat associated with obesity is an important source of molecules including metabolic disorders. Inflammatory cytokines produced in visceral fat cause elevation of serum CRP, which is reported to be positively correlated with a number of metabolic alterations, such as CVD.
In this study, a limitation was that serum ferritin is an acute phase reactant that is elevated in infections and inflammation, although we controlled this problem by measuring serum iron and TIBC, and also excluded participants with any other disease that tends to increase the concentration of iron in the body.
CONCLUSION
In conclusion, although in this study we showed an association between serum ferritin and CRP as a potent cardiovascular risk factor, by using multiple regression analysis, between all independent variables, only WC showed significant correlation with CRP. So, we can suggest that WC is a better surrogate of elevation CRP. On the other hand, the result of this study revealed that elevated level of body iron combined with elevated levels of LDL-c and WC are associated with significantly higher level of CRP.
ACKNOWLEDGMENTS
The present study was funded by the grant number 86-3457 from Shiraz University of Medical Sciences. We gratefully acknowledge all 200 participants for their good cooperation. We also thank staff of Al-zahra Heart Hospital of Shiraz University of Medical Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Rehema A, Zilmer M, Zilmer K, Kullisaar T, Vihalemm T. Could long-term alimentary iron overload have an impact on the parameters of oxidative stress? A study on the basis of a village in southern Estonia. Ann Nutr Metab. 1998;42:40–3. doi: 10.1159/000012716. [DOI] [PubMed] [Google Scholar]
- 2.Glei M, Latunde-Dada GO, Klinder A, Becker TW, Hermann U, Voigt K, et al. Iron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19A. Mutat Res. 2002;519:151–61. doi: 10.1016/s1383-5718(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 3.Fischer JG, Glauert HP, Yin T, Sweeney-Reeves ML, Larmonier N, Black MC. Moderate iron overload enhances lipid peroxidation in livers of rats, but does not affect NF-kappaB activation induced by the peroxisome proliferator, Wy-14, 643. J Nutr. 2002;132:2525–31. doi: 10.1093/jn/132.9.2525. [DOI] [PubMed] [Google Scholar]
- 4.Day SM, Duquaine D, Mundada LV, Menon RG, Khan BV, Rajagopalan S, et al. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation. 2003;107:2601–6. doi: 10.1161/01.CIR.0000066910.02844.D0. [DOI] [PubMed] [Google Scholar]
- 5.Brown SM, Bicknell R. Hypoxia and oxidative stress in breast cancer. Oxidative stress: Its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001;3:323–7. doi: 10.1186/bcr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunez MT, Tapia VS, Toyokuni S, Okada S. Iron-induced oxidative damage in colon carcinoma (Caco-2) cells. Free Rad Res. 2001;34:57–68. doi: 10.1080/10715760100300061. [DOI] [PubMed] [Google Scholar]
- 7.Esterbauer H, Wag G, Puhl H. Lipid peroxidation and its role in atherosclerosis. Br Med Bull. 1993;49:566–76. doi: 10.1093/oxfordjournals.bmb.a072631. [DOI] [PubMed] [Google Scholar]
- 8.Tuomainen TP, Punnonen K, Nyyssönen K, Salonen JT. Association between body iron stores and the risk of acute myocardial infarction in men. Circulation. 1998;97:1461–6. doi: 10.1161/01.cir.97.15.1461. [DOI] [PubMed] [Google Scholar]
- 9.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–92. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salonen JT, Ylä-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, et al. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–7. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 11.Smith LL, Johnson BH. Biological activities of oxysterols. Free Radic Biol Med. 1989;7:285–332. doi: 10.1016/0891-5849(89)90136-6. [DOI] [PubMed] [Google Scholar]
- 12.Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 13.Russell DW. Oxysterol biosynthetic enzymes. Biochim Biophys Acta. 2000;1529:126–35. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 14.Meaney S, Hassan M, Sakinis A, Lütjohann D, von Bergmann D, Wennmalm A, et al. Evidence that the major oxysterols in human circulation originate from distinct pools of cholesterol: A stable isotope study. J Lipid Res. 2001;42:70–8. [PubMed] [Google Scholar]
- 15.Salonen JT. Is there a continuing need for longitudinal epidemiologic research? The Kuopio Ischaemic Heart Disease Risk Factor Study. Ann Clin Res. 1988;20:46–50. [PubMed] [Google Scholar]
- 16.MONICA Manual, Part IV, Event Registration. [Last accessed on 2012 Oct 30]. Available from: http://wwwktl.fi/publications/monica/manual/index.htm .
- 17.Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86:803–11. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- 18.Salonen JT, Nyyssönen K, Salonen R. Body iron stores and the risk of coronary heart disease. N Engl J Med. 1994;331:1159. doi: 10.1056/NEJM199410273311714. [DOI] [PubMed] [Google Scholar]
- 19.Baer DM, Tekawa IS, Hurley LB. Iron stores are not associated with acute myocardial infarction. Circulation. 1994;89:2915–8. doi: 10.1161/01.cir.89.6.2915. [DOI] [PubMed] [Google Scholar]
- 20.Sempos CT, Looker AC, Gillum RF, Makuc DM. Body iron stores and the risk of coronary heart disease. N Engl J Med. 1994;330:1119–24. doi: 10.1056/NEJM199404213301604. [DOI] [PubMed] [Google Scholar]
- 21.Liao Y, Cooper RS, McGee DL. Iron status and coronary heart disease: Negative findings from the NHANES I epidemiologic follow-up study. Am J Epidemiol. 1994;139:704–12. doi: 10.1093/oxfordjournals.aje.a117060. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis. JAMA. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 24.Friiedewald WT, Levi RI, Fredrickson DS. Estimation of the concentration of low density lipoproteins cholesterol in plasma without use of the ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1:1293–4. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- 26.Mainous AG, Wells BJ, Everett CJ, Gill JM, King DA. Association of ferritiin and lipids with, C-reactive protein. Am J Card. 2004;93:559–62. doi: 10.1016/j.amjcard.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314:793–4. doi: 10.1136/bmj.314.7083.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zegrean M. Association of body iron stores with development of cardiovascular disease in the adult population: A systematic review of the literature. Can J Cardiovasc Nurs. 2009;19:26–32. [PubMed] [Google Scholar]
- 29.Bullo M, Garcia-Lorda P, Megias I, Salas-Salvado J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;85:3338–42. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 30.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance and endothelial dysfunction: A potential role for cytokines originating from adipose tissue. Int J Obes. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 31.Santos AC, Lopes C, Guimaraes JT, Barros H. Central obesity as a major determinant of increased high-sensitivity C-reactive protein in metabolic syndrome. Int J Obes. 2005;29:1452–6. doi: 10.1038/sj.ijo.0803035. [DOI] [PubMed] [Google Scholar]
- 32.Shemesh T, Rowley KG, Jenkins A, Brimblecombe J, Best JD, O’Dea K. Differential association of C-reactive protein with adiposity in men and women in an Aboriginal community in northeast Arnhem Land of Australia. Int J Obes. 2007;31:103–8. doi: 10.1038/sj.ijo.0803350. [DOI] [PubMed] [Google Scholar]


